Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: The Role of Sequencing Genetic Profiling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genomic DNA Extraction
2.3. Next-Generation Sequencing Analysis
2.4. Statistical Analysis
3. Results
3.1. Preoperative Clinical Data
3.2. Patient Outcome
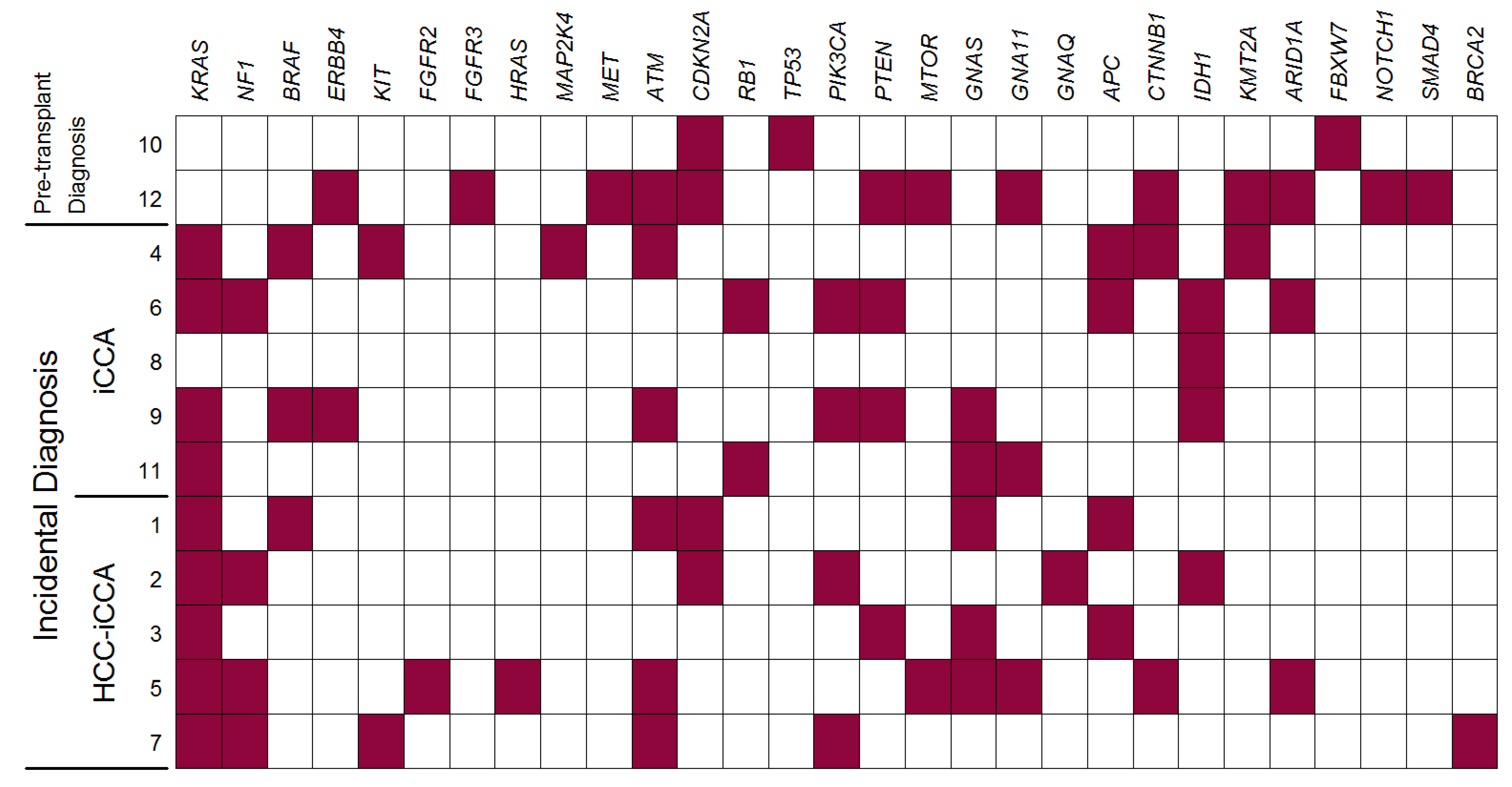
3.3. NGS Analysis
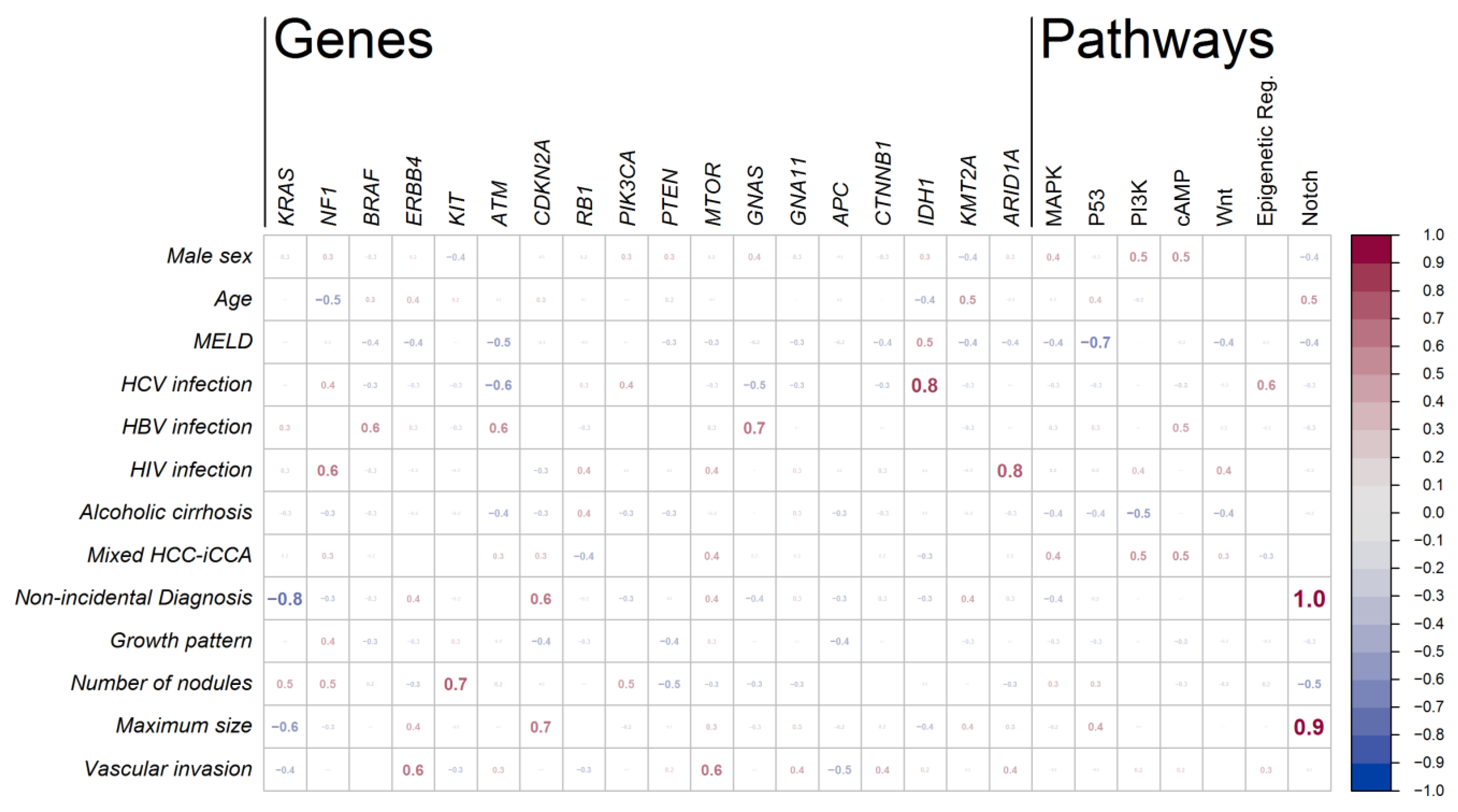
3.4. Associations between Mutations and Clinical Characteristics of the Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruttadauria, S.; Barbara, M.; Liotta, R. Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: An Italian Experience. Updates Surg. 2021, 73, 1587–1588. [Google Scholar] [CrossRef]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Moris, D.P.; Zhang, X.-F.; Bagante, F.; Spolverato, G.; Schmidt, C.; Dilhoff, M.; Pawlik, T.M. Evaluation of the 8th Edition American Joint Commission on Cancer (AJCC) Staging System for Patients with Intrahepatic Cholangiocarcinoma: A Surveillance, Epidemiology, and End Results (SEER) Analysis. J. Surg. Oncol. 2017, 116, 643–650. [Google Scholar] [CrossRef]
- Sirica, A.E.; Gores, G.J.; Groopman, J.D.; Selaru, F.M.; Strazzabosco, M.; Wei Wang, X.; Zhu, A.X. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology 2019, 69, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and Risk Factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the Diagnosis and Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving Concepts and Therapeutic Strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Alvaro, D.; Hassan, C.; Cardinale, V.; Carpino, G.; Fabris, L.; Gringeri, E.; Granata, V.; Mutignani, M.; Morement, H.; Giuliante, F.; et al. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part II: Treatment. Dig. Liver Dis. 2020, 52, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Javle, M.; Lerut, J.; Ohtsuka, M.; Ghobrial, M.; Hibi, T.; Kwan, N.M.; Heimbach, J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined Hepatocellular-Cholangiocarcinoma: An Update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef]
- Garancini, M.; Goffredo, P.; Pagni, F.; Romano, F.; Roman, S.; Sosa, J.A.; Giardini, V. Combined Hepatocellular-Cholangiocarcinoma: A Population-Level Analysis of an Uncommon Primary Liver Tumor: Combined Hepatocellular-Cholangiocarcinoma. Liver Transpl. 2014, 20, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Kim, D.Y.; Choi, J.