Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
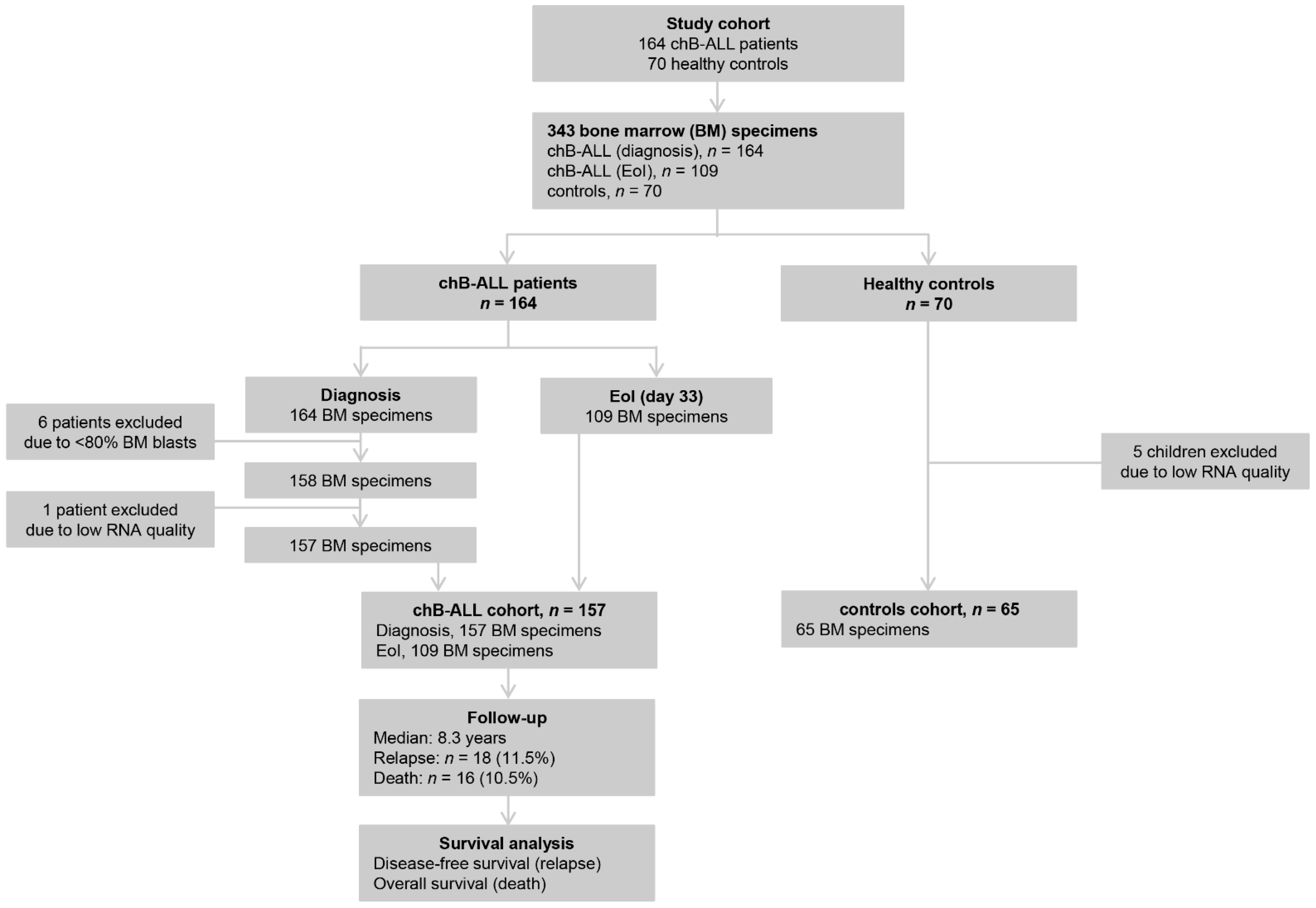
2.1. Study Patients’ Cohort
2.2. Cytogenetics
2.3. Immunophenotype Analysis
2.4. MRD Evaluation
2.5. Isolation of Total RNA
2.6. First-Strand cDNA Synthesis
2.7. Quantitative Real-Time PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Baseline Clinical and Experimental Data
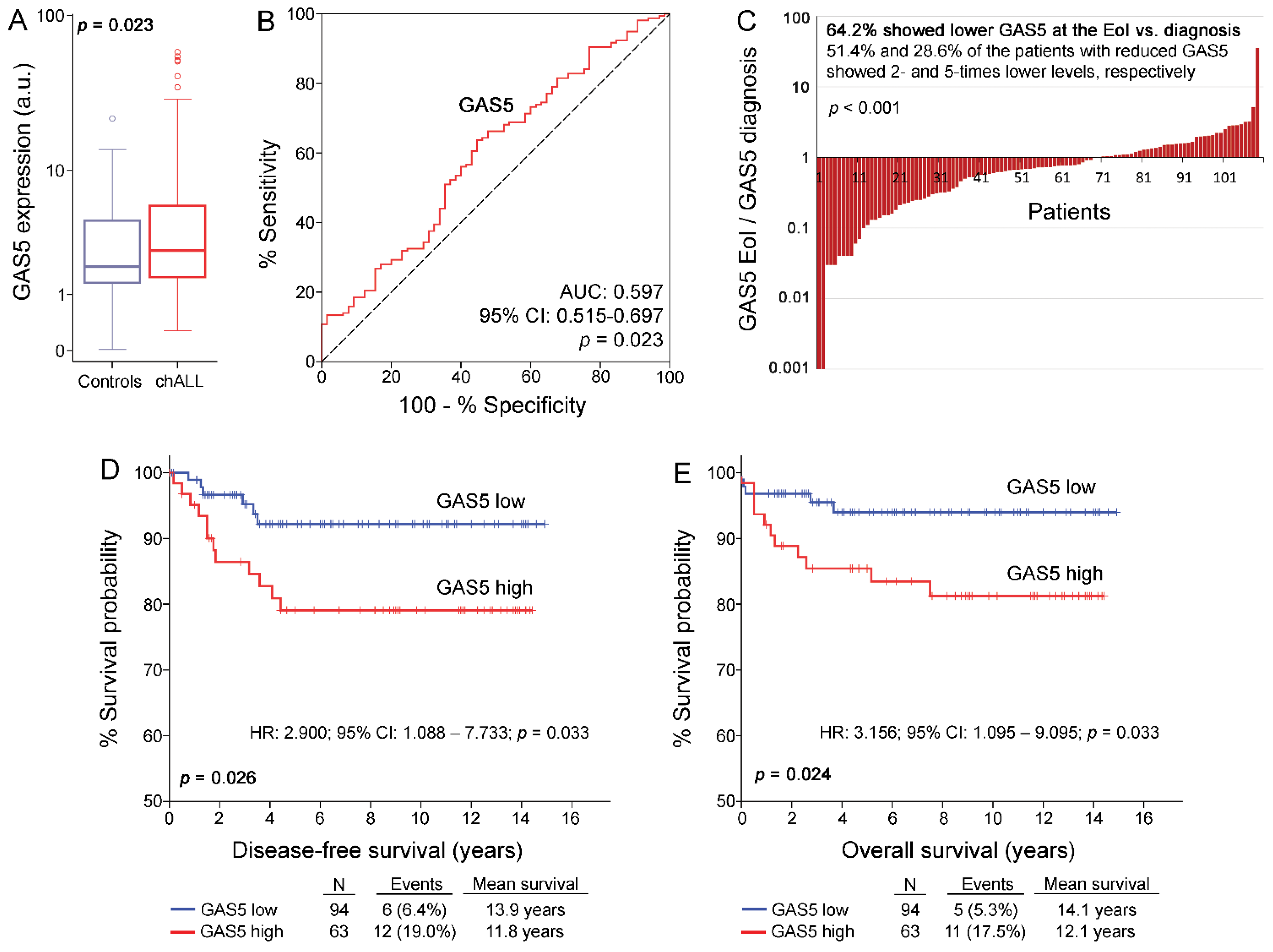
3.2. GAS5 Is Overexpressed in chALL Patients and Decreases to Normal Levels at the EoI
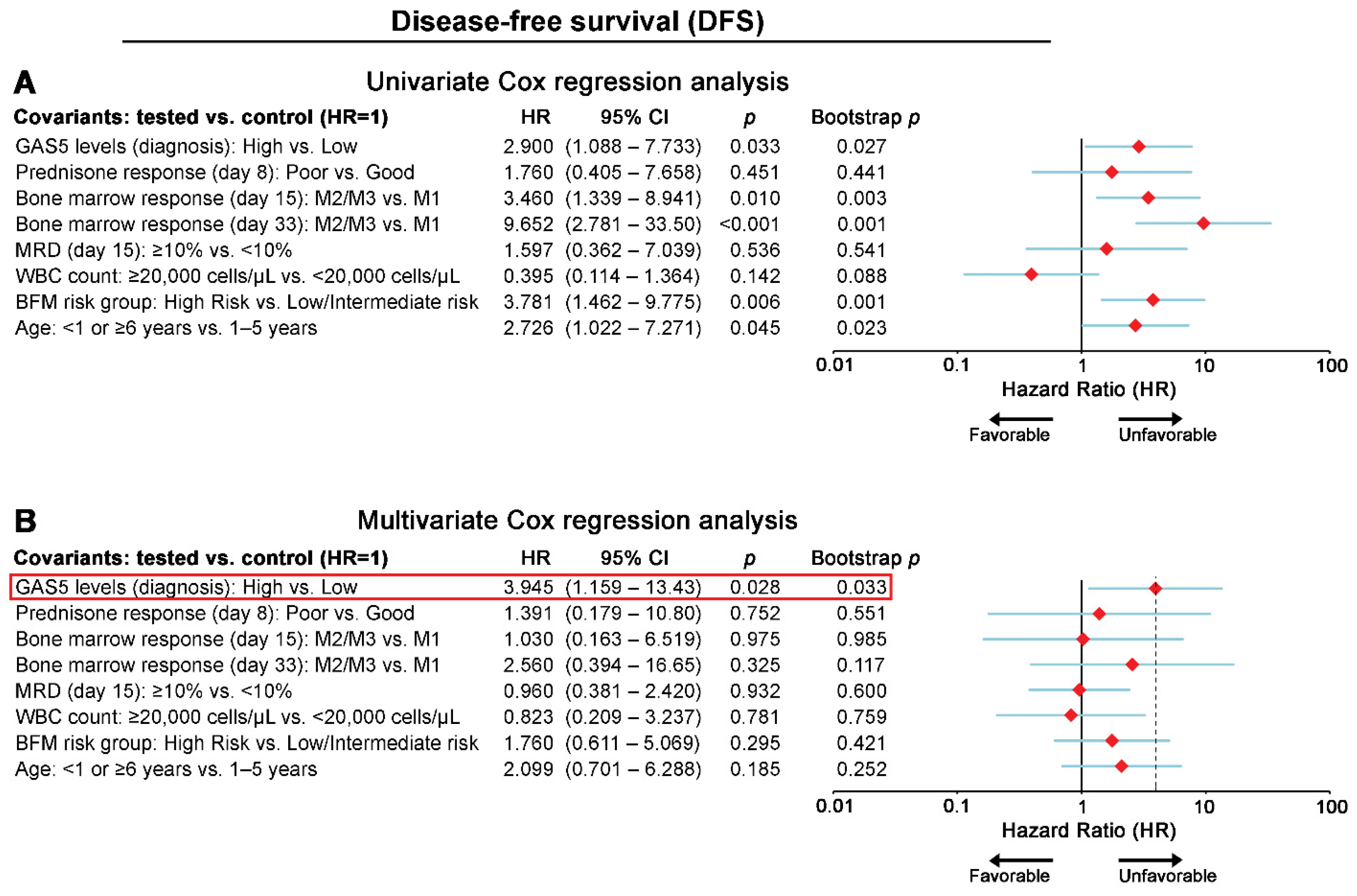
3.3. chALL Patients Overexpressing GAS5 Are at Significantly Higher Risk for Short-Term Relapse and Poor Treatment Outcome
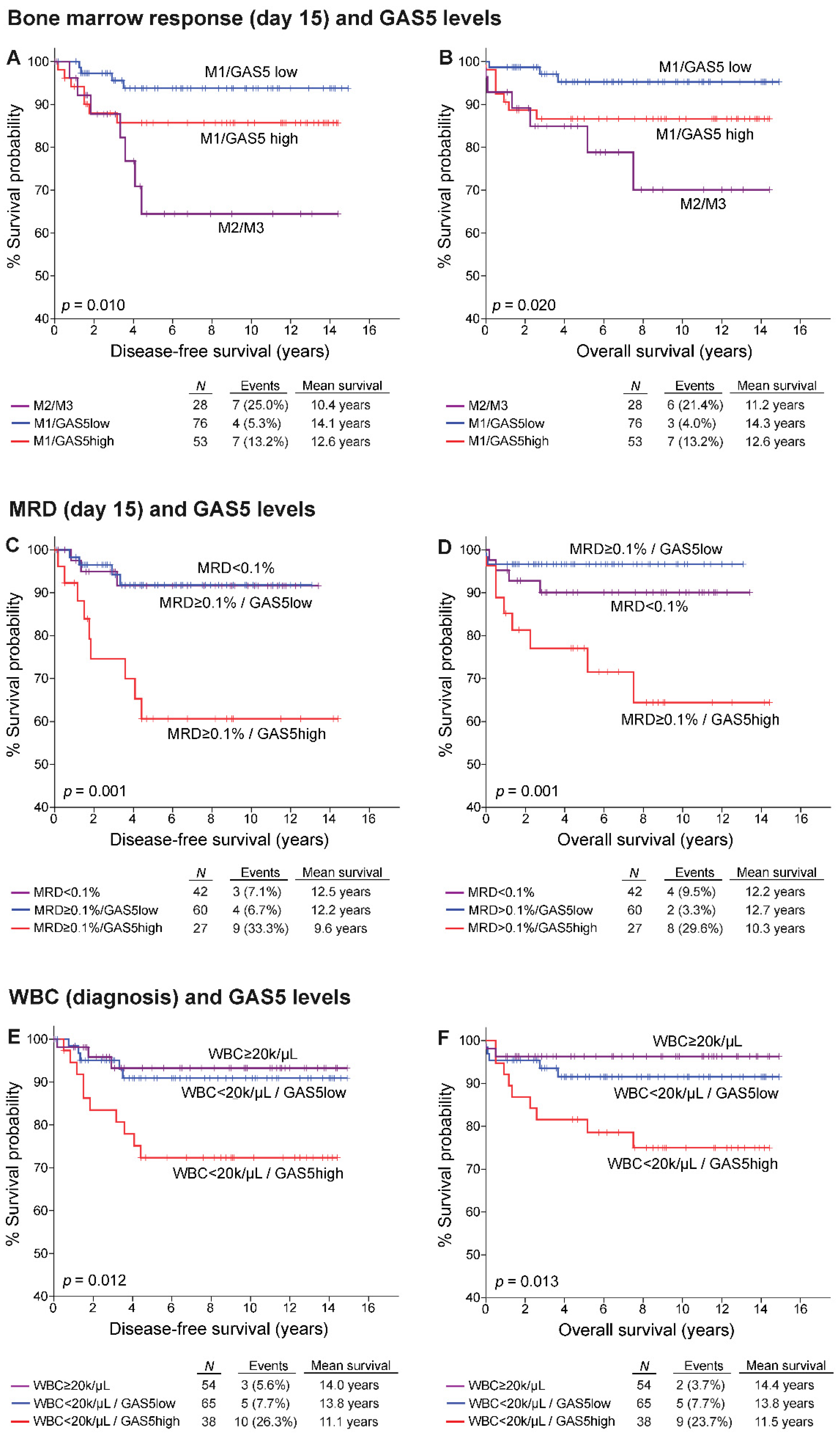
3.4. GAS5 Significantly Benefits the Prognostic Value of chALL Clinically Used Markers
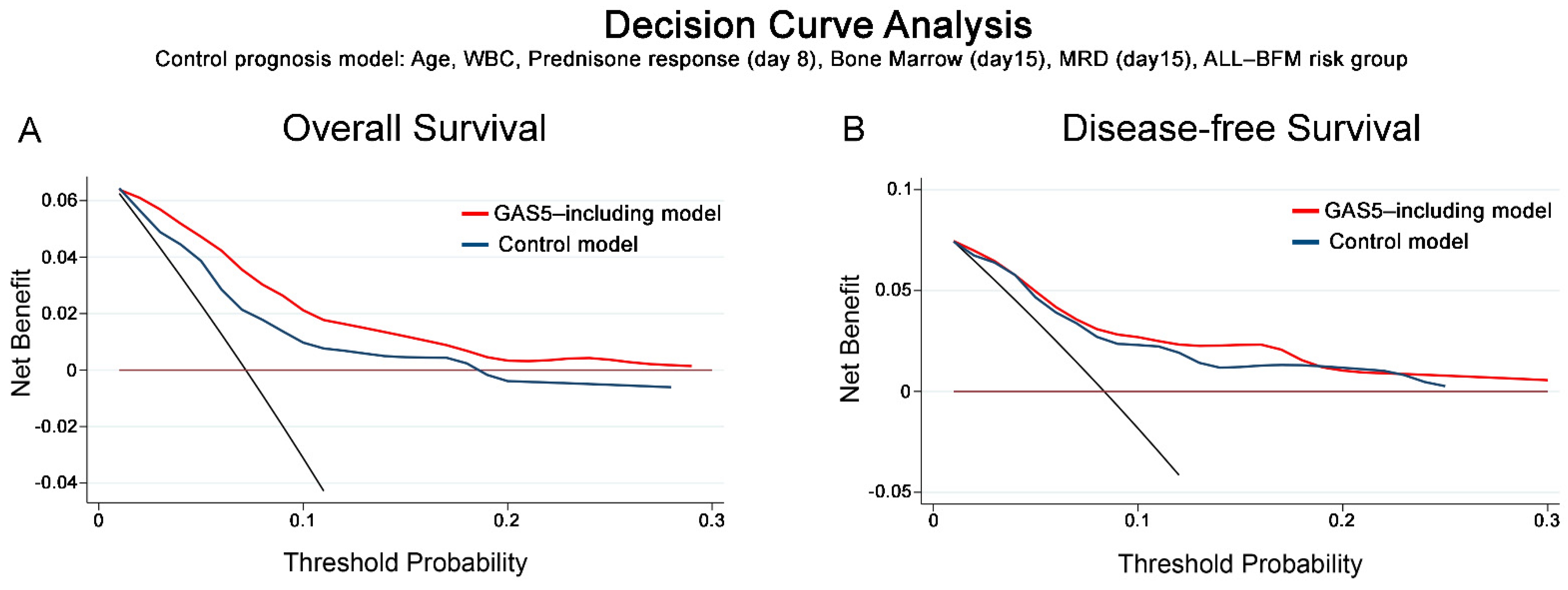
3.5. DCA Demonstrated the Clinical Benefit of GAS5 Evaluation in chALL Prognosis
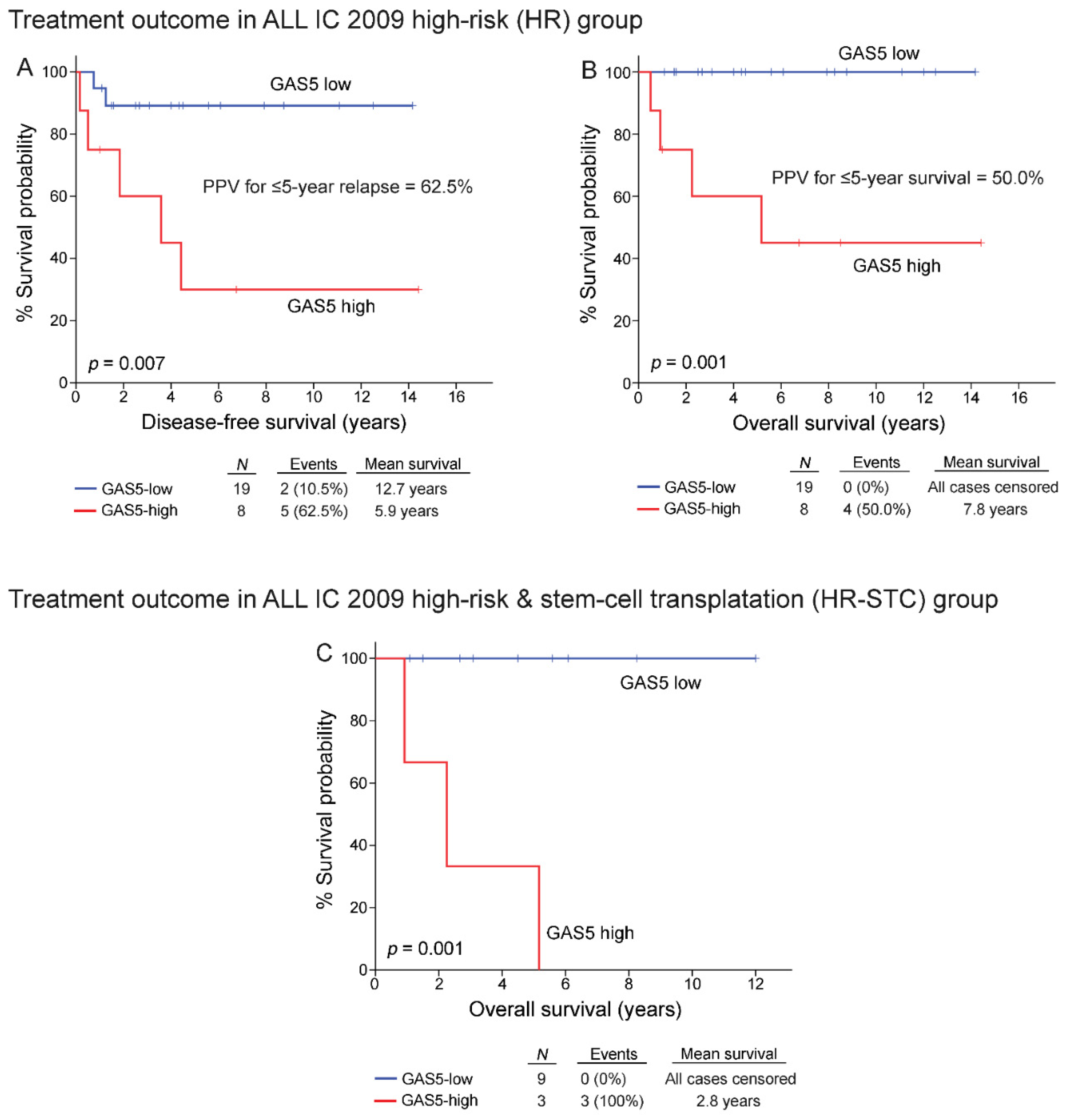
3.6. High-Risk Patients Overexpressing GAS5 Demonstrated Poor Treatment Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. 24), 5178–5189. [Google Scholar] [CrossRef] [Green Version]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955. [Google Scholar] [CrossRef] [Green Version]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaba, H.; Pui, C.H. