Simple Summary
Cartilage oligomeric matrix protein (COMP) is an emerging independent prognostic marker for breast cancer patients. COMP expression by cancer cells affects their metabolism, metastases, and the abundance of cancer stem cell populations. This study assessed the levels of COMP in the sera of metastatic breast cancer patients. Further, matched tumor tissues from the primary tumor and metastases were stained for COMP expression with immunohistochemistry. The levels of serum COMP were highest in the blood of metastatic ER-positive and HER2-positive patients. The expression of COMP in primary tumors correlated with COMP expression in the metastatic loci. Lymph node metastases (LNM) with COMP expression were associated with reduced survival. The expression of COMP in LNM at the time of primary diagnosis could indicate later development of visceral and lung metastases.
Abstract
Cartilage oligomeric matrix protein (COMP) is a regulator of the extracellular matrix and is expressed primarily in the cartilage. Recently, COMP expression was also documented in breast cancer patients both in sera and tumor biopsies, in both of which it could serve as an independent prognostic marker. This study aimed to assess COMP as a potential biomarker in the group of metastatic breast cancer patients. Levels of COMP were measured by ELISA in serum samples of 141 metastatic breast cancer patients. Biopsies from primary tumors, synchronous lymph node metastases, and distant metastases were stained for COMP expression. The levels of serum COMP were higher in patients with ER- and HER2-positive tumors when compared to triple-negative tumors and correlated with the presence of bone and lung metastases, circulating tumor cell count, and clusters. Most of the primary tumors expressing COMP (70%) retained the expression also in the lymph node metastases, which correlated with visceral metastases and reduced survival. In conclusion, COMP appears as a valuable biomarker in metastatic breast cancer patients indicating a more severe stage of the disease. Serum COMP levels were associated with specific types of metastases in patients with metastatic breast cancer emphasizing that further studies are warranted to elucidate its potential role as a monitoring marker.
1. Introduction
Cartilage oligomeric matrix protein (COMP) has been traditionally viewed as an extracellular matrix (ECM) protein that is expressed by chondrocytes of cartilage [1] as well as fibroblasts under fibrotic conditions [2] and contributes to the organization of ECM [3]. COMP is also known as thrombospondin 5, belonging to the thrombospondin family [4]. Recently, several new functions have been ascribed to this large, pentameric molecule. Thus, COMP was shown to contribute to vascular homeostasis since its degradation by ADAMTS-7 [5] regulates vascular remodeling. COMP has also been found in atherosclerotic plaques [6] and lesions contributing to restenosis of the artery [7]. Furthermore, COMP inhibits thrombin [8] and can act as a regulator of the complement system [9,10].
Moreover, COMP has been found in several cancer types in which it is expressed by epithelial cancer cells and in the surrounding matrix. This has so far been observed in breast- [11], prostate- [12], colon- [13,14], and hepatocellular-carcinoma [15]. Most importantly, in all of the types of cancer that have been studied so far, high expression of COMP has been correlated with reduced survival of the patients. Our previous studies in primary breast cancer revealed that COMP expression in breast tumor cells was correlated with reduced breast cancer-specific survival and recurrence-free survival as an independent prognostic marker [11]. Further, COMP expression in cancer cells correlated positively with the presence of lymph node metastases and estrogen/progesterone receptor positivity. We also found increased levels of COMP in sera of metastatic breast cancer (MBC) patients compared with those with early breast cancer. Further, there was correlation between the serum levels of COMP and the presence of liver and bone metastases. Elevated serum levels of COMP appeared to serve as an independent prognostic marker of survival for the metastatic patients [16].
The mechanisms underlying the effects of COMP in breast cancer progression are under investigation. It has been already established that COMP expression in cancer cells increases their invasive potential, alters metabolism (Warburg effect), and renders them resistant to apoptosis [15]. Further, COMP facilitates the interaction between Notch3 and its ligand Jag1, which leads to the generation of a larger population of cancer stem cells [17].