-Y. Cross-Sectional Imaging of Intrahepatic Cholangiocarcinoma: Development, Growth, Spread, and Prognosis. Am. J. Roentgenol. 2017, 209, W64–W75. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G. Multidisciplinary Imaging of Liver Hydatidosis. World J. Gastroenterol. 2012, 18, 1438. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Rodríguez de Lope, C.; Gastaca, M.; Ortiz de Urbina, J.; Suarez, M.A.; Santoyo, J.; Castroagudín, J.F.; Varo, E.; López-Andujar, R.; Palacios, F.; et al. “ Very Early ” Intrahepatic Cholangiocarcinoma in Cirrhotic Patients: Should Liver Transplantation Be Reconsidered in These Patients?: “ Very Early ” Intrahepatic Cholangiocarcinoma. Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; de Lope, C.R.; Gastaca, M.; de Urbina, J.O.; López-Andujar, R.; Palacios, F.; Ramos, E.; Fabregat, J.; Castroagudín, J.F.; Varo, E.; et al. Intrahepatic Cholangiocarcinoma or Mixed Hepatocellular-Cholangiocarcinoma in Patients Undergoing Liver Transplantation: A Spanish Matched Cohort Multicenter Study. Ann. Surg. 2014, 259, 944–952. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Court, C.; Seok Lee, Y.; Lu, D.S.; Naini, B.V.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl. 2018, 24, 1384–1397. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.C.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational Landscape of Intrahepatic Cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Bridgewater, J.; Normanno, N. Practical Considerations in Screening for Genetic Alterations in Cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.C.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef] [Green Version]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision Medicine in Cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 30 November 2021).
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Nelson, L.J.; Ávila, M.A.; Cubero, F.J. Mitogen-Activated Protein Kinases (MAPKs) and Cholangiocarcinoma: The Missing Link. Cells 2019, 8, 1172. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.B.; Thorgeirsson, S.S. Genetic Profiling of Intrahepatic Cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012, 28, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Thomas, H.C.; Toledano, M.B.; Cox, I.J.; Taylor-Robinson, S.D. P53 Mutations in Human Cholangiocarcinoma: A Review. Liver Int. 2005, 25, 704–716. [Google Scholar] [CrossRef]
