The Role of Bitter Taste Receptors in Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Study Selection
3. Results
3.1. Associations between Genetic Variability of Bitter Taste Receptors and Cancer Risk
3.2. Gene and Protein Expression of Bitter Taste Receptors in Cancer
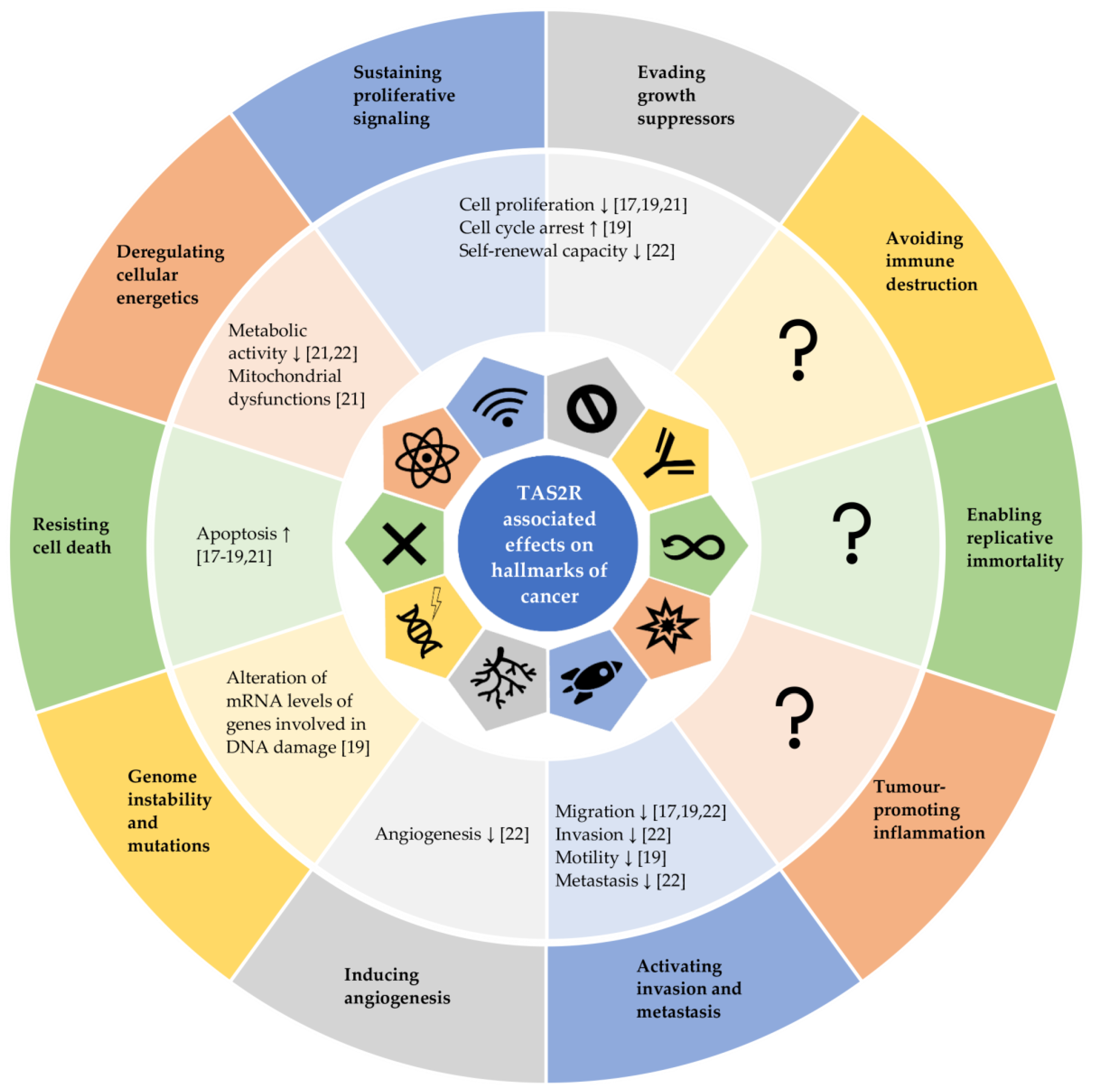
3.3. Functional Role of Bitter Taste Receptors in Cancer Cells and Tissues
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hovan, A.J.; Williams, P.M.; Stevenson-Moore, P.; Wahlin, Y.B.; Ohrn, K.E.O.; Elting, L.S.; Spijkervet, F.K.L.; Brennan, M.T. A systematic review of dysgeusia induced by cancer therapies. Support. Care Cancer 2010, 18, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Spotten, L.E.; Corish, C.; Lorton, C.M.; Dhuibhir, P.M.U.; O’Donoghue, N.C.; O’Connor, B.; Walsh, T.D. Subjective and objective taste and smell changes in cancer. Ann. Oncol. 2017, 28, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Ijpma, I.; Timmermans, E.R.; Renken, R.; Ter Horst, G.J.; Reyners, A.K.L. Metallic Taste in Cancer Patients Treated with Systemic Therapy: A Questionnaire-based Study. Nutr. Cancer 2016, 69, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Omur-Ozbek, P.; Stanek, B.T.; Dietrich, A.M.; Duncan, S.E.; Lee, Y.W.; Lesser, G. Taste and odor abnormalities in cancer patients. J. Support. Oncol. 2009, 7, 58–65. [Google Scholar] [PubMed]
- Tsutsumi, R.; Goda, M.; Fujimoto, C.; Kanno, K.; Nobe, M.; Kitamura, Y.; Abe, K.; Kawai, M.; Matsumoto, H.; Sakai, T.; et al. Effects of chemotherapy on gene expression of lingual taste receptors in patients with head and neck cancer. Laryngoscope 2016, 126, E103–E109. [Google Scholar] [CrossRef]
- DeWys, W.D. Abnormalities of taste as a remote effect of a neoplasm. Ann. N. Y. Acad. Sci. 1974, 230, 427–434. [Google Scholar] [CrossRef]
- DeWys, W.D.; Walters, K. Abnormalities of taste sensation in cancer patients. Cancer 1975, 36, 1888–1896. [Google Scholar] [CrossRef]
- Williams, L.R.; Cohen, M.H. Altered taste thresholds in lung cancer. Am. J. Clin. Nutr. 1978, 31, 122–125. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 2011, 105, 4–13. [Google Scholar] [CrossRef]
- Nissim, I.; Niv, M.Y.; Dagan-Wiener, A. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef]
- Jeruzal-Świątecka, J.; Fendler, W.; Pietruszewska, W. Clinical Role of Extraoral Bitter Taste Receptors. Int. J. Mol. Sci. 2020, 21, 5156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Depoortere, I.; Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.A.; Singh, N.; Arakawa, M.; Duan, K.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Extraoral roles in pathophysiology. Int. J. Biochem. Cell Biol. 2016, 77, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef]