The goal of the current study was to further evaluate COMP as a potential biomarker in metastatic breast cancer patients by assessing the serum COMP levels and COMP expression in relation to the severity of the disease and outcome.
2. Materials and Methods
2.1. Cohort Description
Patients with newly diagnosed MBC were included in a prospective monitoring trial (ClinicalTrials.gov NCT01322893) before the start of systemic therapy. The inclusion criteria were MBC diagnosis, age > 18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and predicted life expectancy > 2 months. Serum samples were taken at a baseline, before the start of systemic therapy and stored at a biobank. Circulating tumor cells (CTCs) were enumerated using the CellSearch system. Clinicopathological data and monitoring of patients were prospectively collected in case report forms. Included patients received systemic therapy according to national clinical guidelines. A response evaluation was performed approximately every third month and the progression versus non-progression was defined according to a modified RECIST criteria [18]. Initial results from the study have previously been published [19].
2.2. Determination of COMP Levels in Sera (S-COMP)
The S-COMP serum levels were measured using an IVD approved ELISA (AnaMar AB, Immunodiagnostic systems) following manufacturer instructions. In brief, serum was diluted (1/10) in the provided sample buffer, added to a precoated 96-well plate together with an enzyme conjugated antibody, and incubated for 2 h at room temperature. The plates were then washed 6 times, developed with TMB substrate, and measured at 450 nm using Cytation 5 (Biotek, Winooski, VT, USA).
2.3. Immunohistochemical (IHC) Detection of COMP in Tumor Tissues
Breast cancer tissues were mounted in a tissue microarray. Antigen retrieval was performed with the Envision Flex high pH kit (Agilent-Dako, Santa Clara, CA, USA) using a PT-link module (Agilent-Dako). The tissues were stained with 0.47 μg/mL rabbit polyclonal affinity purified anti-COMP in-house antibody that was previously evaluated for its specificity [11], utilizing Envision Flex (Agilent-Dako) reagents in the Autostainer Plus system according to the manufacturer’s protocol (Agilent-Dako). Slides were scanned with Aperio Scanner system (Leica) at 40× and the intensity of COMP was evaluated independently by two experienced researchers in a blinded fashion using scores: 0 for negative staining, 1 for low expression, 2 for moderate expression, and 3 for high expression. Staining in the tumor cells was evaluated separately from stroma.
2.4. Statistical Analyses
For comparison of clinicopathological variables, a Pearson’s chi-squared test or a Pearson’s chi-squared test for trend was used. The comparison of COMP expression status between primary tumors, LNM and DM, was performed using the McNemar’s test. Kaplan–Meier curves and log rank tests were used to illustrate and compare survival. Time to progression or death of any cause was calculated from the baseline and patients without events were censored at the last follow-up. The median follow-up time was 49 (27–93) months. All of the statistical analyses were performed using SPSS statistics (version 26.0, IBM Corp., Armonk, NY, USA).
3. Results
3.1. Serum COMP Levels in Patients with Metastatic Breast Cancer and Associations to Clinicopathological Variables
Serum samples that were taken before the start of the systemic therapy were available for 141 patients, as illustrated in the flowchart (Figure 1). Of these 141 patients, 98 had ER-positive (HER2-negative) disease, 15 had HER2-positive (ER-positive or negative) disease, and 25 were diagnosed with triple-negative breast cancer (TNBC). A total of 86 patients had a metastasis-free interval (MFI) of >3 years, 26 had an MFI ≤ 3 years, and 29 patients had de novo MBC (distant metastasis at initial diagnosis). Regarding number of metastatic loci, 98 patients had ≥3, whereas 42 patients had <3. Furthermore, 83 patients were diagnosed with visceral metastasis whereas 58 were not. A total of 72 patients had ≥5 circulating tumor cells (CTC) at baseline, whereas 13 patients had CTC-clusters. The median S-COMP level was 11.67 U/L (range: 0.00–95.24 U/L). For further analyses and correlations with clinicopathological variables, the patients were grouped by dichotomization into groups with high and low S-COMP expression, respectively, using the median as a cut-off. As illustrated in Table 1, S-COMP levels were significantly associated with age, metastasis-free interval (MFI), and histological subtype of the primary tumor. Also, high S-COMP levels were associated with HER2-positive and ER-positive disease whereas low S-COMP levels were seen in patients with TNBC. Regarding distant metastases, high S-COMP levels were significantly associated with the presence of bone metastases whereas low levels of S-COMP were seen in patients with lung metastases. Furthermore, there was a strong correlation of high S-COMP levels and CTC count (≥5CTCs), and with the presence of CTC-clusters at the baseline, where 12 of the 13 patients with CTC-clusters had high S-COMP levels.