- Leelawat, K.; Narong, S.; Udomchaiprasertkul, W.; Leelawat, S.; Tungpradubkul, S. Inhibition of PI3K Increases Oxaliplatin Sensitivity in Cholangiocarcinoma Cells. Cancer Cell Int. 2009, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Baiocchi, L.; Lenci, I.; Milana, M.; Kennedy, L.; Sato, K.; Zhang, W.; Ekser, B.; Ceci, L.; Meadows, V.; Glaser, S.; et al. Cyclic AMP Signaling in Biliary Proliferation: A Possible Target for Cholangiocarcinoma Treatment? Cells 2021, 10, 1692. [Google Scholar] [CrossRef]
- Boulter, L.; Guest, R.V.; Kendall, T.J.; Wilson, D.H.; Wojtacha, D.; Robson, A.J.; Ridgway, R.A.; Samuel, K.; Van Rooijen, N.; Barry, S.T.; et al. WNT Signaling Drives Cholangiocarcinoma Growth and Can Be Pharmacologically Inhibited. J. Clin. Investig. 2015, 125, 1269–1285. [Google Scholar] [CrossRef] [Green Version]
- Salati, M.; Caputo, F.; Baldessari, C.; Galassi, B.; Grossi, F.; Dominici, M.; Ghidini, M. IDH Signalling Pathway in Cholangiocarcinoma: From Biological Rationale to Therapeutic Targeting. Cancers 2020, 12, 3310. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Drill, E.; Pak, L.M.; Nguyen, B.; Sigel, C.S.; Doussot, A.; Shin, P.; Goldman, D.A.; Gonen, M.; Allen, P.J.; et al. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology 2021, 74, 1429–1444. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-Analysis. JAMA Surg. 2014, 149, 565. [Google Scholar] [CrossRef] [Green Version]
- Gruttadauria, S.; Saint Georges Chaumet, M.; Pagano, D.; Marsh, J.W.; Bartoccelli, C.; Cintorino, D.; Arcadipane, A.; Vizzini, G.; Spada, M.; Gridelli, B. Impact of Blood Transfusion on Early Outcome of Liver Resection for Colorectal Hepatic Metastases: Blood Transfusion on Liver Resection. J. Surg. Oncol. 2011, 103, 140–147. [Google Scholar] [CrossRef]
- Gruttadauria, S. Small-for-Size Syndrome in Adult-to-Adult Living-Related Liver Transplantation. World J. Gastroenterol. 2010, 16, 5011. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Colombo, M. Systemic Therapy for Advanced Cholangiocarcinoma: New Options on the Horizon. Hepatoma Res. 2020, 6, 70. [Google Scholar] [CrossRef]
- Taylor, A.C.; Maddirela, D.; White, S.B. Role of Radioembolization for Biliary Tract and Primary Liver Cancer. Surg. Oncol. Clin. N. Am. 2019, 28, 731–743. [Google Scholar] [CrossRef]
- Rimassa, L.; Personeni, N.; Aghemo, A.; Lleo, A. The Immune Milieu of Cholangiocarcinoma: From Molecular Pathogenesis to Precision Medicine. J. Autoimmun. 2019, 100, 17–26. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver Transplantation in Hepatocellular Carcinoma after Tumour Downstaging (XXL): A Randomised, Controlled, Phase 2b/3 Trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
