Simple Summary
Evaluating health-related quality of life (HRQOL) is important because it reflects the impact of treatment from the patient’s perspective. This holds especially true when prognosis is poor, as is the case for gastric cancer patients. This systematic review was performed to evaluate the short- and long-term impacts of current treatments on HRQOL in patients with locally advanced gastric cancer. The results of this review show that surgery and chemoradiotherapy have a significant impact on short-term HRQOL, although this recovers after 6–12 months. More focus on HRQOL in current clinical practice might improve patient counselling and monitoring before, during, and after treatment in locally advanced gastric cancer patients.
Abstract
Background: Current treatment strategies have been designed to improve survival in locally advanced gastric cancer patients. Besides its impact on survival, treatment also affects health-related quality of life (HRQOL), but an overview of reported studies is currently lacking. The aim of this systematic review was therefore to determine the short- and long-term impact of chemotherapy, surgery, and (chemo)radiotherapy on HRQOL in locally advanced, non-metastatic gastric cancer patients. Methods: A systematic review was performed including studies published between January 2000 and February 2021. We extracted studies published in Medline, Embase, and Scopus databases that assessed HRQOL in patients with locally advanced, non-metastatic gastric cancer treated with curative intent. Studies using non-validated HRQOL questionnaires were excluded. Short-term and long-term HRQOL were defined as HRQOL scores within and beyond 6 months after treatment, respectively. Results: Initially, we identified 8705 articles (4037 of which were duplicates, i.e., 46%) and ultimately included 10 articles. Most studies reported that short-term HRQOL worsened in the follow-up period from 6 weeks to 3 months after surgery. However, recovery of HRQOL to preoperative levels occurred after 6 months. After completion of chemoradiotherapy, the same pattern was seen with worse HRQOL after treatment and a recovery of HRQOL after 6–12 months. Conclusions: In patients with locally advanced, non-metastatic gastric cancer, HRQOL deteriorated during the first 3 months after surgery and chemoradiotherapy. However, the long-term data showed a recovery of HRQOL after 6–12 months. To implement HRQOL in clinical decision making in current clinical practice, more research is needed.
1. Introduction
As the fourth cause of cancer-related mortality worldwide, gastric cancer remains a major health problem [1]. Despite intensive treatment, the prognosis remains poor, and the majority of Western patients recur within two years after treatment [2,3,4,5,6]. Current curative treatment options for locally advanced gastric cancer include surgery with postoperative chemoradiotherapy (adjuvant leucovorin + fluorouracil and 45 Gray) based on the Intergroup 0116 trial and perioperative chemotherapy based on the MAGIC trial (epirubicin + cisplatin + fluorouracil) and FLOT4 (fluorouracil + leucovorin + oxaliplatin + docetaxel) trial in Western patients, while in Asia adjuvant chemotherapy is the preferred treatment [7,8,9,10,11,12]. In addition to improving survival, the potentially curative treatments also have an impact on the patient’s health-related quality of life (HRQOL). HRQOL is becoming increasingly important in clinical trials because it reflects the treatment impact from the patient’s perspective [13]. However, little is known about the impact of treatment on HRQOL in patients with locally advanced gastric cancer. Most studies evaluating HRQOL in gastric cancer patients focus on the impact of surgery alone. Deterioration of HRQOL after a gastrectomy has been described [14,15]. However, the impact on HRQOL of current curative treatment strategies is still unclear [14,15]. These considerations prompted us to conduct this systematic review. Besides the impact of treatment on HRQOL, recent studies in gastric cancer have shown that HRQOL data before treatment have prognostic value [16,17]. Worse HRQOL before treatment was associated with worse survival during follow-up in patients with (locally) advanced gastric cancer [16,17]. The importance of measuring HRQOL is evident, not only during but also prior to treatment. Therefore, HRQOL can also play an important role in clinical decision-making. To be able to inform patients on the course of HRQOL after treatment, we wanted to investigate the effects of treatment on both short- and long-term HRQOL.
The aim of this systematic review is to provide an overview of the available literature on HRQOL in patients treated for locally advanced, non-metastatic gastric cancer. We have therefore formulated the following research questions: (1) What is the short-term impact of chemotherapy, surgery, and (chemo)radiotherapy on HRQOL in patients with locally advanced, non-metastatic gastric cancer? (2) What is the long-term impact of treatment on HRQOL in patients with locally advanced, non-metastatic gastric cancer?
