Pathogen-Induced Epigenetic Modifications in Cancers: Implications for Prevention, Detection and Treatment of Cancers in Africa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epigenetics in Cancer Development and Progression
3. Overview of Cancer Epigenetic Studies Conducted in Populations of African Descent
4. Correlating the Prevalence of Tropical Pathogens and Cancer Prevalence
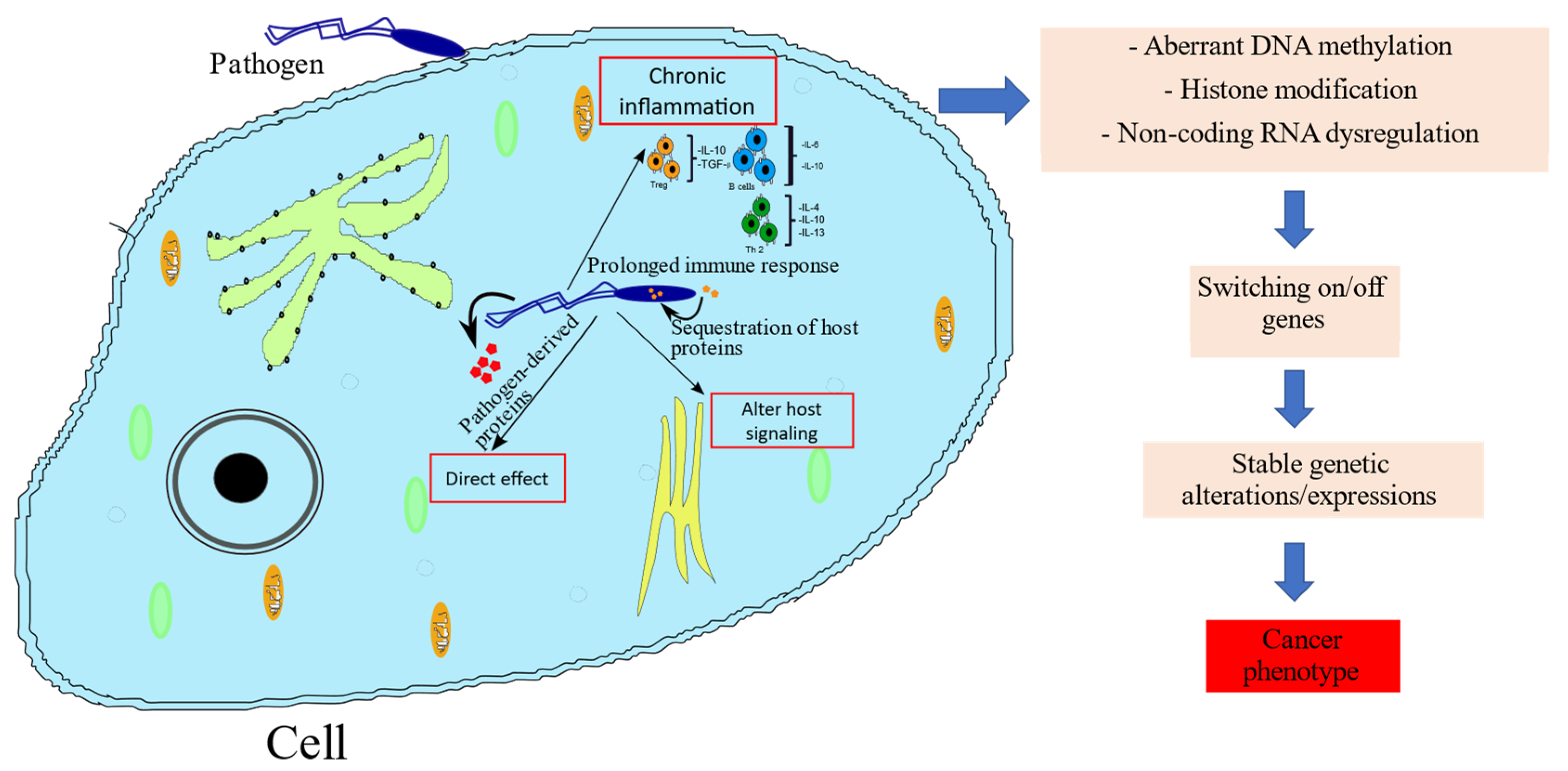
5. Mechanisms by which Pathogens Induce Cancer-Associated Epigenetic Alterations
6. Opportunities for Development of Treatment for Tropical Pathogen-Induced Cancers in Africa
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Cancer in Sub-Saharan Africa. Science 2020, 367, 27–28. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Roser, M.; Ritchie, H. Burden of Disease. Available online: https://ourworldindata.org/burden-of-disease (accessed on 8 November 2021).