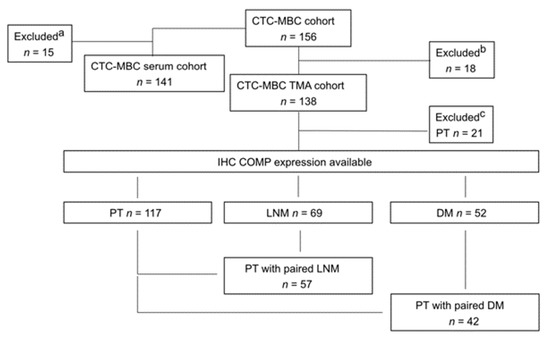
Figure 1.
Flowchart of the study cohort. a Patient excluded due to lack of available serum samples. b Patients excluded due to lack of available tumor tissue. c Patients excluded due to lack of available primary tumor tissue for staining.

Table 1.
S-COMP levels (high/low) in relation to clinicopathological variables in patients with metastatic breast cancer.
3.2. Progression-Free and Overall Survival in Correlation to S-COMP Levels
When analyzing progression-free (PFS) and overall survival (OS) in relation to S-COMP levels there were no significant differences in patients with high versus low S-COMP levels, illustrated by Kaplan–Meier curves (Figure 2).
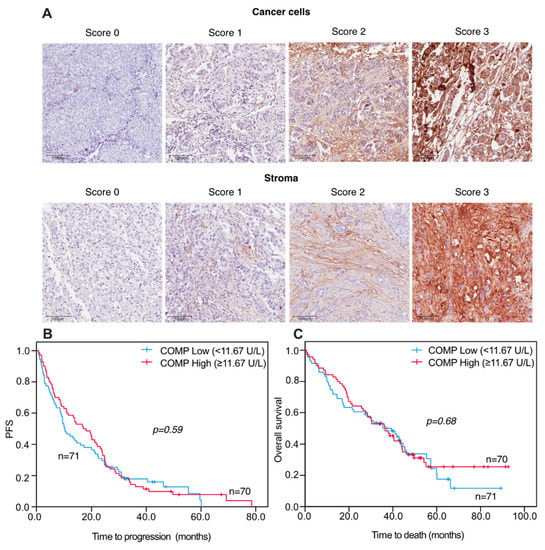
Figure 2.
Representative pictures of TMA IHC stained for COMP expression (A). Progression-free and overall survival in relation to S-COMP levels in metastatic breast cancer patients Kaplan-Meier curves displaying PFS (B) and OS (C) in MBC patients with high or low S-COMP levels, using the median S-COMP level as cut-off. Scale bars: 100 μm.
3.3. Correlations of S-COMP Levels and Comp IHC Expression in Distant Metastases
Comparisons of the S-COMP levels and COMP levels that were detected using immunohistochemistry (IHC expression) in cancer cells and stroma in distant metastases (DM) revealed a positive correlation of high S-COMP levels in patients that had a strong IHC COMP expression in DM stroma (p = 0.01; Table 1). Similarly, a trend for a positive correlation was seen between patients with high S-COMP levels and IHC COMP expression in DM cancer cells, however this was not significant (p = 0.13; Table 1). Comparisons of serum COMP levels to primary tumors and lymph node metastases (LNM) COMP IHC expression were not performed since the serum samples were obtained at diagnosis of metastatic disease and not at the initial breast cancer diagnosis.