2. Materials and Methods
2.1. Study Protocol
We performed a systematic literature review which is registered in the PROSPERO database, an international database of prospectively registered systematic reviews (registration number: CRD42021249987). We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for our protocol and the systematic review [18].
2.2. Search Strategy
A systematic search of the Medline, Embase, and Scopus databases was performed on 12 February 2021. No additional studies have been included after this date. We searched for articles assessing HRQOL in patients with locally advanced, non-metastatic gastric cancer treated with curative intent. The full search strategy is described in the Supplementary Materials.
2.3. Eligibility Criteria
Studies were included if they met the following inclusion criteria: (I) patients with locally advanced gastric cancer (Ib-IVa gastric according to the 8th TNM staging of the American Joint Committee on Cancer) treated with curative intent (surgical resection with (neo)adjuvant chemotherapy or adjuvant (chemo)radiotherapy); (II) the use of a validated HRQOL questionnaire; (III) article written in English language; and (IV) publishing date from the year 2000 and onwards.
In addition, if studies analyzed different stages of gastric cancer, only the locally advanced groups were used in the analysis.
Studies were excluded if: a non-validated questionnaire was used, when no original data was presented, reviews, study protocols, or abstracts with no full text available. Studies researching various stages of gastric cancer were excluded if the proportion of patients exceeded 20% stage Ia. This study investigated the impact of multimodal treatment in gastric cancer, whereas Ia is often treated less intensively.
2.4. Outcomes
Primary outcomes of the study were: (1) short-term impact of treatment on HRQOL; (2) long-term impact of treatment on HRQOL. Based on available literature, short-term HRQOL was defined as HRQOL scores within 6 months after treatment [19]. Long-term HRQOL was defined as HRQOL scores more than 6 months after treatment.
2.5. Data Extraction
All studies (titles, abstracts, and full texts) identified by the search were independently analyzed by two observers (R.v.A. and K.v.d.S.). Conflicting decisions were resolved by discussion and if persistent, screened by a third reviewer (I.W.). R.v.A. and K.v.d.S. extracted the following data of the included studies: author, number of patients included, patient characteristics (age, gender, and tumor stadium), study design, inclusion years, country, HRQOL questionnaires, time points of questionnaires, HRQOL subscales, and treatment strategy. Authors of articles who did not present all data in the original manuscript were contacted for additional results. Unfortunately, we did not receive sufficient data to perform a pooled analysis.
2.6. Risk of Bias Assessment
Risk of Bias was assessed and discussed by two observers (R.v.A. and K.v.d.S.). The revised Cochrane risk of bias tool (RoB2) was used to assess randomized trials [20]. Non-randomized trials were scored by the risk of bias in non-randomized trials of interventions (ROBINS-I tool) and the critical appraisal skills program (CASP) tool was used in cohort studies [21,22]. Additionally, a study population below 20 patients was considered to be at risk of bias based on sample size and therefore excluded from further analyses.
3. Results
3.1. Search
A flowchart of the included articles in this systematic review is displayed in Figure 1. Our search string yielded 8705 articles, of which 4037 duplicates were removed. The remaining articles were scored based on title and abstract, resulting in 276 articles being analyzed for full text. Furthermore, 261 articles were excluded based on incorrect stage of disease, only an abstract being available, use of a non-validated questionnaire, different tumor type, non-English text, inclusion before the year 2000, letter to the editor, insufficient data, and a study protocol (Figure 1). Data was extracted from the remaining 15 articles. Two articles (Goody et al., and Kassam et al.) reported the same study; therefore these were analyzed together [23,24]. We included the additional data we received from Avery et al. [25]. Four articles with a high risk of bias were excluded in the final analysis [26,27,28,29].
