TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tubulin Polymerization Assay
2.2. Cell Culture
2.3. Immunofluorescence Imaging
2.4. Live Cell Imaging
2.5. Image Processing and Statistical Analysis
3. Results
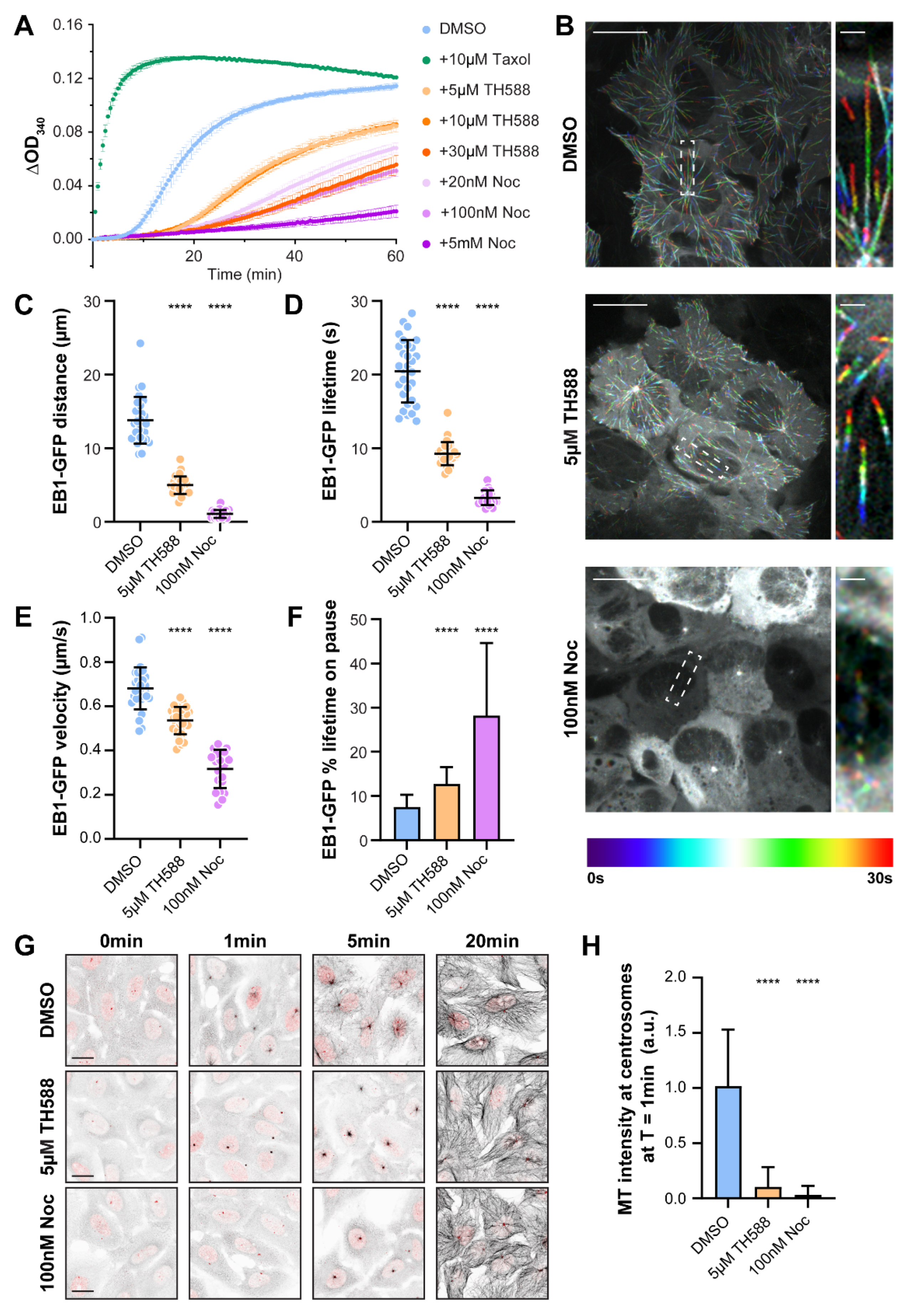
3.1. TH588 Disrupts In Vitro MT Polymerization and in Cellulo MT Nucleation and Reduces MT Dynamics in Interphase Cells
3.2. TH588 Induces Severe Chromosome Congression Problems That Lead to Mitotic Arrest, Followed by the Cell Death or Erroneous Cell Division
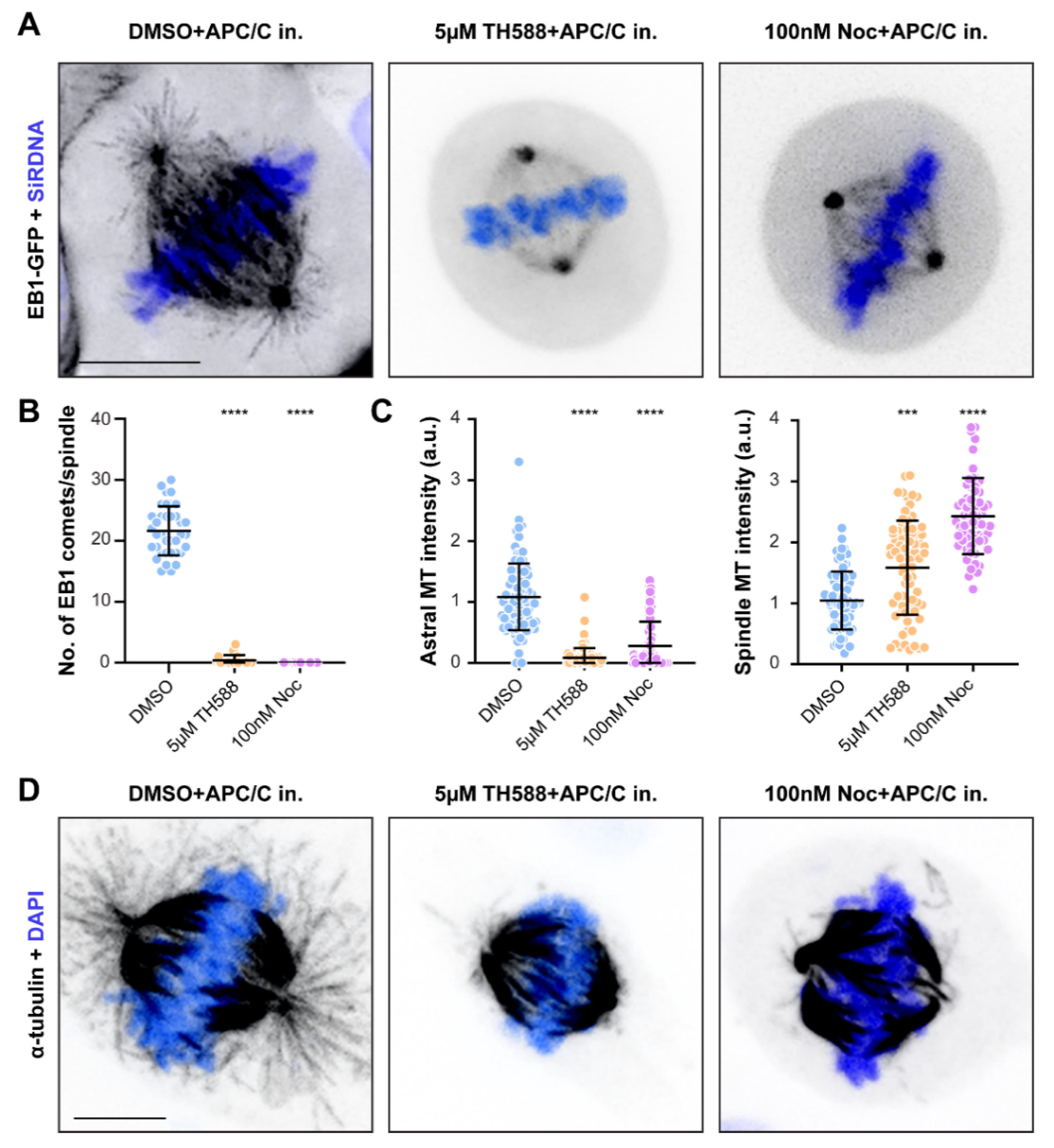
3.3. Similar to Low-Dose Nocodazole, TH588 Disrupts a More Dynamic Array of Astral MTs, While Stabilizing Longer-Lived Kinetochore-MTs
3.4. TH588 and Low Concentration of Nocodazole Similarly Reduce MT Turnover within the Mitotic Spindle
3.5. TH588 Triggers Mitotic Arrest and Erroneous Cell Divisions via Premature Stabilization of Kinetochore-MT End-On Attachments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassimeris, L.; Pryer, N.K.; Salmon, E.D. Real-time observations of microtubule dynamic instability in living cells. J. Cell Biol. 1988, 107, 2223–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horio, T.; Hotani, H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature 1986, 321, 605–607. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Sammak, P.J.; Borisy, G.G. Direct observation of microtubule dynamics in living cells. Nature 1988, 332, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A.; O’Brien, E.T.; Pryer, N.K.; Soboeiro, M.F.; Voter, W.A.; Erickson, H.P.; Salmon, E.D. Dynamic instability of individual microtubules analyzed by video light microscopy: Rate constants and transition frequencies. J. Cell Biol. 1988, 107, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Carlier, M.F.; Pantaloni, D. Kinetic analysis of guanosine 5’-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry 1981, 20, 1918–1924. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Gascoigne, K.E.; Taylor, S.S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 2008, 14, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Orth, J.D.; Kohler, R.H.; Foijer, F.; Sorger, P.K.; Weissleder, R.; Mitchison, T.J. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 2011, 71, 4608–4616. [Google Scholar] [CrossRef] [Green Version]