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report 2020: Cancer Research for Cancer Prevention; International Agency for Research and Cancer: Lyon, France, 2020; Volume 199. [Google Scholar]
- Parkin, D.M.; Hämmerl, L.; Ferlay, J.; Kantelhardt, E.J. Cancer in Africa 2018: The Role of Infections. Int. J. Cancer 2020, 146, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Ushijima, T. Epigenetic Impact of Infection on Carcinogenesis: Mechanisms and Applications. Genome Med. 2016, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Dewals, B.; Hoving, J.C.; Horsnell, W.G.C.; Brombacher, F. Control of Schistosoma Mansoni Egg-Induced Inflammation by IL-4-Responsive CD4(+)CD25(-)CD103(+)Foxp3(-) Cells Is IL-10-Dependent. Eur. J. Immunol. 2010, 40, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Gillette-Ferguson, I.; Hise, A.G.; Sun, Y.; Diaconu, E.; McGarry, H.F.; Taylor, M.J.; Pearlman, E. Wolbachia- and Onchocerca Volvulus-Induced Keratitis (River Blindness) Is Dependent on Myeloid Differentiation Factor 88. Infect. Immunol. 2006, 74, 2442–2445. [Google Scholar] [CrossRef] [Green Version]
- Orengo, J.M.; Leliwa-Sytek, A.; Evans, J.E.; Evans, B.; van de Hoef, D.; Nyako, M.; Day, K.; Rodriguez, A. Uric Acid Is a Mediator of the Plasmodium Falciparum-Induced Inflammatory Response. PLoS ONE 2009, 4, e5194. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Brewster, R.; Tamburini, F.B.; Asiimwe, E.; Oduaran, O.; Hazelhurst, S.; Bhatt, A.S. Surveying Gut Microbiome Research in Africans: Toward Improved Diversity and Representation. Trends Microbiol. 2019, 27, 824–835. [Google Scholar] [CrossRef]
- Rao Malla, R.; Marni, R.; Kumari, S.; Chakraborty, A.; Lalitha, P. Microbiome Assisted Tumor Microenvironment: Emerging Target of Breast Cancer. Clin. Breast Cancer 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting Epigenetic Regulators for Cancer Therapy: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garraway, L.A.; Lander, E.S. Lessons from the Cancer Genome. Cell 2013, 153, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.A.; Issa, J.P.J.; Baylin, S. Targeting the Cancer Epigenome for Therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A. The Cancer Epigenome: Concepts, Challenges, and Therapeutic Opportunities. Science 2017, 355, 1147–1152. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic Modifications and Human Disease. Nat. Biotechnol. 2010, 28, 1057. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.F.; Assenov, Y.; Martin-Subero, J.I.; Balint, B.; Siebert, R.; Taniguchi, H.; Yamamoto, H.; Hidalgo, M.; Tan, A.C.; Galm, O.; et al. A DNA Methylation Fingerprint of 1628 Human Samples. Genome Res. 2012, 22, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, R.; Gupta, S. Epigenetic Modifications in Cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Minucci, S. Alterations of histone modifications in cancer. In Epigenetics in Human Disease, 2nd ed.; Tollefsbol, T., Ed.; Academic Press: Waltham, MA, USA, 2018; pp. 141–217. [Google Scholar]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Liz, J.; Esteller, M. LncRNAs and MicroRNAs with a Role in Cancer Development. Biochim. Biophys. Acta BBA—Gen. Regul. Mech. 2016, 1859, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Lin, L.K.; Zhang, S. Epigenetic Silencing of MicroRNA-335 Contributes to Nasopharyngeal Carcinoma Metastasis. Am. J. Otolaryngol. Head Neck Med. Surg. 2020, 41, 102302. [Google Scholar] [CrossRef] [PubMed]
- Law, C.-T.; Wei, L.L.; Tsang, F.H.; Xu, I.M.; Lai, R.K.; Ho, D.W.; Lee, J.M.; Wong, C.C.; Ng, I.O.; Wong, J.C.; et al. Chromatin Remodeler HELLS Is an Epigenetic Driver for Hepatocellular Carcinoma Progression. In Proceedings of the Cancer Research, American Association for Cancer Research (AACR), Washington, DC, USA, 1–5 April 2017; Volume 77, p. 1022. [Google Scholar]
- Ward, A.K.; Mellor, P.; Smith, S.E.; Kendall, S.; Just, N.A.; Vizeacoumar, F.S.; Sarker, S.; Phillips, Z.; Alvi, R.; Saxena, A.; et al. Epigenetic Silencing of CREB3L1 by DNA Methylation Is Associated with High-Grade Metastatic Breast Cancers with Poor Prognosis and Is Prevalent in Triple Negative Breast Cancers. Breast Cancer Res. 2016, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Roberts, C.W.M. Targeting EZH2 in Cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Z.; Wu, W.K.K.; Wang, M.H.; To, K.F.; Chen, Y.; Yang, W.; Li, M.S.M.; Shin, V.Y.; Tong, J.H.; et al. Epigenetic Silencing of MiR-490-3p Reactivates the Chromatin Remodeler SMARCD1 to Promote Helicobacter Pylori-Induced Gastric Carcinogenesis. Cancer Res. 2015, 75, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissa, S.; Swellam, M.; El-Khouly, I.M.; Kassim, S.K.; Shehata, H.; Mansour, A.; Esmat, M.; Nossier, A.I.; Hamdy, M.A.; Awad, N.M.; et al. Aberrant Methylation of RARβ2and APC Genes in Voided Urine as Molecular Markers for Early Detection of Bilharzial and Nonbilharzial