Metastatic Lymph Node Ratio for Predicting Recurrence in Medullary Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
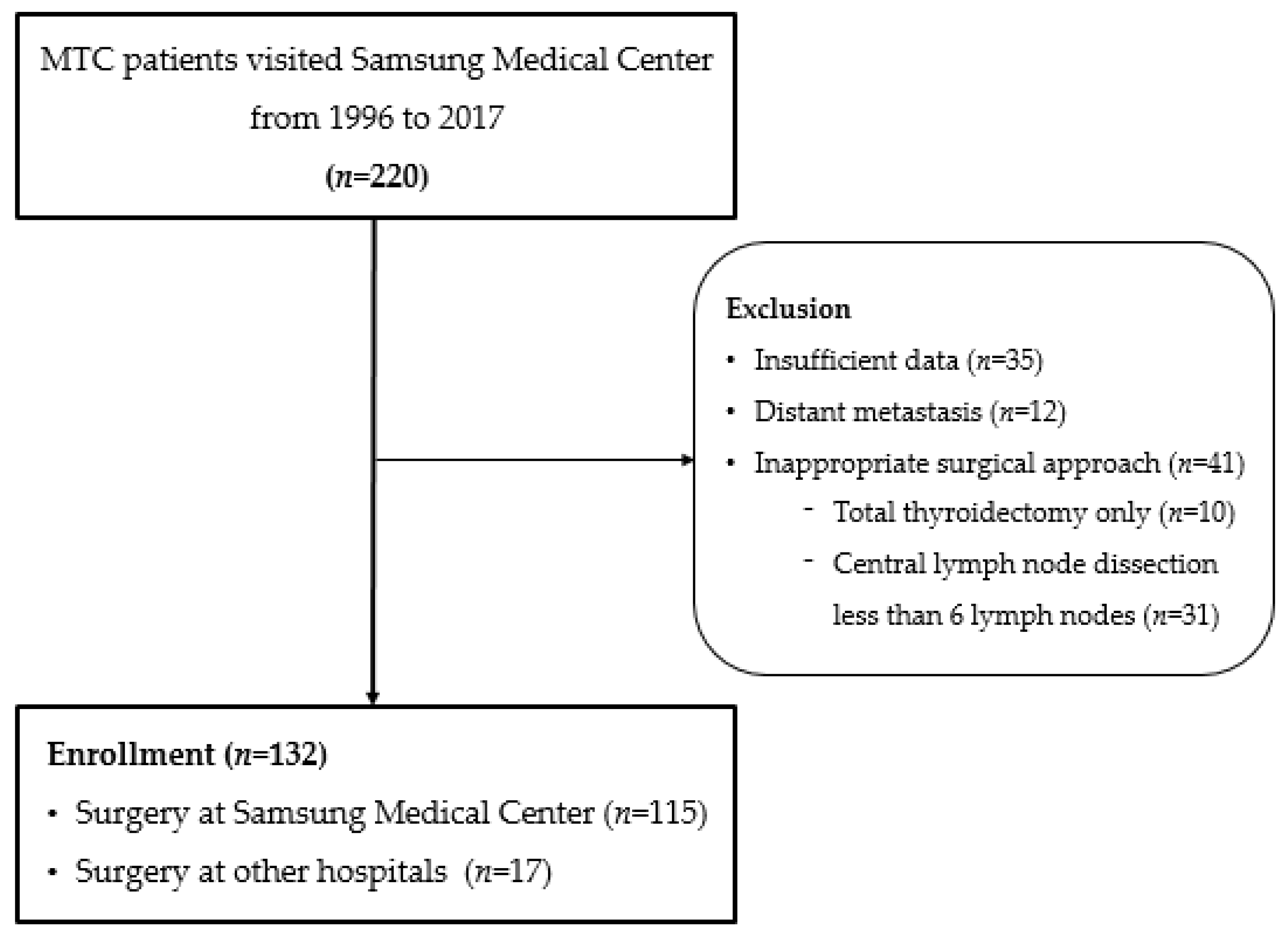
2. Materials and Methods
3. Results