3.4. Concordance and Discordance of IHC COMP Expression in Matched Samples of Primary Tumors, Lymph Node Metastases, and Distant Metastases
To evaluate shifts in COMP expression during tumor progression, matched pairs of primary tumors, synchronous LNM and DM, were analyzed by IHC. McNemar’s analyses were used for the evaluation of concordance and discordance of IHC COMP expression in cancer cells and stroma. As illustrated in Table 2, a significant shift was seen in the COMP IHC stroma expression from the primary tumor to LNM (p = 0.049), revealing that in 70% of the pairs the expression was stable but there were more pairs with a loss of COMP expression from the primary tumor to LNM than with a gain. Similarly, a trend was seen in shifts regarding COMP IHC cancer cell expression from the primary tumor to LNM, however this was not significant (p = 0.077). Regarding shifts from the primary tumor to DM and from LNM to DM, there were no significant shifts.

Table 2.
Shifts in COMP expression from primary tumors to lymph node metastases and distant metastases, evaluated with McNemar’s analyses.
3.5. COMP IHC Expression and Correlations to Clinicopathological Variables
Comparisons of IHC expression in cancer cells and stroma in primary tumors and LNMs with different clinicopathological variables showed a significant correlation of COMP expression in primary tumor cancer cells with age (p = 0.005) and in primary tumor stroma with MFI (p = 0.007) (Table 3). Furthermore, there were significant correlations between high IHC COMP expression in the primary tumor cancer cells (p = 0.013) as well as in the primary tumor stroma (p = 0.013) and the presence of liver metastases. When analyzing possible correlations between LNM IHC COMP expression and clinicopathological variables there were significant correlations to performance status (p = 0.023) and furthermore a high COMP IHC expression in stroma correlated to visceral metastases (p = 0.03) and presence of lung metastases (p = 0.039). Associations of cancer cell and stroma COMP expression in DM showed no significant associations to the respective clinicopathological variables (Table S1).

Table 3.
COMP expression in primary tumors and lymph node metastases in relation to clinicopathological variables in patients with metastatic breast cancer. Bold indicates p values < 0.05.
3.6. COMP IHC Expression in Relation to Survival
For survival analyses, a combined variable was used that assessed the COMP IHC expression in both the cancer cells and stroma. When analyzing PFS and OS in relation to COMP IHC expression, no significant correlations were seen for primary tumors and DM COMP expression in cancer cells and stroma (Figure 3A,B). However, when analyzing COMP IHC expression in LNM cancer cells and stroma, there were significant associations with PFS and OS where a high IHC COMP expression was associated with a worse outcome (PFS, p = 0.040 and OS, p = 0.041; Figure 4A,B). Furthermore, when analyzing the survival from the date of primary tumor diagnosis, there was also a strong correlation between high COMP IHC expression in LNM and worse survival (p = 0.019; Figure 4C,D).
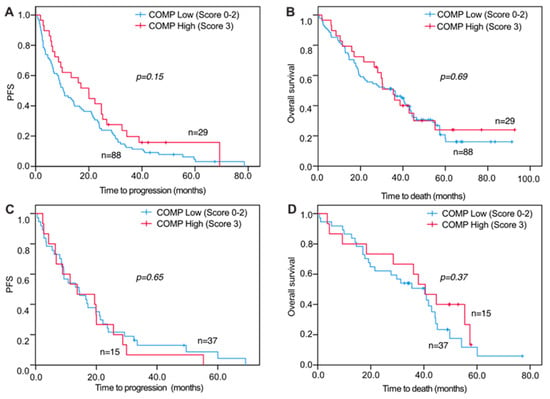
Figure 3.