Bladder Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in Cancer. Carcinogenesis 2009, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Defining Driver DNA Methylation Changes in Human Cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Luo, H.; Krawczyk, M.; Wei, W.; Wang, W.; Wang, J.; Flagg, K.; Hou, J.; Zhang, H.; Yi, S.; et al. DNA Methylation Markers for Diagnosis and Prognosis of Common Cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 7414–7419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özdağ, H.; Teschendorff, A.E.; Ahmed, A.A.; Hyland, S.J.; Blenkiron, C.; Bobrow, L.; Veerakumarasivam, A.; Burtt, G.; Subkhankulova, T.; Arends, M.J.; et al. Differential Expression of Selected Histone Modifier Genes in Human Solid Cancers. BMC Genom. 2006, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.C.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B Driver Variants Define Chondroblastoma and Giant Cell Tumour of Bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef]
- Wu, G.; Broniscer, A.; McEachron, T.A.; Lu, C.; Paugh, B.S.; Becksfort, J.; Qu, C.; Ding, L.; Huether, R.; Parker, M.; et al. Somatic Histone H3 Alterations in Paediatric Diffuse Intrinsic Pontine Gliomas and Non-Brainstem Glioblastomas. Nat. Genet. 2012, 44, 251–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and Prioritization of Somatic Mutations in Diffuse Large B-Cell Lymphoma (DLBCL) by Whole-Exome Sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884. [Google Scholar] [CrossRef] [Green Version]
- Papillon-Cavanagh, S.; Lu, C.; Gayden, T.; Mikael, L.G.; Bechet, D.; Karamboulas, C.; Ailles, L.; Karamchandani, J.; Marchione, D.M.; Garcia, B.A.; et al. Impaired H3K36 Methylation Defines a Subset of Head and Neck Squamous Cell Carcinomas. Nat. Genet. 2017, 49, 180–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Bellone, S.; Lopez, S.; Thakral, D.; Schwab, C.; English, D.P.; Black, J.; Cocco, E.; Choi, J.; Zammataro, L.; et al. Mutational Landscape of Uterine and Ovarian Carcinosarcomas Implicates Histone Genes in Epithelial–Mesenchymal Transition. Proc. Natl. Acad. Sci. USA 2016, 113, 12238–12243. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.W.; Müller, M.M.; Koletsky, M.S.; Cordero, F.; Lin, S.; Banaszynski, L.A.; Garcia, B.A.; Muir, T.W.; Becher, O.J.; Allis, C.D. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science 2013, 340, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, H.J.; Esteller, M. Non-Coding RNAs, Epigenetics, and Cancer: Tying It All Together. Cancer Metastasis Rev. 2018, 37, 55–73. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent Deletions and Down-Regulation of Micro- RNA Genes MiR15 and MiR16 at 13q14 in Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Gregory, R. MicroRNA Biogenesis Pathways in Cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Tishkoff, S.A.; Reed, F.A.; Friedlaender, F.R.; Ranciaro, A.; Froment, A.; Hirbo, J.B.; Awomoyi, A.A.; Bodo, J.; Doumbo, O.; Ibrahim, M.; et al. The Genetic Structure and History of Africans and African Americans. Science 2010, 324, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Reed, F.A.; Tishkoff, S.A. African Human Diversity, Origins and Migrations. Curr. Opin. Genet. Dev. 2006, 16, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Ding, Y.B.; Liu, X.Q.; Chen, X.M.; Cheng, S.Q.; Li, L.B.; Ma, M.F.; He, J.L.; Wang, Y.X. Racial/Ethnic Disparities in Human DNA Methylation. Biochim. Biophys. Acta Rev. Cancer 2014, 1846, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.; Ramsay, M. Epigenetics and the Burden of Noncommunicable Disease: A Paucity of Research in Africa. Epigenomics 2015, 7, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodson, K.; Hayes, R.; Wideroff, L.; Villaruz, L.; Tangrea, J. Hypermethylation of GSTP1, CD44, and E-Cadherin Genes in Prostate Cancer among US Blacks and Whites. Prostate 2003, 55, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kwabi-Addo, B.; Wang, S.; Chung, W.; Jelinek, J.; Patierno, S.R.; Wang, B.D.; Andrawis, R.; Lee, N.H.; Apprey, V.; Issa, J.P.; et al. Identification of Differentially Methylated Genes in Normal Prostate Tissues from African American and Caucasian Men. Clin. Cancer Res. 2010, 16, 3539–3547. [Google Scholar] [CrossRef] [Green Version]
- Theodore, S.C.; Davis, M.; Zhao, F.; Wang, H.; Chen, D.; Rhim, J.; Dean-Colomb, W.; Turner, T.; Ji, W.; Zeng, G.; et al. MicroRNA Profiling of Novel African American and Caucasian Prostate Cancer Cell Lines Reveals a Reciprocal Regulatory Relationship of MiR-152 and DNA Methyltranferase 1. Oncotarget 2014, 5, 3512–3525. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ji, P.; Zhang, Y.; LaComb, J.F.; Tian, X.; Li, E.; Williams, J.L. Aberrant DNA Methylation: Implications in Racial Health Disparity. PLoS ONE 2016, 11, e0153125. [Google Scholar] [CrossRef]