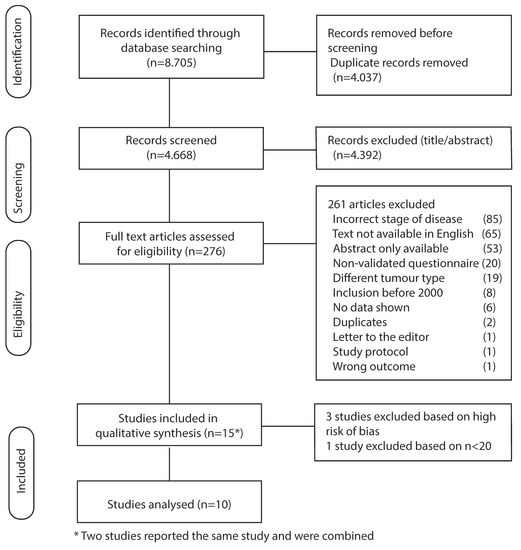
Figure 1.
Flowchart of included studies.
Baseline demographics are described in Table 1. Of the 10 included studies, the year of publication varied between 2010 and 2020. Most included studies investigated the impact of surgery on HRQOL (n = 8), followed by the impact of chemoradiotherapy (n = 2) and chemotherapy (n = 2) on HRQOL. There were no studies evaluating the impact of perioperative chemotherapy on HRQOL. Based on the risk of bias analysis, 4 of the 10 studies scored as having “some concerns” and 6 as having “low concerns”.

Table 1.
Included studies, baseline demographics.
3.2. HRQOL Measures
The EORTC cancer-specific questionnaire (QLQ-C30) was used in the majority of the studies (n = 7, 70%), followed by the QLQ-STO22 (n = 4, 40%), EQ-5D (n = 1, 10%), FACT-G and FACT-Ga (n = 1, 10%), and PGSAS-45 (n = 1, 10%).
3.3. Impact of Surgery on HRQOL
This section included eight studies that examined the impact of surgery on HRQOL in gastric cancer patients. A summary of all the outcomes is displayed in Table 2.

Table 2.
Summary of studies evaluating HRQOL.
Short-term HRQOL was impaired from 6 weeks to 3 months after surgery as described by Avery et al., and Munene et al. [25,30]. In a cohort study by Avery et al., HRQOL was evaluated in 58 patients undergoing potentially curative surgery. HRQOL was significantly worse 6 weeks after surgery compared to before surgery (except for cognitive and emotional functioning) [25]. Munene et al., reported similar results regarding the outcome of HRQOL. They evaluated HRQOL (FACT-G and FACT-Ga) in 43 patients treated in the majority with total gastrectomy and D2, D3 lymphadenectomy. HRQOL was deteriorated 3 months postoperatively [30]. However, in the long term, Avery et al., and Munene et al., did report a recovery of HRQOL to (almost) preoperative levels 6 months after surgery in [25,30]. Hereafter, HRQOL persisted until a recurrence was discovered or prior to death [25,30].
The long-term impact on HRQOL was investigated by Jakstaite et al., 6 to 18 months after gastric surgery. Almost half of the patients also received adjuvant chemotherapy. Patients aged 65 years and older had better global HRQOL scores and social functioning. No significant impact on HRQOL was seen based on gender, clinical stage, or adjuvant chemotherapy [31]. Kinami et al., also investigated long-term impacts on HRQOL (PGSAS-45) in patients who underwent a distal partial gastrectomy > 1 year ago. Locally advanced stage gastric cancer patients, in which the majority also received adjuvant chemotherapy before, showed similar HRQOL compared to early-stage gastric cancer (except for the dumping subscale which was found to be dependent on remnant stomach size) [32].
The impact of the type of surgery on HRQOL yielded mixed results. Munene et al., concluded that there was no difference in HRQOL between patients who underwent a partial or total gastrectomy [30]. Huang et al., did not describe a difference in HRQOL between a laparoscopic-assisted total gastrectomy compared to a totally laparoscopic total gastrectomy (except for the symptom subscales pain and dysphagia, which were worse in laparoscopic-assisted total gastrectomy) [33]. However, Brenkman et al., showed, in a multivariate analysis, that patients who received a distal gastrectomy or minimal invasive surgery had better HRQOL, especially on the gastrointestinal subscales [34]. Park et al., described worse HRQOL in patients who underwent a total gastrectomy compared to distal gastrectomy > 1 years after surgery [35].
Both Brenkman et al., and Xia et al., evaluated HRQOL in gastric cancer patients compared to the general population or healthy controls [34,36]. Brenkman et al., described an impaired HRQOL in most subscales in patients after a gastrectomy (> 1 year after treatment, compared to the general population) [34]. Xia et al., observed a similar association in which patients with gastric cancer had significantly worse HRQOL on all the subscales of the EQ-5D compared to healthy controls [36].