Progression-free and overall survival of metastatic breast cancer patients in relation to COMP expression in primary tumors and distant metastases. Kaplan-Meier curves displaying PFS and OS in patients with high (IHC score 3) or low (IHC score 0, 1, or 2) COMP expression in primary tumors (A,B) and distant metastases (C,D).
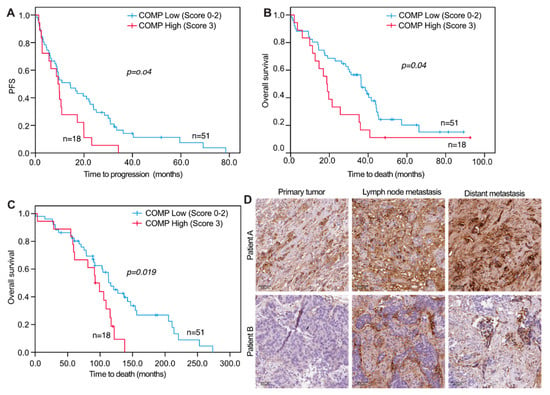
Figure 4.
Progression-free and overall survival of metastatic breast cancer patients in relation to COMP expression in lymph node metastases. Kaplan-Meier curves displaying PFS (A) and OS (B) from the date of metastatic disease in patients with high (IHC score 3) or low (IHC score 0, 1, or 2) COMP expression in LNM. Kaplan-Meier curves displaying OS (C) from the date of initial breast cancer diagnosis in patients with high (IHC score 3) or low (IHC score 0, 1, or 2) COMP expression in LNM. (D) Representative images of IHC COMP expression from the primary tumor, LNM, and distant metastasis. The tumor of patient A retained expression of COMP in metastases while the tumor of patient B gained COMP expression in metastases. Scale bars: 50 μm.
4. Discussion
We recently revealed that COMP levels in the serum of breast cancer patients could serve as an independent prognostic marker. Patients with metastatic disease had higher serum levels of COMP compared with those in the early stages [16]. In the current study, we investigated the associations between serum COMP levels and the clinicopathological characteristics of MBC patients and prognosis. Moreover, we assessed COMP expression by immunohistochemistry in matched pairs of primary tumors, LNM and DM, revealing that high COMP expression in LNM was associated with an inferior prognosis.
The serum level of COMP is a well-established marker of cartilage turnover [20]. Studies in rheumatoid arthritis patients revealed that patients with serum levels of COMP <12 U/L are at a lower risk of joint destruction [21,22]. In accordance with this, the median serum COMP levels in MBC patients were 11.67 U/L. This indicates that the cut-off that was used for cartilage turnover evaluation could also be applied to evaluate breast cancer patient levels of expression.
In previous studies, COMP expression by the tumor cells but not in stroma was associated with a worse prognosis of patients with early breast cancer [11,16]. COMP affects the migration, invasion, and metabolism of the breast cancer cells as well as the abundance of cancer stem cells [17]. Moreover, several studies reported similar results for prostate [12], colon [13], and hepatocellular [15] cancer patients. In the current study, high serum levels of COMP were mostly found in ER-positive and HER2-positive MBC patients. These subgroups of advanced breast cancer patients (ER+ or HER2+) are treated nowadays with targeted systemic therapy as well as chemotherapy and have a better prognosis compared to patients with triple-negative disease [23]. Thus, less aggressive strategies could be selected during therapy if a robust monitoring marker was identified. The present study is the third [11,16] showing a strong correlation between high serum levels of COMP and ER-positivity as well as an increased number of bone and liver metastases, raising concerns about managing those patients. The evaluation of serum COMP levels could potentially identify those MBC patients who have specific types of metastases and hypothetically lead to a more aggressive therapeutic strategy. Thus, longitudinal evaluation of serum COMP levels during therapy would be of interest to monitor the treatment response in patients with ER positive advanced disease to improve the tailoring of systemic therapy.