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010, 11, 1096–1106. [Google Scholar] [CrossRef] [Green Version]
- Pui, C.H.; Evans, W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheschowitsch, K.; Leite, J.A.; Assreuy, J. New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front. Endocrinol 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrappe, M.; Reiter, A.; Zimmermann, M.; Harbott, J.; Ludwig, W.D.; Henze, G.; Gadner, H.; Odenwald, E.; Riehm, H. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Leukemia 2000, 14, 2205–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stary, J.; Zimmermann, M.; Campbell, M.; Castillo, L.; Dibar, E.; Donska, S.; Gonzalez, A.; Izraeli, S.; Janic, D.; Jazbec, J.; et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: Results of the randomized intercontinental trial ALL IC-BFM 2002. J. Clin. Oncol. 2014, 32, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Aguirre, M.; Torres-Lopez, L.; Pottosin, I.; Dobrovinskaya, O. Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol. Front. Oncol. 2021, 11, 617937. [Google Scholar] [CrossRef]
- Scheijen, B. Molecular mechanisms contributing to glucocorticoid resistance in lymphoid malignancies. Cancer Drug Resist. 2019, 2, 647–664. [Google Scholar] [CrossRef] [Green Version]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Schneider, C.; King, R.M.; Philipson, L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Gibb, E.A.; Vucic, E.A.; Enfield, K.S.; Stewart, G.L.; Lonergan, K.M.; Kennett, J.Y.; Becker-Santos, D.D.; MacAulay, C.E.; Lam, S.; Brown, C.J.; et al. Human cancer long non-coding RNA transcriptomes. PLoS ONE 2011, 6, e25915. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5’-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol. Cell Biol. 1998, 18, 6897–6909. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.J.; Chatterjee, S.; Teresky, A.; Levine, A.J. The gas5 gene is disrupted by a frameshift mutation within its longest open reading frame in several inbred mouse strains and maps to murine chromosome 1. Mamm. Genome 1998, 9, 773–774. [Google Scholar] [CrossRef]
- Pickard, M.R.; Williams, G.T. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes 2015, 6, 484–499. [Google Scholar] [CrossRef] [Green Version]