3.4. Impact of Postoperative Chemoradiotherapy on HRQOL
The impact of chemoradiotherapy on HRQOL was investigated in the following studies and summarized in Table 2.
Short-term HRQOL was worse after chemoradiotherapy as described by Zygogianni et al., and Goody/Kassam et al. [23,24,37]. Zygogianni et al., evaluated the short-term impact of three-dimensional multifield radiotherapy (n = 62) versus anteroposterior (AP/PA) radiotherapy (n = 35) on HRQOL based on the QLQ-C30 questionnaire. Patients who were treated with AP/PA radiotherapy had significantly worse global QOL, diarrhea, and loss of appetite compared to patients treated with multifield radiotherapy. Global HRQOL scores were nearly two times higher in the multifield radiotherapy group [37]. The short-term impact of chemoradiotherapy on HRQOL was also described by Goody/Kassam et al., and showed a similar trend as after surgery. After chemoradiation, HRQOL deteriorated compared to HRQOL before treatment. The long-term impact, at 6–12 months of follow-up, showed recovery of HRQOL. No significant differences were seen compared to HRQOL prior to chemoradiation [23,24].
3.5. Impact of Chemotherapy on HRQOL
The impact of chemotherapy on HRQOL in locally advanced gastric cancer was evaluated in the following studies (Table 2).
The short-term impact of chemotherapy on HRQOL was not evaluated in the included studies. The long-term impact on HRQOL was described by Jakstaite et al., and Brenkman et al. [31,34]. Adjuvant chemotherapy had no significant impact on HRQOL in the study of Jakstaite et al. [31]. Brenkman et al., described, based on a multivariate analysis, that patients who received neoadjuvant treatment had better HRQOL after gastrectomy on the following subscales: global QOL, nausea and vomiting, pain, dyspnea, diarrhea, reflux, and eating restrictions [34].
4. Discussion
The aim of this systematic review was to investigate the impact of chemotherapy, surgery, and (chemo)radiotherapy on short- and long-term HRQOL in locally advanced, non-metastatic gastric cancer patients. Our systematic review is the first systematic review investigating HRQOL in locally advanced gastric cancer patients. We found that HRQOL was impaired until 6 months of follow-up but from 6 months onwards, HRQOL recovered to pretreatment levels in most patients.
The impact of treatment is reflected as an impaired short-term HRQOL. Within studies researching the impact of surgery, a decline in physical, mental, and functioning scores were seen from 6 weeks to 3 months after surgery. This pattern was also observed after chemoradiotherapy. However, the long-term impact (after 6 months) showed a recovery of HRQOL for both surgery and chemoradiotherapy [23,24,25,30]. These results can play an important role in daily practice, particularly when discussing the expected dynamic course of HRQOL with patients and thereby clarify the expectations of the patient.
We observed that HRQOL in locally advanced gastric cancer patients was worse compared to healthy controls or the general population [34,36]. This discrepancy might be due to an already impaired HRQOL due to the cancer diagnosis at baseline. The prognostic role of pretreatment HRQOL was not addressed in this systematic review, but also has a potential added value as previously described [16,17]. This further emphasizes the importance of assessing baseline HRQOL.
Another interesting finding of this systematic review was that HRQOL deteriorates when disease recurs and prior to death [25,30]. This outcome is relevant, as HRQOL questionnaires can play a potential role in monitoring locally advanced gastric cancer patients. So, HRQOL can be important not only after treatment but also during follow-up in monitoring disease progression.
In this systematic review, the impact of the type of surgery on HRQOL is unclear, as contradictory conclusions were made. Two studies did not describe a difference between the type of surgery [30,33]. This outcome is in contrast to the results of Park et al., and Brenkman et al. [34,35]. Park et al., described worse long-term HRQOL in patients who underwent a total gastrectomy compared to distal gastrectomy, in both functioning (physical and role) as well as seven symptom scores (fatigue, pain, reflux, eating restrictions, anxiety, taste, and body image) [35]. Brenkman et al., also described a better HRQOL in patients undergoing distal gastrectomy, as well as treatment with minimal invasive surgery in a multivariate analysis [34].