- Kok, B.P.; Galmozzi, A.; Littlejohn, N.K.; Albert, V.; Godio, C.; Kim, W.; Kim, S.M.; Bland, J.S.; Grayson, N.; Fang, M.; et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018, 16, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Tiroch, J.; Sterneder, S.; Di Pizio, A.; Lieder, B.; Hoelz, K.; Holik, A.-K.; Pignitter, M.; Behrens, M.; Somoza, M.; Ley, J.P.; et al. Bitter Sensing TAS2R50 Mediates the trans-Resveratrol-Induced Anti-inflammatory Effect on Interleukin 6 Release in HGF-1 Cells in Culture. J. Agric. Food Chem. 2021. [Google Scholar] [CrossRef]
- Singh, N.; Shaik, F.A.; Myal, Y.; Chelikani, P. Chemosensory bitter taste receptors T2R4 and T2R14 activation attenuates proliferation and migration of breast cancer cells. Mol. Cell. Biochem. 2020, 465, 199–214. [Google Scholar] [CrossRef]
- Martin, L.T.P.; Nachtigal, M.W.; Selman, T.; Nguyen, E.; Salsman, J.; Dellaire, G.; Dupré, D.J. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol. Cell. Biochem. 2018, 454, 203–214. [Google Scholar] [CrossRef]
- Salvestrini, V.; Ciciarello, M.; Pensato, V.; Simonetti, G.; Laginestra, M.A.; Bruno, S.; Pazzaglia, M.; De Marchi, E.; Forte, D.; Orecchioni, S.; et al. Denatonium as a Bitter Taste Receptor Agonist Modifies Transcriptomic Profile and Functions of Acute Myeloid Leukemia Cells. Front. Oncol. 2020, 10, 1225. [Google Scholar] [CrossRef]
- Stern, L.; Giese, N.; Hackert, T.; Strobel, O.; Schirmacher, P.; Felix, K.; Gaida, M.M. Overcoming chemoresistance in pancreatic cancer cells: Role of the bitter taste receptor T2R10. J. Cancer 2018, 9, 711–725. [Google Scholar] [CrossRef]
- Carey, R.M.; McMahon, D.B.; Miller, Z.A.; Kim, T.; Rajasekaran, K.; Gopallawa, I.; Newman, J.G.; Basu, D.; Nead, K.T.; White, E.A.; et al. T2R bitter taste receptors regulate apoptosis and may be associated with survival in head and neck squamous cell carcinoma. Mol. Oncol. 2021. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, Y.-S.; Lee, K.E.; Park, T.H.; Kim, Y. Anti-cancer stemness and anti-invasive activity of bitter taste receptors, TAS2R8 and TAS2R10, in human neuroblastoma cells. PLoS ONE 2017, 12, e0176851. [Google Scholar] [CrossRef]