- Zasadil, L.M.; Andersen, K.A.; Yeum, D.; Rocque, G.B.; Wilke, L.G.; Tevaarwerk, A.J.; Raines, R.T.; Burkard, M.E.; Weaver, B.A. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci. Transl. Med. 2014, 6, 229ra243. [Google Scholar] [CrossRef] [Green Version]
- Komlodi-Pasztor, E.; Sackett, D.; Wilkerson, J.; Fojo, T. Mitosis is not a key target of microtubule agents in patient tumors. Nat. Rev. Clin. Oncol. 2011, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.; Koolmeister, T.; Jemth, A.S.; Eshtad, S.; Jacques, S.A.; Strom, C.E.; Svensson, L.M.; Schultz, N.; Lundback, T.; Einarsdottir, B.O.; et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef]
- Ikejiri, F.; Honma, Y.; Kasukabe, T.; Urano, T.; Suzumiya, J. TH588, an MTH1 inhibitor, enhances phenethyl isothiocyanate-induced growth inhibition in pancreatic cancer cells. Oncol. Lett. 2018, 15, 3240–3244. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Kawatani, M.; Muroi, M.; Kondoh, Y.; Futamura, Y.; Aono, H.; Tanaka, M.; Honda, K.; Osada, H. Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival. Sci. Rep. 2016, 6, 26521. [Google Scholar] [CrossRef] [Green Version]
- Moukengue, B.; Brown, H.K.; Charrier, C.; Battaglia, S.; Baud’huin, M.; Quillard, T.; Pham, T.M.; Pateras, I.S.; Gorgoulis, V.G.; Helleday, T.; et al. TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model. EBioMedicine 2020, 53, 102704. [Google Scholar] [CrossRef]
- Oksvold, M.P.; Berglund, U.W.; Gad, H.; Bai, B.; Stokke, T.; Rein, I.D.; Pham, T.; Sanjiv, K.; Oy, G.F.; Norum, J.H.; et al. Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma. Sci. Rep. 2021, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
- Sanjiv, K.; Calderon-Montano, J.M.; Pham, T.M.; Erkers, T.; Tsuber, V.; Almlof, I.; Hoglund, A.; Heshmati, Y.; Seashore-Ludlow, B.; Nagesh Danda, A.; et al. MTH1 inhibitor TH1579 induces oxidative DNA damage and mitotic arrest in acute myeloid leukemia. Cancer Res. 2021. [Google Scholar] [CrossRef]
- Sakumi, K.; Furuichi, M.; Tsuzuki, T.; Kakuma, T.; Kawabata, S.; Maki, H.; Sekiguchi, M. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem. 1993, 268, 23524–23530. [Google Scholar] [CrossRef]