The impact of chemotherapy is also under debate as Jakstaite et al., did not find an impact of adjuvant chemotherapy on HRQOL [31]. However, Brenkman et al., described, based on a multivariate analysis, that patients treated with neoadjuvant treatment had better HRQOL [34]. These results may differ due to a difference in HRQOL measured in time. Besides these contradictory results, too little is known about the short-term impact on HRQOL of (neo)adjuvant chemotherapy. Therefore, more research is needed to elucidate the effect of chemotherapy on both short -and long-term HRQOL.
Current treatment usually consists of perioperative chemotherapy. However, prospective studies that investigate the impact of perioperative chemotherapy on HRQOL in patients with locally advanced gastric cancer are currently non-existent. Hence, there is a great need for more research on this topic. Within the CRITICS study, HRQOL has been investigated as well. The CRITICS study compared perioperative chemotherapy to preoperative chemotherapy, surgery, and postoperative chemoradiotherapy [4]. The first results show that after postoperative chemo(radio)therapy, the chemotherapy group had significantly better physical functioning (p = 0.020, ES = 0.25) and less dysphagia (p = 0.010, ES = 0.35) compared to the chemoradiotherapy group [38].
Despite the small number of articles describing HRQOL in locally advanced gastric cancer, this study shows a trend in HRQOL similar to other cancers, e.g., esophageal cancer. Treatment of esophageal cancer (including chemoradiotherapy, surgery, and neoadjuvant therapy combined with surgery) also resulted in a short-term deterioration of HRQOL, specified as four months from the start of therapy [15]. Similar to the studies included in this systematic review, a statistically and clinically significant increase in fatigue was found in patients receiving chemoradiotherapy as well as a significant decrease in physical, role, and social functioning, and an increase in loss of appetite and diarrhea in patients receiving surgery. Within the long term, classified at 12 months, comparable results were found for the locally advanced gastric cancer patients. The functioning scores also recovered to baseline, whereas in patients receiving surgery, diarrhea remained. Patients receiving neoadjuvant therapy and surgery reported better short-term HRQOL, compared to other therapies; however, no differences in HRQOL were found compared to surgery alone in the long term. When long-term HRQOL in esophageal cancer patients was compared to healthy controls, HRQOL remained impaired, which is similar to the results of Brenkman et al., and Xia et al. [34,36,39]. The overall similarity between both cancers supports the trend shown in the HRQOL of locally advanced gastric cancer as presented in this study.
This systematic review has some limitations. First, there is a lack of high-quality studies investigating the quality of life in patients with locally advanced gastric cancer. Therefore, we only included a very small number of articles, hindering the possibility to perform a pooled analysis. We requested additional data, but unfortunately, we did not receive sufficient data to perform a pooled analysis. Second, there is also a difference in the quality of the included studies. Most studies are not randomized, and 4 of the 10 (40%) studies were scored as having “some concerns” in the Risk of Bias analysis [25,32,33,35].
A strength of this study is the focus on patients with locally advanced gastric cancer treated with curative intent, which is, to our knowledge, the first systematic review investigating this. Furthermore, we performed an extensive systematic review, including only studies published from the year 2000 and onwards. In addition, we only included studies that assessed HRQOL with validated questionnaires.
5. Conclusions
In conclusion, this is the first systematic review investigating HRQOL in locally advanced, non-metastatic gastric cancer patients. The impact that treatment has on HRQOL in locally advanced, non-metastatic gastric cancer worsened after surgery and chemoradiotherapy but recovered after 6–12 months. This dynamic course of HRQOL is of added value in current clinical practice, to clarify the patient’s expectations. More research is needed to implement HRQOL in clinical decision-making.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13235934/s1, Table S1: search strategy.