- Behrens, M.; Somoza, V. Gastrointestinal taste receptors: Could tastants become drugs? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chakraborty, R.; Bhullar, R.P.; Chelikani, P. Differential expression of bitter taste receptors in non-cancerous breast epithelial and breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 499–503. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Singh, N.; Upadhyaya, J.; Arakawa, M.; Dakshinamurti, S.; Duan, K.; Chelikani, P.; Sikarwar, A.S.; Bhullar, R.P. Analysis of the expression of human bitter taste receptors in extraoral tissues. Mol. Cell. Biochem. 2016, 426, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gaida, M.M.; Mayer, C.; Dapunt, U.; Stegmaier, S.; Schirmacher, P.; Wabnitz, G.H.; Hänsch, G.M. Expression of the bitter receptor T2R38 in pancreatic cancer: Localization in lipid droplets and activation by a bacteria-derived quorum-sensing molecule. Oncotarget 2016, 7, 12623–12632. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.; Subramaniam, D.; Panovich, P.; Standing, D.; Krishnamachary, B.; Kaushik, G.; Thomas, S.M.; Dhar, A.; Weir, S.J.; Jensen, R.A.; et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Kawano, Y.; Nagata, M.; Kohno, T.; Ichimiya, A.; Iwakiri, T.; Okumura, M.; Arimori, K. Caffeine Increases the Antitumor Effect of Cisplatin in Human Hepatocellular Carcinoma Cells. Biol. Pharm. Bull. 2012, 35, 400–407. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Osarieme, E.D.; Modupe, D.T.; Oluchukwu, O.P. The Anticancer Activity of Caffeine—A Review. Arch. Clin. Biomed. Res. 2019, 3, 3. [Google Scholar] [CrossRef][Green Version]
- Rabzia, A.; Khazaei, M.; Rashidi, Z.; Khazaei, M.R. Synergistic Anticancer Effect of Paclitaxel and Noscapine on Human Prostate Cancer Cell Lines. Iran. J. Pharm. Res. 2017, 16, 1432–1442. [Google Scholar]
- Tomar, R.; Sahni, A.; Chandra, I.; Tomar, V.; Chandra, R. Review of Noscapine and its Analogues as Potential Anti-Cancer Drugs. Mini-Rev. Org. Chem. 2018, 15, 345–363. [Google Scholar] [CrossRef]
- Ru, P.; Steele, R.; Nerurkar, P.V.; Phillips, N.; Ray, R.B. Bitter Melon Extract Impairs Prostate Cancer Cell-Cycle Progression and Delays Prostatic Intraepithelial Neoplasia in TRAMP Model. Cancer Prev. Res. 2011, 4, 2122–2130. [Google Scholar] [CrossRef]
- Yung, M.M.H.; Ross, F.A.; Hardie, D.G.; Leung, T.H.Y.; Zhan, J.; Ngan, H.Y.S.; Chan, D.W. Bitter Melon (Momordica Charantia) Extract Inhibits Tumorigenicity and Overcomes Cisplatin-Resistance in Ovarian Cancer Cells through Targeting AMPK Signaling Cascade. Integr. Cancer Ther. 2016, 15, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Luo, G.; Somoza, V. Food Systems Biology Database (FSBI-DB); Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2021; Available online: https://fsbi-db.de/ (accessed on 4 October 2021).