- Rai, P.; Onder, T.T.; Young, J.J.; McFaline, J.L.; Pang, B.; Dedon, P.C.; Weinberg, R.A. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc. Natl. Acad. Sci. USA 2009, 106, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Gul, N.; Karlsson, J.; Tangemo, C.; Linsefors, S.; Tuyizere, S.; Perkins, R.; Ala, C.; Zou, Z.; Larsson, E.; Bergo, M.O.; et al. The MTH1 inhibitor TH588 is a microtubule-modulating agent that eliminates cancer cells by activating the mitotic surveillance pathway. Sci. Rep. 2019, 9, 14667. [Google Scholar] [CrossRef] [Green Version]
- Patterson, J.C.; Joughin, B.A.; Prota, A.E.; Muhlethaler, T.; Jonas, O.H.; Whitman, M.A.; Varmeh, S.; Chen, S.; Balk, S.P.; Steinmetz, M.O.; et al. VISAGE Reveals a Targetable Mitotic Spindle Vulnerability in Cancer Cells. Cell Syst. 2019, 9, 74–92.e78. [Google Scholar] [CrossRef] [Green Version]
- Rudd, S.G.; Gad, H.; Sanjiv, K.; Amaral, N.; Hagenkort, A.; Groth, P.; Strom, C.E.; Mortusewicz, O.; Berglund, U.W.; Helleday, T. MTH1 Inhibitor TH588 Disturbs Mitotic Progression and Induces Mitosis-Dependent Accumulation of Genomic 8-oxodG. Cancer Res. 2020, 80, 3530–3541. [Google Scholar] [CrossRef] [Green Version]
- Castoldi, M.; Popov, A.V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr. Purif. 2003, 32, 83–88. [Google Scholar] [CrossRef]
- Mirigian, M.; Mukherjee, K.; Bane, S.L.; Sackett, D.L. Measurement of in vitro microtubule polymerization by turbidity and fluorescence. Methods Cell Biol. 2013, 115, 215–229. [Google Scholar] [CrossRef]
- Vit, G.; Duro, J.; Rajendraprasad, G.; Hertz, E.P.T.; Kauffeldt Holland, L.K.; Weisser, M.B.; McEwan, B.C.; Lopez-Mendez, B.; Montoya, G.; Mailand, N.; et al. Cellular toxicity of iHAP1 and DT-061 does not occur through PP2A-B56 targeting. bioRxiv 2021. [Google Scholar] [CrossRef]
- DeLuca, J.G. Kinetochore-microtubule dynamics and attachment stability. Methods Cell Biol. 2010, 97, 53–79. [Google Scholar] [CrossRef] [PubMed]
- Steblyanko, Y.; Rajendraprasad, G.; Osswald, M.; Eibes, S.; Jacome, A.; Geley, S.; Pereira, A.J.; Maiato, H.; Barisic, M. Microtubule poleward flux in human cells is driven by the coordinated action of four kinesins. EMBO J. 2020, 39, e105432. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Rajendraprasad, G.; Wang, N.; Eibes, S.; Gao, J.; Yu, H.; Wu, G.; Tu, X.; Huang, H.; Barisic, M.; et al. Molecular basis of vasohibins-mediated detyrosination and its impact on spindle function and mitosis. Cell Res. 2019, 29, 533–547. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Orr, B.; Rajendraprasad, G.; Pereira, A.J.; Lemos, C.; Lima, J.T.; Guasch Boldu, C.; Ferreira, J.G.; Barisic, M.; Maiato, H. alpha-Tubulin detyrosination impairs mitotic error correction by suppressing MCAK centromeric activity. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Vinopal, S.; Cernohorska, M.; Sulimenko, V.; Sulimenko, T.; Vosecka, V.; Flemr, M.; Draberova, E.; Draber, P. gamma-Tubulin 2 nucleates microtubules and is downregulated in mouse early embryogenesis. PLoS ONE 2012, 7, e29919. [Google Scholar] [CrossRef] [Green Version]
- Barisic, M.; Sohm, B.; Mikolcevic, P.; Wandke, C.; Rauch, V.; Ringer, T.; Hess, M.; Bonn, G.; Geley, S. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol. Biol. Cell 2010, 21, 1968–1981. [Google Scholar] [CrossRef] [Green Version]
- Barisic, M.; Aguiar, P.; Geley, S.; Maiato, H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat. Cell Biol. 2014, 16, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.H.; Held, M.; Janssens, V.; Hutchins, J.R.; Hudecz, O.; Ivanova, E.; Goris, J.; Trinkle-Mulcahy, L.; Lamond, A.I.; Poser, I.; et al. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 2010, 12, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Heesbeen, R.G.; Tanenbaum, M.E.; Medema, R.H. Balanced activity of three mitotic motors is required for bipolar spindle assembly and chromosome segregation. Cell Rep. 2014, 8, 948–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, M.A.; Thrower, D.; Wilson, L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J. Cell Sci. 1992, 102 Pt 3, 401–416. [Google Scholar] [CrossRef]
- Vasquez, R.J.; Howell, B.; Yvon, A.M.; Wadsworth, P.; Cassimeris, L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 1997, 8, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Bakhoum, S.F.; Thompson, S.L.; Manning, A.L.; Compton, D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009, 11, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Hoebeke, J.; Van Nijen, G.; De Brabander, M. Interaction of oncodazole (R 17934), a new antitumoral drug, with rat brain tubulin. Biochem Biophys Res. Commun. 1976, 69, 319–324. [Google Scholar] [CrossRef]
- De Brabander, M.J.; Van de Veire, R.M.; Aerts, F.E.; Borgers, M.; Janssen, P.A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976, 36, 905–916. [Google Scholar]