- Tani, H.; Torimura, M.; Akimitsu, N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS ONE 2013, 8, e55684. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Williams, G.T. Growth arrest on inhibition of nonsense-mediated decay is mediated by noncoding RNA GAS5. BioMed Res. Int. 2013, 2013, 358015. [Google Scholar] [CrossRef] [PubMed]
- Isken, O.; Maquat, L.E. Quality control of eukaryotic mRNA: Safeguarding cells from abnormal mRNA function. Genes Dev. 2007, 21, 1833–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [Green Version]
- Hudson, W.H.; Pickard, M.R.; de Vera, I.M.; Kuiper, E.G.; Mourtada-Maarabouni, M.; Conn, G.L.; Kojetin, D.J.; Williams, G.T.; Ortlund, E.A. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat. Commun. 2014, 5, 5395. [Google Scholar] [CrossRef] [Green Version]
- Mourtada-Maarabouni, M.; Hedge, V.L.; Kirkham, L.; Farzaneh, F.; Williams, G.T. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J. Cell Sci. 2008, 121, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Mourtada-Maarabouni, M.; Hasan, A.M.; Farzaneh, F.; Williams, G.T. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Mol. Pharmacol. 2010, 78, 19–28. [Google Scholar] [CrossRef]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The lncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pui, C.H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, G.I.; Hatziagapiou, K.; Zaravinos, A. The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance. Int. J. Mol. Sci. 2020, 21, 7633. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xie, H.; Zhan, H.; Li, J.; Liu, Y.; Huang, W. Prognostic Values of Long Noncoding RNA GAS5 in Various Carcinomas: An Updated Systematic Review and Meta-Analysis. Front. Physiol. 2017, 8, 814. [Google Scholar] [CrossRef] [Green Version]
- Avgeris, M.; Tsilimantou, A.; Levis, P.K.; Tokas, T.; Sideris, D.C.; Stravodimos, K.; Ardavanis, A.; Scorilas, A. Loss of GAS5 tumour suppressor lncRNA: An independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br. J. Cancer 2018, 119, 1477–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dorr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Bhatia, R. Stem cell quiescence. Clin. Cancer Res. 2011, 17, 4936–4941. [Google Scholar] [CrossRef] [Green Version]