- Mehrotra, J.; Ganpat, M.M.; Kanaan, Y.; Fackler, M.J.; McVeigh, M.; Lahti-Domenici, J.; Polyak, K.; Argani, P.; Naab, T.; Garrett, E.; et al. Estrogen Receptor/Progesterone Receptor-Negative Breast Cancers of Young African-American Women Have a Higher Frequency of Methylation of Multiple Genes than Those of Caucasian Women. Clin. Cancer Res. 2004, 10, 2052–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seema Sethi, R.S. Differential Expression of MicroRNAs in Papillary Thyroid Carcinoma and Their Role in Racial Disparity. J. Cancer Sci. Ther. 2015, 7, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, G.L.; Shoji, Y.; Darcy, K.; Litzi, T.; Berchuck, A.; Hamilton, C.A.; Conrads, T.P.; Risinger, J.I. MicroRNAs in Endometrial Cancers from Black and White Patients. Am. J. Obstet. Gynecol. 2015, 212, 191.e1–191.e10. [Google Scholar] [CrossRef] [PubMed]
- McHayle, A.; Boles, A.; Diarra, L.; Mitchell, K.A. Epigenetics, Regulation of Cancer Immunotherapy Response Genes, and Race: A Comparison of DNA Methylation in Non-Small Cell Lung Cancers from African Americans and European Americans (Abstract). Am. Assoc. Cancer Res. 2018, 78, 3325. [Google Scholar] [CrossRef]
- Vandeven, N.; Nghiem, P. Pathogen-Driven Cancers and Emerging Immune Therapeutic Strategies. Cancer Immunol. Res. 2014, 2, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.C.; Tishkoff, S.A. African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annu. Rev. Genom. Hum. Genet. 2008, 9, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Hillyar, C.; Rallis, K.S.; Varghese, J. Advances in Epigenetic Cancer Therapeutics. Cureus 2020, 12, e11725. [Google Scholar] [CrossRef]
- de Vries, J.; Pepper, M. Genomic Sovereignty and the African Promise: Mining the African Genome for the Benefit of Africa. J. Med. Ethics 2012, 38, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, M. Africa: Continent of Genome Contrasts with Implications for Biomedical Research and Health. FEBS Lett. 2012, 586, 2813–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoll, L.J.; Hogan, D.A.; Leong, J.M.; Heitman, J.; Condit, R.C. Pearls Collections: What We Can Learn about Infectious Disease and Cancer. PLoS Pathog. 2018, 14, e1006915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.K.; Pagano, J.S.; Khalili, K. Viruses and Human Cancers: A Long Road of Discovery of Molecular Paradigms. Clin. Microbiol. Rev. 2014, 27, 463–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; pp. 1–83. [Google Scholar]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2021; pp. 1–112. [Google Scholar]
- Ayee, R.; Ofori, M.E.O.; Wright, E.; Quaye, O. Epstein Barr Virus Associated Lymphomas and Epithelia Cancers in Humans. J. Cancer 2020, 11, 1737–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayee, R.; Ofori, M.E.O.; Tagoe, E.A.; Languon, S.; Searyoh, K.; Armooh, L.; Bilson-Amoah, E.; Baidoo, K.; Kitcher, E.; Wright, E.; et al. Genotypic Characterization of Epstein Barr Virus in Blood of Patients with Suspected Nasopharyngeal Carcinoma in Ghana. Viruses 2020, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.F. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Xiao, Y.; Zhou, R.; Liao, Y.; Zhou, J.; Ma, X. Prognostic Significance of Helicobacter pylori—Infection in Gastric Diffuse Large B-Cell Lymphoma. BMC Cancer 2019, 19, 842. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Waterboer, T.; Idahl, A.; Brenner, N.; Brinton, L.A.; Butt, J.; Coburn, S.B.; Hartge, P.; Hufnagel, K.; Inturrisi, F.; et al. Antibodies against Chlamydia Trachomatis and Ovarian Cancer Risk in Two Independent Populations. J. Natl. Cancer Inst. 2019, 111, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.S.; Muñoz, N.; Herrero, R.; Eluf-Neto, J.; Ngelangel, C.; Franceschi, S.; Bosch, F.X.; Walboomers, J.M.M.; Peeling, R.W. Evidence for Chlamydia Trachomatis as a Human Papillomavirus Cofactor in the Etiology of Invasive Cervical Cancer in Brazil and the Philippines. J. Infect. Dis. 2002, 185, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, E.; Rother, M.; Kerr, M.C.; Al-Zeer, M.A.; Abu-Lubad, M.; Kessler, M.; Brinkmann, V.; Loewer, A.; Meyer, T.F. Chlamydia Infection Depends on a Functional MDM2-P53 Axis. Nat. Commun. 2014, 5, 5201. [Google Scholar] [CrossRef] [Green Version]
- Lien, Y.C.; Wang, J.Y.; Lee, M.C.; Shu, C.C.; Chen, H.Y.; Hsieh, C.H.; Lee, C.H.; Lee, L.N.; Chao, K.M. Urinary Tuberculosis Is Associated with the Development of Urothelial Carcinoma but Not Renal Cell Carcinoma: A Nationwide Cohort Study in Taiwan. Br. J. Cancer 2013, 109, 2933–2940. [Google Scholar] [CrossRef]
- Marbjerg, L.H.; Øvrehus, A.L.H.; Johansen, I.S. Schistosomiasis-Induced Squamous Cell Bladder Carcinoma in an HIV-Infected Patient. Int. J. Infect. Dis. 2015, 40, 113–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, K.; Hsieh, M.H. Understanding Urogenital Schistosomiasis-Related Bladder Cancer: An Update. Front. Med. 2018, 5, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 10 November 2021).