| Incidentally Diagnosed on Pathologic Examination | Pre-Transplant Diagnosed | ||
|---|---|---|---|
| HCC-iCCA | iCCA | ||
| N | 5 | 5 | 2 |
| Male sex | 5 (100) | 4 (80) | 1 (50) |
| Age, median (IQR) § | 60 (55–60) | 62 (58–65) | 65, 67 § |
| MELD, median (IQR) § | 14 (11–15) | 10 (9–11) | 9, 6 § |
| Waiting list time, days § | 132 (93–147) | 177 (51–186) | 4, 114 § |
| Etiology of liver disease | |||
| HCV infection | 1 (20) | 1 (20) | 0 (0) |
| HBV infection | 1 (20) | 1 (20) | 0 (0) |
| HCV-HIV co-infection | 0 (0) | 1 (20) | 0 (0) |
| HBV-HIV co-infection | 1 (20) | 0 (0) | 0 (0) |
| Alcoholic cirrhosis | 0 (0) | 1 (20) | 0 (0) |
| Unknown etiology | 2 (40) | 1 (20) | 2 (100) |
| Pretransplant oncologic diagnosis | |||
| Hepatocellular carcinoma | 4 (80) | 4 (80) | 0 (0) |
| Intrahepatic cholangiocarcinoma | 0 (0) | 0 (0) | 2 (100) |
| No liver tumor | 1 (20) | 1 (20) | |
| Liver cirrhosis | 5 (100) | 5 (100) | 1 (50) |
| Radiological diagnosis of liver tumor | 4 (80) | 4 (80) | 2 (100) |
| Radiological evidence of lymph nodes enlargement | 5 (100) | 3 (60) | 2 (100) |
| Previous treatments | |||
| Laparoscopic hepatic resection | 0 (0) | 1 (20) | 0 (0) |
| Laparoscopic microwave thermal ablation | 0 (0) | 1 (20) | 0 (0) |
| Transarterial chemo-embolization | 3 (60) | 2 (40) | 0 (0) |
| Transartherial radio-embolization | 0 (0) | 0 (0) | 2 (100) |
| Neoadjuvant chemotherapy | 0 (0) | 0 (0) | 2 (100) |
| Anatomopathological findings | |||
| Mixed HCC-iCCA | 5 (100) | 0 (0) | 1 (50) |
| Macroscopic growth pattern | |||
| Mass forming type | 3 (60) | 4 (80) | 2 (100) |
| Intraductal growing type | 2 (40) | 0 (0) | 0 (0) |
| Periductal infiltrating type | 0 (0) | 1 (20) | 0 (0) |
| Number of nodules | |||
| Monofocal tumor | 1 (20) | 0 (0) | 2 (100) |
| 2 nodules | 2 (40) | 4 (67) | 0 (0) |
| 3 nodules | 0 (0) | 0 (0) | 0 (0) |
| 4 nodules | 2 (40) | 1 (17) | 0 (0) |
| Size of the greatest nodule, cm § | 1.8 (1.6–2.5) | 2.5 (2.0–3.0) | Both 8 cm § |
| Vascular invasion | 1 (20) | 2 (40) | 1 (50) |
| Portal vein invasion | 1 (20) | 1 (20) | 1 (50) |
| Hepatic vein invasion | 1 (20) | 1 (20) | 1 (50) |
| Serous membrane | 1 (20) | 1 (20) | 1 (50) |
| Pathway GENE | Mutations (N = 92) | Patients (N = 12) |
|---|---|---|
| MAPK pathway | ||
| Overall | 27 (29) | 10 (83) |
| KRAS | 10 (11) | 9 (75) |
| NF1 | 5 (5) | 4 (33) |