- Cheshier, S.H.; Morrison, S.J.; Liao, X.; Weissman, I.L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1999, 96, 3120–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.S.; Gnamlin, P.; Durden, B.; Gupta, V.K.; Kesh, K.; Garrido, V.T.; Dudeja, V.; Saluja, A.; Banerjee, S. Long non-coding RNA GAS5 acts as proliferation “brakes” in CD133+ cells responsible for tumor recurrence. Oncogenesis 2019, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Xiao, D. Long non-coding RNA GAS5 is critical for maintaining stemness and induces chemoresistance in cancer stem-like cells derived from HCT116. Oncol. Lett. 2020, 19, 3431–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, J.; Tian, G.; Cheung, H.H.; Wei, W.; Lee, T.L. Gas5 is an essential lncRNA regulator for self-renewal and pluripotency of mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res. Ther. 2018, 9, 71. [Google Scholar] [CrossRef]
- Gasic, V.; Stankovic, B.; Zukic, B.; Janic, D.; Dokmanovic, L.; Krstovski, N.; Lazic, J.; Milosevic, G.; Lucafo, M.; Stocco, G.; et al. Expression Pattern of Long Non-coding RNA Growth Arrest-specific 5 in the Remission Induction Therapy in Childhood Acute Lymphoblastic Leukemia. J. Med. Biochem. 2019, 38, 292–298. [Google Scholar] [CrossRef]

| Variables | N | % |
|---|---|---|
| Gender | ||
| Male | 89 | 56.7 |
| Female | 68 | 43.3 |
| Age | ||