- Kaewpitoon, N.; Kaewpitoon, S.J.; Pengsaa, P.; Sripa, B. Opisthorchis viverrini: The Carcinogenic Human Liver Fluke. World J. Gastroenterol. 2008, 14, 666–674. [Google Scholar] [CrossRef]
- Bollati, V.; Fabris, S.; Pegoraro, V.; Ronchetti, D.; Mosca, L.; Deliliers, G.L.; Motta, V.; Bertazzi, P.A.; Baccarelli, A.; Neri, A. Differential repetitive DNA methylation in multiple myeloma molecular subgroups. Carcinogenesis 2009, 30, 1330–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.S.; Pak, J.H.; Kim, J.B.; Bahk, Y.Y. Clonorchis sinensis, an Oriental Liver Fluke, as a Human Biological Agent of Cholangiocarcinoma: A Brief Review. BMB Rep. 2016, 49, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.S.; Tesfamariam, S.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-Induced Hepatocellular Carcinoma in Developing Countries: Geographical Distribution, Mechanism of Action and Prevention (Review). Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wu, F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Massey, T.E.; Smith, G.B.J.; Tam, A.S. Mechanism of Aflatoxin B1 Lung Tumorigenesis. Exp. Lung Res. 2000, 26, 673–683. [Google Scholar]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of Different Mycotoxins on Humans, Cell Genome and Their Involvement in Cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [Green Version]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The Fungal Mycobiome Promotes Pancreatic Oncogenesis via Activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Kinnaird, J.; Weir, W.; Durrani, Z.; Pillai, S.; Baird, M.; Shiels, B. A Bovine Lymphosarcoma Cell Line Infected with Theileria annulata Exhibits an Irreversible Reconfiguration of Host Cell Gene Expression. PLoS ONE 2013, 8, e66833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeseman, K.; Weitzman, J. Host–Parasite Interactions: An Intimate Epigenetic Relationship. Cell Microbiol. 2015, 17, 1121–1132. [Google Scholar] [CrossRef]
- Kaneda, A.; Matsusaka, K.; Aburatani, H.; Fukayama, M. Epstein-Barr Virus Infection as an Epigenetic Driver of Tumorigenesis. Cancer Res. 2012, 72, 3445–3450. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Shinjo, K.; Shimizu, Y.; Sano, T.; Yamao, K.; Gao, W. Hepatitis Virus Infection Affects DNA Methylation in Mice with Humanized Livers. Gastroentrology 2014, 146, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.; Ladu, S.; Gorden, A.; Farini, M.; Lee, J.; Conner, E. Mechanistic and Prognostic Significance of Aberrant Methylation in the Molecular Pathogenesis of Human Hepatocellular Carcinoma. J. Clin. Investig. 2007, 117, 2713–2722. [Google Scholar] [CrossRef] [Green Version]
- Ushijima, T. Epigenetic Field for Cancerization. J. Biochem. Mol. Biol. 2007, 40, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T.; Tsukamoto, T.; Toyoda, T.; Mori, A.; Tanaka, H.; Maekita, T. Inflammatory Processes Triggered by Helicobacter pylori Infection Cause Aberrant DNA Methylation in Gastric Epithelial Cells. Cancer Res. 2010, 70, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Bodily, J.M.; Mehta, K.P.M.; Laimins, L.A. Human Papillomavirus E7 Enhances Hypoxia-Inducible Factor 1-Mediated Transcription by