- Lambert, J.D.; VanDusen, S.R.; Cockroft, J.E.; Smith, E.C.; Greenwood, D.C.; Cade, J.E. Bitter taste sensitivity, food intake, and risk of malignant cancer in the UK Women’s Cohort Study. Eur. J. Nutr. 2019, 58, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, J. Genetic Variation in the TAS2R38 Bitter Taste Receptor and Gastric Cancer Risk in Koreans. Sci. Rep. 2016, 6, 26904. [Google Scholar] [CrossRef]
- Basson, M.D.; Bartoshuk, L.M.; Dichello, S.Z.; Panzini, L.; Weiffenbach, J.M.; Duffy, V.B. Association Between 6-n-Propylthiouracil (PROP) Bitterness and Colonic Neoplasms. Dig. Dis. Sci. 2005, 50, 483–489. [Google Scholar] [CrossRef]
- Schembre, S.M.; Cheng, I.; Wilkens, L.R.; Albright, C.L.; Marchand, L.L.; Le Marchand, L. Variations in bitter-taste receptor genes, dietary intake, and colorectal adenoma risk. Nutr. Cancer 2013, 65, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, M.; Saito, H.; Isono, K.; Goto, T.; Shirakawa, H.; Shoji, N.; Satoh-Kuriwada, S.; Sasano, T.; Okada, R.; Kudoh, K.; et al. Genotyping Analysis of Bitter-Taste Receptor Genes TAS2R38 and TAS2R46 in Japanese Patients with Gastrointestinal Cancers. J. Nutr. Sci. Vitaminol. 2017, 63, 148–154. [Google Scholar] [CrossRef]
- Carrai, M.; Steinke, V.; Vodicka, P.; Pardini, B.; Rahner, N.; Holinski-Feder, E.; Morak, M.; Schackert, H.K.; Görgens, H.; Stemmler, S.; et al. Association Between TAS2R38 Gene Polymorphisms and Colorectal Cancer Risk: A Case-Control Study in Two Independent Populations of Caucasian Origin. PLoS ONE 2011, 6, e20464. [Google Scholar] [CrossRef]
- Campa, D.; Vodicka, P.; Pardini, B.; Naccarati, A.; Carrai, M.; Vodickova, L.; Novotny, J.; Hemminki, K.; Försti, A.; Barale, R.; et al. A gene-wide investigation on polymorphisms in the taste receptor 2R14 (TAS2R14) and susceptibility to colorectal cancer. BMC Med Genet. 2010, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Barontini, J.; Antinucci, M.; Tofanelli, S.; Cammalleri, M.; Monte, M.D.; Gemignani, F.; Vodicka, P.; Marangoni, R.; Vodickova, L.; Kupcinskas, J.; et al. Association between polymorphisms of TAS2R16 and susceptibility to colorectal cancer. BMC Gastroenterol. 2017, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.; Yang, S.; Lee, E.K.; Hwangbo, Y.; Kim, J. Genetic variations in TAS2R3 and TAS2R4 bitterness receptors modify papillary carcinoma risk and thyroid function in Korean females. Sci. Rep. 2018, 8, 15004. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Variations in the bitterness perception-related genes TAS2R38 and CA6 modify the risk for colorectal cancer in Koreans. Oncotarget 2017, 8, 21253–21265. [Google Scholar] [CrossRef][Green Version]
- Choi, J.-H.; Kim, J. TAS2R38 Bitterness Receptor Genetic Variation and Risk of Gastrointestinal Neoplasm: A Meta-Analysis. Nutr. Cancer 2019, 71, 585–593. [Google Scholar] [CrossRef]