| BRAF | 3 (3) | 3 (25) |
| ERBB4 | 2 (2) | 2 (17) |
| KIT | 2 (2) | 2 (17) |
| FGFR2 | 1 (1) | 1 (8) |
| FGFR3 | 1 (1) | 1 (8) |
| HRAS | 1 (1) | 1 (8) |
| MAP2K4 | 1 (1) | 1 (8) |
| MET | 1 (1) | 1 (8) |
| P53 pathway | ||
| Overall | 17 (18) | 10 (83) |
| ATM | 10 (11) | 6 (50) |
| CDKN2A | 4 (4) | 4 (33) |
| RB1 | 2 (2) | 2 (17) |
| TP53 | 1 (1) | 1 (8) |
| PI3K-Akt/mTOR pathway | ||
| Overall | 14 (15) | 7 (58) |
| PIK3CA | 8 (9) | 4 (33) |
| PTEN | 4 (4) | 4 (33) |
| MTOR | 2 (2) | 2 (17) |
| cAMP pathway | ||
| Overall | 9 (10) | 7 (58) |
| GNAS | 5 (5) | 5 (42) |
| GNA11 | 3 (3) | 3 (25) |
| GNAQ | 1 (1) | 1 (8) |
| Wnt pathway | ||
| Overall | 9 (10) | 6 (50) |
| APC | 6 (7) | 4 (33) |
| CTNNB1 | 3 (3) | 3 (25) |
| Epigenetic regulation | ||
| Overall | 6 (7) | 6 (50) |
| IDH1 | 4 (4) | 4 (33) |
| KMT2A | 2 (2) | 2 (17) |
| Chromatin remodeling | ||
| ARID1A | 6 (7) | 3 (25) |
| Notch pathway | ||
| Any mutation | 2 (2) | 2 (17) |
| FBXW7 | 1 (1) | 1 (8) |
| NOTCH1 | 1 (1) | 1 (8) |
| TGFβ pathway | ||
| SMAD4 | 1 (1) | 1 (8) |
| DNA Repair | ||
| BRCA2 | 1 (1) | 1 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruttadauria, S.; Barbera, F.; Pagano, D.; Liotta, R.; Miraglia, R.; Barbara, M.; Bavetta, M.G.; Cammà, C.; Petridis, I.; Di Carlo, D.; et al. Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: The Role of Sequencing Genetic Profiling. Cancers 2021, 13, 6049. https://doi.org/10.3390/cancers13236049
Gruttadauria S, Barbera F, Pagano D, Liotta R, Miraglia R, Barbara M, Bavetta MG, Cammà C, Petridis I, Di Carlo D, et al. Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: The Role of Sequencing Genetic Profiling. Cancers. 2021; 13(23):6049. https://doi.org/10.3390/cancers13236049
Chicago/Turabian StyleGruttadauria, Salvatore, Floriana Barbera, Duilio Pagano, Rosa Liotta, Roberto Miraglia, Marco Barbara, Maria Grazia Bavetta, Calogero Cammà, Ioannis Petridis, Daniele Di Carlo, and et al. 2021. "Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: The Role of Sequencing Genetic Profiling" Cancers 13, no. 23: 6049. https://doi.org/10.3390/cancers13236049
APA StyleGruttadauria, S., Barbera, F., Pagano, D., Liotta, R., Miraglia, R., Barbara, M., Bavetta, M. G., Cammà, C., Petridis, I., Di Carlo, D., Conaldi, P. G., & Di Francesco, F. (2021). Liver Transplantation for Unresectable Intrahepatic Cholangiocarcinoma: The Role of Sequencing Genetic Profiling. Cancers, 13(23), 6049. https://doi.org/10.3390/cancers13236049