- Thery, M.; Racine, V.; Pepin, A.; Piel, M.; Chen, Y.; Sibarita, J.B.; Bornens, M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 2005, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, B.; Sarangapani, K.K.; Powers, A.F.; Nelson, C.R.; Reichow, S.L.; Arellano-Santoyo, H.; Gonen, T.; Ranish, J.A.; Asbury, C.L.; Biggins, S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 2010, 468, 576–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, A.D.; Powers, A.F.; Gestaut, D.R.; Gonen, T.; Davis, T.N.; Asbury, C.L. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat. Cell Biol. 2007, 9, 832–837. [Google Scholar] [CrossRef] [Green Version]
- King, J.M.; Nicklas, R.B. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J. Cell Sci. 2000, 113 Pt 21, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, R.B.; Koch, C.A. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 1969, 43, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cane, S.; Ye, A.A.; Luks-Morgan, S.J.; Maresca, T.J. Elevated polar ejection forces stabilize kinetochore-microtubule attachments. J. Cell Biol. 2013, 200, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drpic, D.; Pereira, A.J.; Barisic, M.; Maresca, T.J.; Maiato, H. Polar Ejection Forces Promote the Conversion from Lateral to End-on Kinetochore-Microtubule Attachments on Mono-oriented Chromosomes. Cell Rep. 2015, 13, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Aher, A.; Kok, M.; Sharma, A.; Rai, A.; Olieric, N.; Rodriguez-Garcia, R.; Katrukha, E.A.; Weinert, T.; Olieric, V.; Kapitein, L.C.; et al. CLASP Suppresses Microtubule Catastrophes through a Single TOG Domain. Dev. Cell 2018, 46, 40–58.e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bassam, J.; Kim, H.; Brouhard, G.; van Oijen, A.; Harrison, S.C.; Chang, F. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev. Cell 2010, 19, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Girao, H.; Okada, N.; Rodrigues, T.A.; Silva, A.O.; Figueiredo, A.C.; Garcia, Z.; Moutinho-Santos, T.; Hayashi, I.; Azevedo, J.E.; Macedo-Ribeiro, S.; et al. CLASP2 binding to curved microtubule tips promotes flux and stabilizes kinetochore attachments. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Lawrence, E.J.; Arpag, G.; Norris, S.R.; Zanic, M. Human CLASP2 specifically regulates microtubule catastrophe and rescue. Mol. Biol. Cell 2018, 29, 1168–1177. [Google Scholar] [CrossRef]