Inhibiting Binding of Histone Deacetylases. Cancer Res. 2011, 71, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Das, K.; Saha, S.; Mazumdar, M.; Manna, A.; Chakraborty, S.; Mukherjee, S.; Khan, P.; Adhikary, A.; Mohanty, S.; et al. Nuclear Matrix Protein SMAR1 Represses C-Fos-Mediated HPV18 E6 Transcription through Alteration of Chromatin Histone Deacetylation. J. Biol. Chem. 2014, 289, 29074–29085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivière, L.; Gerossier, L.; Ducroux, A.; Dion, S.; Deng, Q.; Michel, M.-L.; Buendia, M.-A.; Hantz, O.; Neuveut, C. HBx Relieves Chromatin-Mediated Transcriptional Repression of Hepatitis B Viral CccDNA Involving SETDB1 Histone Methyltransferase. J. Hepatol. 2015, 63, 1093–1102. [Google Scholar] [CrossRef]
- Gu, H.; Roizman, B. The Two Functions of Herpes Simplex Virus 1 ICP0, Inhibition of Silencing by the CoREST/REST/HDAC Complex and Degradation of PML, Are Executed in Tandem. J. Virol. 2009, 83, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-J.; Kim, Y.-E.; Pham, H.T.; Kim, E.T.; Chung, Y.-H.; Ahn, J.-H. Functional Interaction of the Human Cytomegalovirus IE2 Protein with Histone Deacetylase 2 in Infected Human Fibroblasts. J. Gen. Virol. 2007, 88, 3214–3223. [Google Scholar] [CrossRef]
- Tang, Q.; Maul, G.G. Mouse Cytomegalovirus Immediate-Early Protein 1 Binds with Host Cell Repressors to Relieve Suppressive Effects on Viral Transcription and Replication during Lytic Infection. J. Virol. 2003, 77, 1357–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, H.; Gubellini, F.; Rivera-Calzada, A.; Braun, N.; Connery, S.; Dujeancourt, A. Structure of a Type IV Secretion System. Nature 2014, 508, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.-Y.; Galli, S.J.; et al. Butyrate Inhibits Human Mast Cell Activation via Epigenetic Regulation of FcεRI-Mediated Signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Soldati, D. The Transcription Machinery and the Molecular Toolbox to Control Gene Expression in Toxoplasma Gondii and Other Protozoan Parasites. Microbes Infect. 2005, 7, 1376–1384. [Google Scholar] [CrossRef] [Green Version]
- Croken, M.M.; Nardelli, S.C.; Kim, K. Chromatin Modifications, Epigenetics, and How Protozoan Parasites Regulate Their Lives. Trends Parasitol. 2012, 28, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Hakimi, M.-A.; Deitsch, K.W. Epigenetics in Apicomplexa: Control of Gene Expression during Cell Cycle Progression, Differentiation and Antigenic Variation. Curr. Opin. Microbiol. 2007, 10, 357–362. [Google Scholar] [CrossRef]
- Pennini, M.E.; Pai, R.K.; Schultz, D.C.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis 19-KDa Lipoprotein Inhibits IFN-γ-Induced Chromatin Remodeling of MHC2TA by TLR2 and MAPK Signaling. J. Immunol. 2006, 176, 4323–4330. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Díaz, E.; Jordà, M.; Peinado, M.A.; Rivero, A. Epigenetics of Host-Pathogen Interactions: The Road Ahead and the Road Behind. PLoS Pathog. 