- Behrens, M.; Gunn, H.C.; Ramos, P.C.M.; Meyerhof, W.; Wooding, S.P. Genetic, Functional, and Phenotypic Diversity in TAS2R38-Mediated Bitter Taste Perception. Chem. Senses 2013, 38, 475–484. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.B.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Gentiluomo, M.; Lu, Y.; Canzian, F.; Campa, D. Genetic variants in taste-related genes and risk of pancreatic cancer. Mutagenesis 2019, 34, 391–394. [Google Scholar] [CrossRef]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP Bitterness Depends on More Than the TAS2R38 Gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef]
- Lipchock, S.V.; Mennella, J.; Spielman, A.I.; Reed, D.R. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am. J. Clin. Nutr. 2013, 98, 1136–1143. [Google Scholar] [CrossRef]
- Melis, M.; Atzori, E.; Cabras, S.; Zonza, A.; Calò, C.; Muroni, P.; Nieddu, M.; Padiglia, A.; Sogos, V.; Tepper, B.J.; et al. The Gustin (CA6) Gene Polymorphism, rs2274333 (A/G), as a Mechanistic Link between PROP Tasting and Fungiform Taste Papilla Density and Maintenance. PLoS ONE 2013, 8, e74151. [Google Scholar] [CrossRef] [PubMed]
- Bruford, E.A.; Braschi, B.; Denny, P.; Jones, T.E.M.; Seal, R.L.; Tweedie, S. Guidelines for human gene nomenclature. Nat. Genet. 2020, 52, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. Chemosensory alterations and cancer therapies. In National Institutes of Health Consensus Development Conference on Oral Compliaction of Cancer Therapies: Diagnosis, Prevention, and Treatment; NCI Monographs: Bethesda, MD, USA, 1990; pp. 179–184. [Google Scholar]
| Cancer Type | Cells or Tissues | TAS2Rs/TAS2Rs Regulation | Agonists Used in Functional Assay with Cell Lines | Ref. |
|---|---|---|---|---|
| Breast cancer | MCF-7 and MDA-MB-231 vs. MCF-10A | TAS2R4 ↓, TAS2R4 ↓ No differences for TAS2R1, 10, 20 (49), 38 at gene and protein level | Quinine, dextromethorphan, PTC (verified with MCF-7, MDA-MB-231, MCF-10A) | [24] |
| Breast cancer | MDA-MB-231 vs. MCF-10A | TAS2R14 ↑, TAS2R20 ↑ No differences for other TAS2Rs | [25] | |
| Breast cancer | Breast cancer tissues vs. non-cancerous breast tissues | TAS2R4 ↓ TAS2R14 ↑ TAS2R14 ↑ | Quinine, apigenin (verified with MDA-MB-231 andMCF-10A) | [17] |
| Ovarian cancer | OVCAR4, OVCAR8, ovarian cystadenocarcinoma tissue vs. normal fallopian tube tissue; SKOV3 and IGROV1 vs. normal uterine tissue | TAS2R1 ↓ in OVCAR8, TAS2R4 and 14 ↓ in OVCAR8 and SKOV3, TAS2R10 and 14 ↓ in cancer tissue, TAS2R38 ↓ in all analysed cell lines and cancer tissue | Noscapine and diphenhydramine for TAS2R14 (verified with SKOV3) | [18] |