Author Contributions
Conceptualization, R.M.v.A., K.v.d.S. and I.W.; methodology, R.M.v.A., K.v.d.S., W.S. and I.W.; formal analysis, R.M.v.A., K.v.d.S. and I.W.; writing—original draft preparation, R.M.v.A. and K.v.d.S.; writing—review and editing, R.M.v.A., K.v.d.S., I.W., W.S., E.P.M.J., J.W.v.S. and M.V.; supervision, J.W.v.S., M.V. and I.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Avery et al., for the additional data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gunderson, L.L. Gastric cancer—Patterns of relapse after surgical resection. Semin. Radiat. Oncol. 2002, 12, 150–161. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Kranenbarg, E.M.-K.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.M.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- de Steur, W.; van Amelsfoort, R.; Hartgrink, H.; Putter, H.; Kranenbarg, E.M.-K.; van Grieken, N.; van Sandick, J.; Claassen, Y.; Braak, J.; Jansen, E.; et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann. Oncol. 2020, 32, 360–367. [Google Scholar] [CrossRef]
- van der Kaaij, R.T.; Koemans, W.J.; van Putten, M.; Snaebjornsson, P.; Luijten, J.C.H.B.M.; van Dieren, J.M.; Cats, A.; Lemmens, V.E.P.P.; Verhoevend, R.H.A.; van Sandick, J.W. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur. J. Cancer 2020, 130, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated Analysis of SWOG-Directed Intergroup Study 0116: A Phase III Trial of Adjuvant Radiochemotherapy versus Observation after Curative Gastric Cancer Resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 versus Surgery Alone in Stage II or III Gastric Cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Noh, S.H.; Park, S.R.; Yang, H.-K.; Chung, H.; Chung, I.-J.; Kim, S.-W.; Kim, H.-H.; Choi, J.-H.; Kim, H.-K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Basch, E. Missing Patients’ Symptoms in Cancer Care Delivery—The Importance of Patient-Reported Outcomes. JAMA Oncol. 2016, 2, 433–434. [Google Scholar] [CrossRef]
- McCall, M.D.; Graham, P.J.; Bathe, O.F. Quality of life: A critical outcome for all surgical treatments of gastric cancer. World J. Gastroenterol. 2016, 22, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Boorn, H.G.V.D.; Stroes, C.I.; Zwinderman, A.H.; Eshuis, W.J.; Hulshof, M.C.; van Etten-Jamaludin, F.S.; Sprangers, M.A.; van Laarhoven, H.W. Health-related quality of life in curatively-treated patients with esophageal or gastric cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. 2020, 154, 103069. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, M.S.; Kim, Y.S.; Hong, J.; Nam, E.; Park, J.; Cho, E.K.; Shin, D.B.; Lee, J.H.; Lee, W.K. Self-reported health-related quality of life predicts survival for patients with advanced gastric cancer treated with first-line chemotherapy. Qual. Life Res. 2008, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Norman, A.R.; Cunningham, D.; Waters, J.S.; Oates, J.; Ross, P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 2004, 22, 2395–2403. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Toms, C.; Phty, S.D.B.; Yeo David, M.B.B.S.; Pulitano, C.; Sandroussi, C. Quality of Life Instruments and Trajectories after Pancreatic Cancer Resection: A Systematic Review. Pancreas 2021, 50, 1137–1153. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Nadelson, S.; Nadelson, L. Evidence-Based Practice Article Reviews Using CASP Tools: A Method for Teaching EBP. Worldviews Evid. -Based Nurs. 2014, 11, 344–346. [Google Scholar] [CrossRef]
- Goody, R.B.; MacKay, H.; Pitcher, B.; Oza, A.; Siu, L.L.; Kim, J.; Wong, R.K.S.; Chen, E.; Swallow, C.; Knox, J.; et al. Phase 1/2 Study of the Addition of Cisplatin to Adjuvant Chemotherapy with Image Guided High-Precision Radiation Therapy for Completely Resected Gastric Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; MacKay, H.; Buckley, C.; Fung, S.; Pintilie, M.; Kim, J.; Ringash, J. Evaluating the Impact on Quality of Life of Chemoradiation in Gastric Cancer. Curr. Oncol. 2010, 17, 77–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avery, K.; Hughes, R.; McNair, A.; Alderson, D.; Barham, P.; Blazeby, J. Health-related quality of life and survival in the 2years after surgery for gastric cancer. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kundes, M.F.; Kement, M.; Yegen, F.; Alkan, M.; Kaya, S.; Kaptanoglu, L. Effects of clinical factors on quality of life following curative gastrectomy for gastric cancer. Niger. J. Clin. Pr. 2019, 22, 661–668. [Google Scholar]