- Majumdar, S.; Kim, T.; Chen, Z.; Munyoki, S.; Tso, S.C.; Brautigam, C.A.; Rice, L.M. An isolated CLASP TOG domain suppresses microtubule catastrophe and promotes rescue. Mol. Biol. Cell 2018, 29, 1359–1375. [Google Scholar] [CrossRef]
- Chen, Y.; Hancock, W.O. Kinesin-5 is a microtubule polymerase. Nat. Commun. 2015, 6, 8160. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, H.; McAinsh, A.D. Kinesin-12 motors cooperate to suppress microtubule catastrophes and drive the formation of parallel microtubule bundles. Proc. Natl. Acad. Sci. USA 2016, 113, E1635–E1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Gonzalez, P.; Pines, J.; Desai, A. Spindle assembly checkpoint activation and silencing at kinetochores. Semin. Cell Dev. Biol. 2021, 117, 86–98. [Google Scholar] [CrossRef]
- Maiato, H.; Gomes, A.M.; Sousa, F.; Barisic, M. Mechanisms of Chromosome Congression during Mitosis. Biology 2017, 6, 13. [Google Scholar] [CrossRef]
- Maresca, T.J.; Salmon, E.D. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 2009, 184, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.S.; Takagaki, K.; Kumada, K.; Hirayama, Y.; Noda, T.; Hirota, T. Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 2009, 184, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, K.S.K.; Jo, M.; Nagasaka, K.; Takahashi, M.; Shindo, N.; Shibata, K.; Tanaka, K.; Masumoto, H.; Fukagawa, T.; Hirota, T. Kinetochore stretching-mediated rapid silencing of the spindle-assembly checkpoint required for failsafe chromosome segregation. Curr. Biol. 2021, 31, 1581–1591.e1583. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendraprasad, G.; Eibes, S.; Boldú, C.G.; Barisic, M. TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle. Cancers 2021, 13, 5995. https://doi.org/10.3390/cancers13235995
Rajendraprasad G, Eibes S, Boldú CG, Barisic M. TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle. Cancers. 2021; 13(23):5995. https://doi.org/10.3390/cancers13235995
Chicago/Turabian StyleRajendraprasad, Girish, Susana Eibes, Claudia Guasch Boldú, and Marin Barisic. 2021. "TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle" Cancers 13, no. 23: 5995. https://doi.org/10.3390/cancers13235995
APA StyleRajendraprasad, G., Eibes, S., Boldú, C. G., & Barisic, M. (2021). TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle. Cancers, 13(23), 5995. https://doi.org/10.3390/cancers13235995