The levels of COMP in serum correlated with the deposition of COMP in the stroma of DM. A similar trend was observed between the levels of serum COMP and the expression of COMP by the cancer cells of DM. This demonstrates a correlation between the blood levels of COMP and the local expression of COMP in the distant metastasis. Furthermore, high S-COMP levels correlated with the presence of bone metastases, indicating a possible role of COMP in bone metastatic progression of breast cancer.
A significant association between serum COMP and the number and clusters of CTCs was found, supporting the hypothesis that serum COMP can be a marker of a more severe stage of metastatic disease. The presence of ≥5 CTCs carries a strong prognostic value in MBC and the presence of CTC-clusters indicates an even worse prognosis [19]. CTC-clusters have been shown to have a much higher metastatic potential compared to single CTCs [24] and the strong correlation of high S-COMP levels and CTC-clusters suggests COMP as being involved in metastatic progression. If CTCs and CTC-clusters express COMP or can secrete it into the circulation is currently not known but would be interesting to explore in future studies.
One advantage of the current study is that it includes matched tumor tissue that was collected from the same patient from both the primary tumor, synchronous LNM and DM. Analysis of this material revealed that most of the LNM cells retained the expression of COMP as identified in the primary tumor in both the cancer (73%) and stromal cells (70%). From the remaining paired samples, more LNM lost the expression of COMP (21% and 23%, respectively) than gained it (7% for both the cancer and stromal cells). Despite this loss, COMP expression in cancer and stromal cells in LNM was associated with progression-free and overall survival from the date of metastasis as well as from the primary breast cancer diagnosis. These findings suggest that COMP may have a role in the intravasation of the tumor cells from the primary tumor already at the time of initial breast cancer diagnosis. These data are in accordance with published studies showing that the expression of COMP by cancer cells induced the epithelial to mesenchymal transition [25]. In addition, COMP expression in primary tumors correlated with liver metastases confirming previous observations [16]. COMP is a crucial cartilage ECM component binding mainly to collagens [26,27]. The liver, lymph nodes, and other collagen-rich tissues could be providing COMP-expressing cancer cells a better locus for metastases. COMP is not only binding to collagen but also other main components of the ECM [3,28], making the hypothesis more complicated. Further studies are needed to thoroughly dissect the relations between ECM composition and COMP expression by the metastatic cancer cells.
High expression of COMP in the stroma of LNM correlated with visceral metastases and the presence of lung metastases. LNM in breast cancer patients are regional metastases that are commonly identified at the time of initial cancer diagnosis. In contrast, DM are found in later, more advanced stages of the disease. One may hypothesize that COMP expression in LNM contributes to the development of distant metastases either by affecting the intravasation of the cancer cells to the bloodstream or the lymphatic system [29] or by providing an advantage to the cancer cells to spread to collagen-rich metastatic locus.
It is clear that breast cancer cells can express COMP since we detected it in an intracellular location when using IHC. Further, COMP is deposited in the stroma of the tumors, but future studies must be designed to address the source of COMP in the stroma; this could be fibroblasts as these were previously shown to express COMP under certain conditions [2]. The initial dogma that the ECM proteins are only secreted by the stromal cells in the tumor microenvironment has been disputed. Recent studies addressing this question found that the source of ECM could be either the stroma cells or the cancer cells [30,31]. Interestingly, in one of these studies COMP has been predicted to be expressed by the cancer cells rather than the stromal cells [30].
One weakness of the current study lies in the limited number of patients that were included, and, therefore, further studies including larger cohorts will be needed to fine-tune conclusions on how to best use COMP as biomarker in the management of breast cancer. The main limitation of the study is the lack of a group of non-metastatic patients. Moreover, a cohort including all patients with metastatic breast cancer is heterogeneous in the sense that the underlying biology is different between the subtypes, and patients vary regarding the extent of the metastatic disease and the therapy that was received. Lastly, for some patients, tissue material was not collected from all of the metastatic sites.
Taken together, COMP emerges as a biomarker of advanced breast cancer progression, especially in ER-positive and HER2-positive patients.