2012, 8, e1003007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moen, E.L.; Zhang, X.; Mu, W.; Delaney, S.M.; Wing, C.; Mcquade, J.; Myers, J.; Godley, L.A.; Eileen Dolan, M.; Zhang, W. Genome-Wide Variation of Cytosine Modifications between European and African Populations and the Implications for Complex Traits. Genetics 2013, 194, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mofolo, N.; Betshu, O.; Kenna, O.; Koroma, S.; Lebeko, T.; Claassen, F.M.; Joubert, G. Knowledge of Prostate Cancer among Males Attending a Urology Clinic, a South African Study. SpringerPlus 2015, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Azim, S.; Zubair, H.; Khan, M.A.; Singh, S.; Carter, J.E.; Rocconi, R.P.; Singh, A.P. Epigenetic Basis of Cancer Health Disparities: Looking beyond Genetic Differences. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zakharia, F.; Basu, A.; Absher, D.; Assimes, T.L.; Go, A.S.; Hlatky, M.A.; Iribarren, C.; Knowles, J.W.; Li, J.; Narasimhan, B.; et al. Characterizing the Admixed African Ancestry of African Americans. Genome Biol. 2009, 10, R141.1–R141.11. [Google Scholar] [CrossRef] [Green Version]
- Alegría-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and Lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Costa-Pinheiro, P.; Montezuma, D.; Henrique, R.; Jerónimo, C. Diagnostic and Prognostic Epigenetic Biomarkers in Cancer. Epigenomics 2015, 7, 1003–1015. [Google Scholar] [CrossRef]
- Gonzalgo, M.L.; Pavlovich, C.P.; Lee, S.M.; Nelson, W.G. Prostate Cancer Detection by GSTP1 Methylation Analysis of Postbiopsy Urine Specimens. Clin. Cancer Res. 2003, 9, 2673–2677. [Google Scholar] [PubMed]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder Cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Hoque, M.O.; Begum, S.; Topaloglu, O.; Jeronimo, C.; Mambo, E.; Westra, W.H.; Califano, J.A.; Sidransky, D. Quantitative Detection of Promoter Hypermethylation of Multiple Genes in the Tumor, Urine, and Serum DNA of Patients with Renal Cancer. Cancer Res. 2004, 64, 5511–5517. [Google Scholar] [CrossRef]
- Kloten, V.; Becker, B.; Winner, K.; Schrauder, M.G.; Fasching, P.A.; Anzeneder, T.; Veeck, J.; Hartmann, A.; Knüchel, R.; Dahl, E. Promoter Hypermethylation of the Tumor-Suppressor Genes ITIH5, DKK3, and RASSF1A as Novel Biomarkers for Blood-Based Breast Cancer Screening. Breast Cancer Res. 2013, 15, R4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partin, A.W.; Neste, L.V.; Klein, E.A.; Marks, L.S.; Gee, J.R.; Troyer, D.A.; Rieger-Christ, K.; Jones, J.S.; Magi-galluzzi, C.; Mangold, A.; et al. Clinical Validation of an Epigenetic Assay to Predict Negative Histopathological Results in Repeat Prostate Biopsies. J. Urol. 2014, 192, 1081–1087. [Google Scholar] [CrossRef]
- Issa, J.J.; Garcia-Manero, G.; Giles, F.J.; Mannari, R.; Thomas, D.; Faderl, S.; Bayar, E.; Lyons, J.; Rosenfeld, C.S.; Cortes, J.; et al. Phase 1 Study of Low-Dose Prolonged Exposure Schedules of the Hypomethylating Agent 5-Aza-2 J -Deoxycytidine (Decitabine) in Hematopoietic Malignancies. Am. Soc. Hematol. 2004, 103, 1635–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzola, M.; Calzetta, L.; Matera, M.G.; Hanania, N.A.; Rogliani, P. How Does Race/Ethnicity Influence Pharmacological Response to Asthma Therapies? Expert Opin. Drug Metab. Toxicol. 2018, 14, 435–446. [Google Scholar] [CrossRef]