| 1–6 years | 82 | 52.2 |
| 6–10 years | 26 | 16.6 |
| <1 or ≥10 years | 49 | 31.2 |
| WBC | ||
| <20,000 cells/μL | 103 | 65.6 |
| ≥20,000 cells/μL | 54 | 34.4 |
| High Hyperdiploidy (>50 chrom.) | ||
| Yes | 26 | 17.3 |
| No | 124 | 82.7 |
| Unknown | 7 | |
| ETV6-RUNX1/t(12;21)(p13;q22) | ||
| Negative | 114 | 73.1 |
| Positive | 42 | 26.9 |
| Unknown | 1 | |
| Philadelphia chromosome | ||
| Negative | 154 | 98.1 |
| Positive | 3 | 1.9 |
| Bone Marrow on day 15 | ||
| M1 (blasts <5%) | 129 | 82.2 |
| M2 (blasts 5–25%) | 20 | 12.7 |
| M3 (blasts ≥25%) | 8 | 5.1 |
| MRD on day 15 | ||
| <0.1% | 42 | 32.6 |
| ≥0.1%–<10% | 76 | 58.9 |
| ≥10% | 11 | 8.5 |
| Unknown | 28 | |
| MRD on day 33 | ||
| Negative (<0.01%) | 93 | 81.6 |
| Positive (≥0.01%) | 21 | 18.4 |
| Unknown | 43 | |
| Prednisone response on day 8 | ||
| Good (<1000 blasts) | 145 | 92.4 |
| Poor (≥1000 blasts) | 12 | 7.6 |
| ALL IC - BFM risk groups | ||
| Standard Risk | 20 | 12.7 |
| Intermediate Risk | 110 | 70.1 |
| High Risk | 27 | 17.2 |
| Stem Cell Transplantation | ||
| No | 140 | 89.2% |
| Yes | 17 | 10.8% |
| Disease relapse | ||
| Relapse-free | 139 | 88.5 |
| Relapse | 18 | 11.5 |
| Patients’ survival | ||