| Endometrial cancer | HEC-1a vs. normal uterine tissue | TAS2R1, 14, 38 ↓ | [18] | |
| Prostate cancer | PC3, LNCAP, DU145 vs. BPH1 | TAS2R1, 4, 10, 14 ↓ in PC3, LNCAP, DU145, TAS2R38 ↓ in LNCAP, DU145 | Noscapine for TAS2R14 (verified with PC3, DU145) | [18] |
| Pancreatic cancer | Primary human pancreatic tumour tissues, T3M4, SU.86.86, PANC-1, MiaPaCa-2, Colo357, Capan-1, BxPC-3, AsPC-1 | TAS2R10 TAS2R10 | Caffeine (verified with BxPC-3 and PANC-1) | [20] |
| Pancreatic cancer | Tumour infiltrating leukocytes; pancreatic tumour tissues vs. non-cancerous pancreatic tissues; SU.86.86, T3M4, MiaPaCa-2, and RLT | TAS2R38 | PTC, N-acetyl-dodecanoyl-homoserine lactone (AHL-12) (verified with SU.86.86 and RLT) | [26] |
| Neuroblastoma | SK-N-BE(2)C vs. SH-SY5Y | TAS2R8 ↓ TAS2R10 ↓ | Denatonium benzoate (verified with SK-N-BE(2)C) | [22] |
| Acute myeloid leukemia | Primary acute myeloid leukemia cells, in silico gene expression profiling of samples from a database, OCI-AML3, THP-1 | Analyses of 18 to 24 TAS2Rs out of 25 TAS2Rs & identification of all TAS2Rs but not in all approaches | Denatonium benzoate, quinine (verified with primary acute myeloidleukemia cells, OCI-AML3, THP-1) | [19] |
| Head and neck squamous cell carcinoma | Cancerous vs. non-cancerous tissues; SCC4, SCC15, SCC47, SCC90, SCC152,OCTT2 and VU147T | Analysis of all TAS2Rs, highest gene expression of TAS2R4, 14, 19, 20, 30, 43, 45 in cancer cell lines, TAS2R4, 8, 10, 13, 14, 30, 42, 46 in SCC4, SCC47, TAS2R4, 8, 10, 30, 39 in SCC47, SCC90, SCC152 | Denatonium benzoate, quinine, thujone, diphenidol, flufenamic acid, parthenolide, N-3-oxo-dodecanoyl-L-homoserine lactone (3-oxo-C12HSL) (verified in different settings with the used cancer cell lines) | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehentner, S.; Reiner, A.T.; Grimm, C.; Somoza, V. The Role of Bitter Taste Receptors in Cancer: A Systematic Review. Cancers 2021, 13, 5891. https://doi.org/10.3390/cancers13235891
Zehentner S, Reiner AT, Grimm C, Somoza V. The Role of Bitter Taste Receptors in Cancer: A Systematic Review. Cancers. 2021; 13(23):5891. https://doi.org/10.3390/cancers13235891
Chicago/Turabian StyleZehentner, Sofie, Agnes T. Reiner, Christoph Grimm, and Veronika Somoza. 2021. "The Role of Bitter Taste Receptors in Cancer: A Systematic Review" Cancers 13, no. 23: 5891. https://doi.org/10.3390/cancers13235891
APA StyleZehentner, S., Reiner, A. T., Grimm, C., & Somoza, V. (2021). The Role of Bitter Taste Receptors in Cancer: A Systematic Review. Cancers, 13(23), 5891. https://doi.org/10.3390/cancers13235891