- Roberti, A.; Valdes, A.F.; Torrecillas, R.; Fraga, M.F.; Fernandez, A.F. Epigenetics in Cancer Therapy and Nanomedicine. Clin. Epigenet. 2019, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Silmon De Monerri, N.C.; Kim, K. Pathogens Hijack the Epigenome: A New Twist on Host-Pathogen Interactions. Am. J. Pathol. 2014, 184, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, R.J.; Boyle, P.; Milburn, A.; Ngoma, T.; Arbuthnott, J.; McCaffrey, R.; Kerr, S.H.; Kerr, D.J. The Challenge of Cancer Control in Africa. Nat. Rev. Cancer 2008, 8, 398–403. [Google Scholar] [CrossRef]
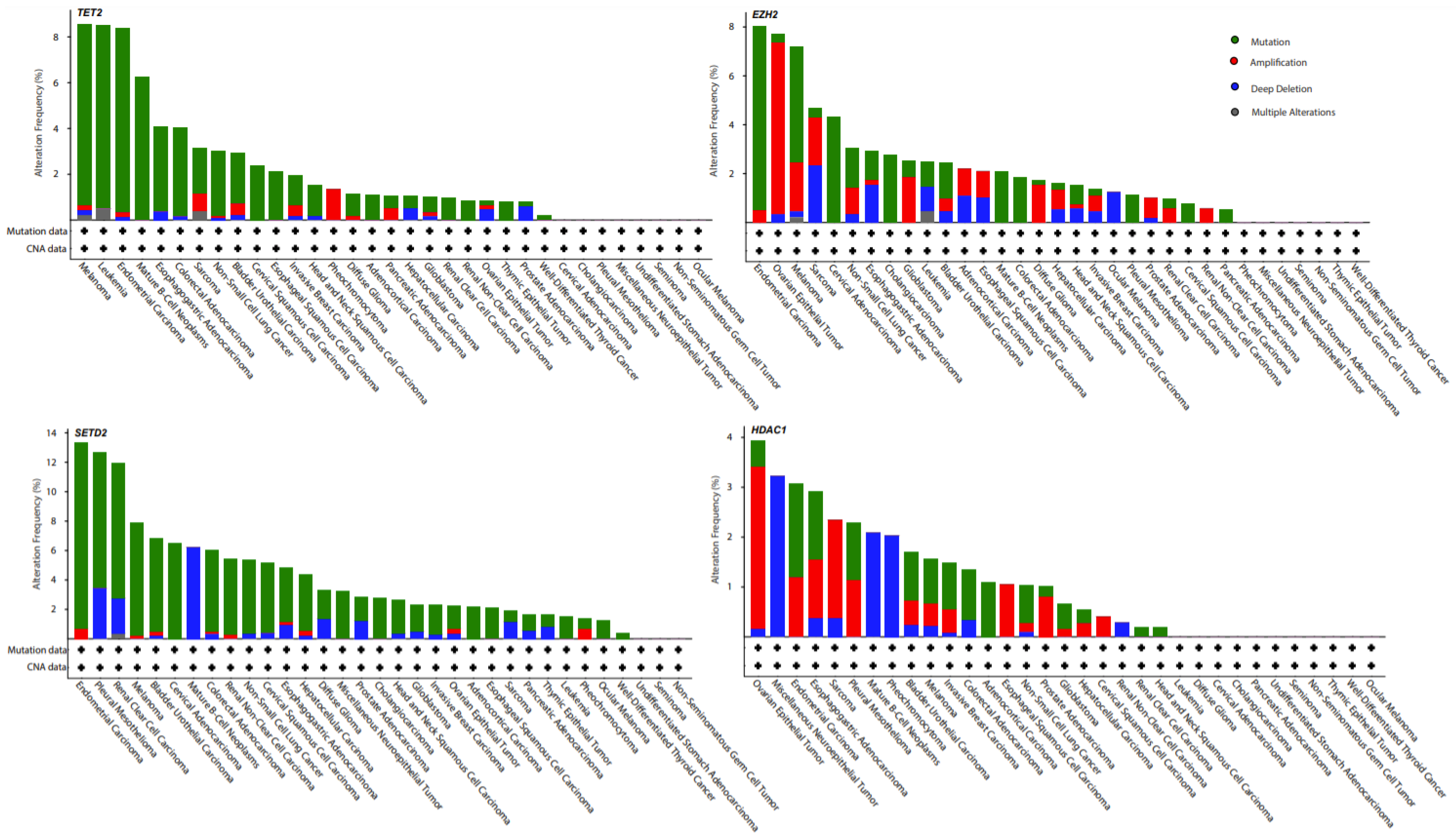
| Type of Cancer | Epigenetic Modification | Genes/miRNA Involved | Differences between African Americans and Caucasians | Link with Disease Progression | References |
|---|---|---|---|---|---|
| Prostate | Hypermethylation | CD44, GSTP1 | Higher frequency of CD44 hypermethylation in African Americans | Not indicated | [51] |
| Hypermethylation | Nkx2-5, TIMP3 AR, RARβ2, SPARC | Higher methylation in African Americans | Yes | [52] | |
| Hypermethylated | miR-152 | Higher methylation in African Americans | Yes | [53] | |
| Colorectal | Hypermethylation | CHL1, NELL1, GDF1, ARHGEF4, ITGA4 | Higher methylation in African Americans | Not indicated | [54] |
| Hypermethylation | miR-9, miR-124, miR-137, miR-548, miR-663, miR-2682, miR-6130 | Higher methylation in African Americans | Not indicated | [54] | |
| Breast | Hypermethylation | CDH13, HIN-1, TWIST, RAR-β, RASSF1A | Higher methylation in African Americans | Not indicated | [55] |
| Thyroid | miRNA Upregulation | miR-31, miR-221 | Upregulated in Caucasians downregulated in African Americans | Yes | [56] |
| Endometrial | RNA Down-regulation | miR-337 | Downregulated in Caucasians compared with African Americans | Not indicated | [57] |
| Lung | Hypomethylation | SHISA4, RAD1 | Decreased DNA methylation in African Americans | Yes | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djomkam Zune, A.L.; Olwal, C.O.; Tapela, K.; Owoicho, O.; Nganyewo, N.N.; Lyko, F.; Paemka, L. Pathogen-Induced Epigenetic Modifications in Cancers: Implications for Prevention, Detection and Treatment of Cancers in Africa. Cancers 2021, 13, 6051. https://doi.org/10.3390/cancers13236051
Djomkam Zune AL, Olwal CO, Tapela K, Owoicho O, Nganyewo NN, Lyko F, Paemka L. Pathogen-Induced Epigenetic Modifications in Cancers: Implications for Prevention, Detection and Treatment of Cancers in Africa. Cancers. 2021; 13(23):6051. https://doi.org/10.3390/cancers13236051
Chicago/Turabian StyleDjomkam Zune, Alexandra Lindsey, Charles Ochieng’ Olwal, Kesego Tapela, Oloche Owoicho, Nora Nghochuzie Nganyewo, Frank Lyko, and Lily Paemka. 2021. "Pathogen-Induced Epigenetic Modifications in Cancers: Implications for Prevention, Detection and Treatment of Cancers in Africa" Cancers 13, no. 23: 6051. https://doi.org/10.3390/cancers13236051
APA StyleDjomkam Zune, A. L., Olwal, C. O., Tapela, K., Owoicho, O., Nganyewo, N. N., Lyko, F., & Paemka, L. (2021). Pathogen-Induced Epigenetic Modifications in Cancers: Implications for Prevention, Detection and Treatment of Cancers in Africa. Cancers, 13(23), 6051. https://doi.org/10.3390/cancers13236051