- Pruthi, D.S.; Ahmad, M.; Gupta, M.; Bansal, S.; Nautiyal, V.; Saini, S. Assessment of quality of life in resectable gastric cancer patients undergoing chemoradiotherapy as adjuvant treatment Letter to the Editor. S. Asian J. Cancer 2018, 07, 16–20. [Google Scholar] [CrossRef]
- Shu, P.; Tang, H.; Zhou, B.; Wang, R.; Xu, Y.; Shao, J.; Qi, M.; Xia, Y.; Huang, W.; Liu, S. Effect of Yiqi Huayu Jiedu decoction on stages II and III gastric cancer: A multicenter, prospective, cohort study. Medicine 2019, 98, e17875. [Google Scholar] [CrossRef]
- Kim, K.C.; Yook, J.; Eisenbraun, J.; Kim, B.; Huber, R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—a randomized, controlled pilot study. BMC Complementary Altern. Med. 2012, 12, 172. [Google Scholar] [CrossRef]
- Munene, G.; Francis, W.; Garland, S.N.; Pelletier, G.; Mack, L.A.; Bathe, O.F. The quality of life trajectory of resected gastric cancer. J. Surg. Oncol. 2011, 105, 337–341. [Google Scholar] [CrossRef]
- Jakstaite, G.; Samalavicius, N.E.; Smailyte, G.; Lunevicius, R. The quality of life after a total gastrectomy with extended lymphadenectomy and omega type oesophagojejunostomy for gastric adenocarcinoma without distant metastases. BMC Surg. 2012, 12, 11. [Google Scholar] [CrossRef]
- Kinami, S.; Nakamura, N.; Zhiyong, J.; Miyata, T.; Fujita, H.; Takamura, H.; Ueda, N.; Iida, Y.; Kosaka, T. Severity of postgastrectomy syndrome and quality of life after advanced gastric cancer radical gastrectomy. Mol. Clin. Oncol. 2020, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-N.; Huang, C.-M.; Zheng, C.-H.; Li, P.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Chen, Q.-Y.; Cao, L.-L.; et al. Digestive tract reconstruction using isoperistaltic jejunum-later-cut overlap method after totally laparoscopic total gastrectomy for gastric cancer: Short-term outcomes and impact on quality of life. World J. Gastroenterol. 2017, 23, 7129–7138. [Google Scholar] [CrossRef]
- Brenkman, H.J.F.; Tegels, J.J.W.; Ruurda, J.P.; Luyer, M.D.P.; Kouwenhoven, E.A.; Draaisma, W.A.; van der Peet, D.L.; Wijnhoven, B.P.L.; Stoot, J.H.M.B. Factors influencing health-related quality of life after gastrectomy for cancer. Gastric Cancer 2017, 21, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Park, K.B.; Park, J.Y.; Lee, S.S.; Chung, H.Y.; Kwon, O.K. Chronological changes in quality of life and body composition after gastrectomy for locally advanced gastric cancer. Ann. Surg. Treat. Res. 2020, 98, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zeng, H.; Liu, Q.; Liu, S.; Zhang, Z.; Liu, Y.; Guo, G.; Song, G.; Zhu, Y.; Wu, X.; et al. Health-related quality of life and health utility score of patients with gastric cancer: A multi-centre cross-sectional survey in China. Eur. J. Cancer Care 2020, 29, e13283. [Google Scholar] [CrossRef]
- Zygogianni, A.; Fotineas, A.; Platoni, K.; Patatoukas, G.; Dilvoi, M.; Antypas, C.; Armpilia, C.; Arkadopoulos, N.; Psyrri, A.; Koukourakis, G.; et al. A five split-field three dimensional conformal technique versus an anterior-posterior on in postoperative radiotherapy for gastric carcinoma: A multicenter comparative study using quality of life measurements as well as clinical and dosimetric parameters. JBUON 2018, 23, 1020–1028. [Google Scholar]
- Amelsfoort, R.M.v. Quality of Life in the CRITICS Gastric Cancer Study; IGCC: Prague, Czech Republic, 2018. [Google Scholar]
- Staal, E.F.C.; van Sandick, J.W.; van Tinteren, H.; Cats, A.; Aaronson, N.K. Health-related quality of life in long-term esophageal cancer survivors after potentially curative treatment. J. Thorac. Cardiovasc. Surg. 2010, 140, 777–783. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).