5. Conclusions
High COMP expression in lymph node metastases in breast cancer has prognostic implications suggesting that COMP is involved in the progression to a more aggressive metastatic disease. The serum level of COMP appears to be a valuable marker of breast cancer progression as it could distinguish ER or HER2 positive patients with advanced disease. Patients with ER- and HERs-positive MBC and an increased serum level of COMP could potentially benefit from applying more aggressive therapeutic strategies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13235876/s1, Table S1: COMP expression in distant metastases.
Author Contributions
K.S.P.: performed the measurements of COMP in serum and contributed to manuscript writing. C.H.: evaluated the IHC stainings. L.R.: oversaw the study and contributed to manuscript writing. A.-M.L.: performed the statistical analyses, oversaw the study, and contributed to manuscript writing A.M.B.: evaluated the IHC stainings, oversaw the study and contributed to manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Cancerfonden (20 0686), Malmö Hospital Cancer Foundation, the Swedish Cancer and Allergy Fund, the Swedish Government Funds for Clinical research (ALF), the Erling Persson Foundation and the Berta Kamprad foundation.
Institutional Review Board Statement
Ethical approval was obtained from Lund University ethical committee (LU 2010/135) and the study was performed according to the Helsinki declaration and Good Clinical Practice (GCP).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Acknowledgments
The authors thank Kristina Ekström-Holka for expert help with immunohistochemical staining and Kristina Lövgren for invaluable work with tissue micro-array construction and sectioning of slides.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heinegård, D. Fell-Muir Lecture: Proteoglycans and more-from molecules to biology. Int. J. Exp. Pathol. 2009, 90, 575–586. [Google Scholar] [CrossRef]
- Agarwal, P.; Schulz, J.-N.; Blumbach, K.; Andreasson, K.; Heinegård, D.; Paulsson, M.; Mauch, C.; Eming, S.A.; Eckes, B.; Krieg, T. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013, 32, 325–331. [Google Scholar] [CrossRef]
- Acharya, C.; Yik, J.H.; Kishore, A.; Van Dinh, V.; Di Cesare, P.E.; Haudenschild, D. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. 2014, 37, 102–111. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Kong, W. ADAMTS-7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao [Acta Physiol. Sin.] 2010, 62, 285–294. [Google Scholar]
- Riessen, R.; Fenchel, M.; Chen, H.; Axel, D.I.; Karsch, K.R.; Lawler, J. Cartilage oligomeric matrix protein (Thrombospondin-5) is expressed by human vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2001, 21, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultman, K.; Edsfeldt, A.; Björkbacka, H.; Dunér, P.; Sundius, L.; Nitulescu, M.; Persson, A.; Boyle, J.J.; Nilsson, J.; Hultgårdh-Nilsson, A.; et al. Cartilage oligomeric matrix protein associates with a vulnerable plaque phenotype in human atherosclerotic plaques. Stroke 2019, 50, 3289–3292. [Google Scholar] [CrossRef]
- Liang, Y.; Fu, Y.; Qi, R.; Wang, M.; Yang, N.; He, L.; Yu, F.; Zhang, J.; Yun, C.-H.; Wang, X.; et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood 2015, 126, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Happonen, K.E.; Saxne, T.; Aspberg, A.; Mörgelin, M.; Heinegård, D.; Blom, A.M. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 3574–3583. [Google Scholar] [CrossRef] [Green Version]
- Happonen, K.E.; Saxne, T.; Geborek, P.; Andersson, M.; A Bengtsson, A.; Hesselstrand, R.; Heinegård, D.; Blom, A.M. Serum COMP-C3b complexes in rheumatic diseases and relation to anti-TNF-α treatment. Arthritis Res. Ther. 2012, 14, R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englund, E.; Bartoschek, M.; Reitsma, B.; Jacobsson, L.; Escudero-Esparza, A.; Orimo, A.; Leandersson, K.; Hagerling, C.; Aspberg, A.; Storm, P.; et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene 2016, 35, 5585–5596. [Google Scholar] [CrossRef]