| Alive | 141 | 89.8 |
| Dead | 16 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xagorari, M.; Marmarinos, A.; Kossiva, L.; Baka, M.; Doganis, D.; Servitzoglou, M.; Tsolia, M.; Scorilas, A.; Avgeris, M.; Gourgiotis, D. Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL. Cancers 2021, 13, 6064. https://doi.org/10.3390/cancers13236064
Xagorari M, Marmarinos A, Kossiva L, Baka M, Doganis D, Servitzoglou M, Tsolia M, Scorilas A, Avgeris M, Gourgiotis D. Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL. Cancers. 2021; 13(23):6064. https://doi.org/10.3390/cancers13236064
Chicago/Turabian StyleXagorari, Marieta, Antonios Marmarinos, Lydia Kossiva, Margarita Baka, Dimitrios Doganis, Marina Servitzoglou, Maria Tsolia, Andreas Scorilas, Margaritis Avgeris, and Dimitrios Gourgiotis. 2021. "Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL" Cancers 13, no. 23: 6064. https://doi.org/10.3390/cancers13236064
APA StyleXagorari, M., Marmarinos, A., Kossiva, L., Baka, M., Doganis, D., Servitzoglou, M., Tsolia, M., Scorilas, A., Avgeris, M., & Gourgiotis, D. (2021). Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL. Cancers, 13(23), 6064. https://doi.org/10.3390/cancers13236064