- Englund, E.; Canesin, G.; Papadakos, K.S.; Vishnu, N.; Persson, E.; Reitsma, B.; Anand, A.; Jacobsson, L.; Helczynski, L.; Mulder, H.; et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget 2017, 8, 98298–98311. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-S.; Zhang, M.; Liu, X.-N.; Zhu, F.-X.; Zhu, F.-M.; Ouyang, S.-W.; Li, S.-B.; Song, C.-L.; Sun, H.-M.; et al. Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway. J. Cancer Res. Clin. Oncol. 2018, 144, 1049–1063. [Google Scholar] [CrossRef]
- Nfonsam, V.N.; Jecius, H.C.; Janda, J.; Omesiete, P.N.; Elquza, E.; Scott, A.J.; Nfonsam, L.E.; Jandova, J. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg. Endosc. 2019, 34, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Wang, Y.; Sun, L.; Liu, Z.; Wang, L.; Song, T.; Yao, Y.; Liu, Q.; Tu, K. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J. Exp. Clin. Cancer Res. 2018, 37, 231. [Google Scholar] [CrossRef]
- Papadakos, K.; Darlix, A.; Jacot, W.; Blom, A.M. High levels of cartilage oligomeric matrix protein in the serum of breast cancer patients can serve as an independent prognostic marker. Front. Oncol. 2019, 9, 1141. [Google Scholar] [CrossRef]
- Papadakos, K.; Bartoschek, M.; Rodriguez, C.; Gialeli, C.; Jin, S.-B.; Lendahl, U.; Pietras, K.; Blom, A.M. Cartilage oligomeric matrix protein initiates cancer stem cells through activation of Jagged1-Notch3 signaling. Matrix Biol. 2019, 81, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.-M.; Jansson, S.; Bendahl, P.-O.; Jörgensen, C.L.T.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Rydén, L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018, 20, 48. [Google Scholar] [CrossRef]
- Saxne, T.; Heinegård, D. Cartilage oligomeric matrix protein: A novel marker of cartilage turnover detectable in synovial fluid and blood. Rheumatology 1992, 31, 583–591. [Google Scholar] [CrossRef]
- Forslind, K.; Eberhardt, K.; Jonsson, A.; Saxne, T. Increased serum concentrations of cartilage oligomeric matrix protein. A prognostic marker in early rheumatoid arthritis. Rheumatology 1992, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.; André, F.; Barrios, C.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Hou, H.; Liu, T.; Su, S.; Xi, X.; Liao, Y.; Xie, R.; Jin, G.; Liu, X.; Zhu, L.; et al. Cartilage oligomeric matrix protein promotes epithelial-mesenchymal transition by interacting with Transgelin in Colorectal Cancer. Theranostics 2020, 10, 8790–8806. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Rosenberg, K.; Olsson, H.; Mörgelin, M.; Heinegård, D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 1998, 273, 20397–20403. [Google Scholar] [CrossRef] [Green Version]
- Dicesare, P.; Hauser, N.; Lehman, D.; Pasumarti, S.; Paulsson, M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994, 354, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, A.; Bulte, C.; Kalaichelvan, A.; Cheng, M.; Krishnamachary, B.; Bhujwalla, Z.M.; Jiang, L.; Glunde, K. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci. Rep. 2015, 5, 10002. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Clauser, K.; Öhlund, D.; Rickelt, S.; Huang, Y.; Gupta, M.; Mani, D.R.; Carr, S.A.; Tuveson, D.A.; Hynes, R.O. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19609–19618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, C.; Öhlund, D.; Rickelt, S.; Lidström, T.; Huang, Y.; Hao, L.; Zhao, R.T.; Franklin, O.; Bhatia, S.N.; Tuveson, D.A.; et al. Cancer cell–derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. 2020, 80, 1461–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).