3.1. Baseline Characteristics of the Study Population
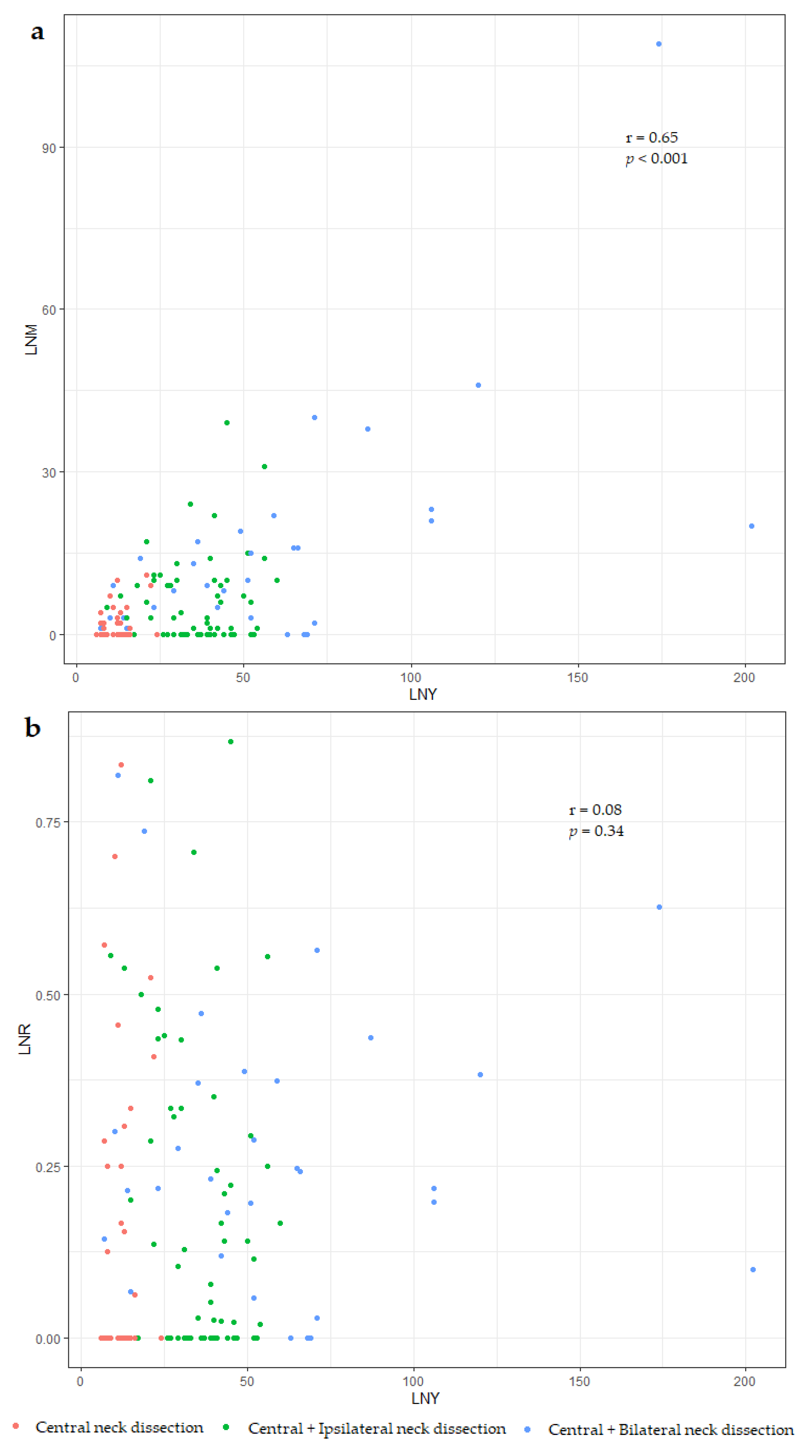
3.2. Differences between the Number and Ratio of Metastatic Lymph Nodes (LNM vs. LNR)
3.3. Prognostic Factors Influencing Disease-Free Survival
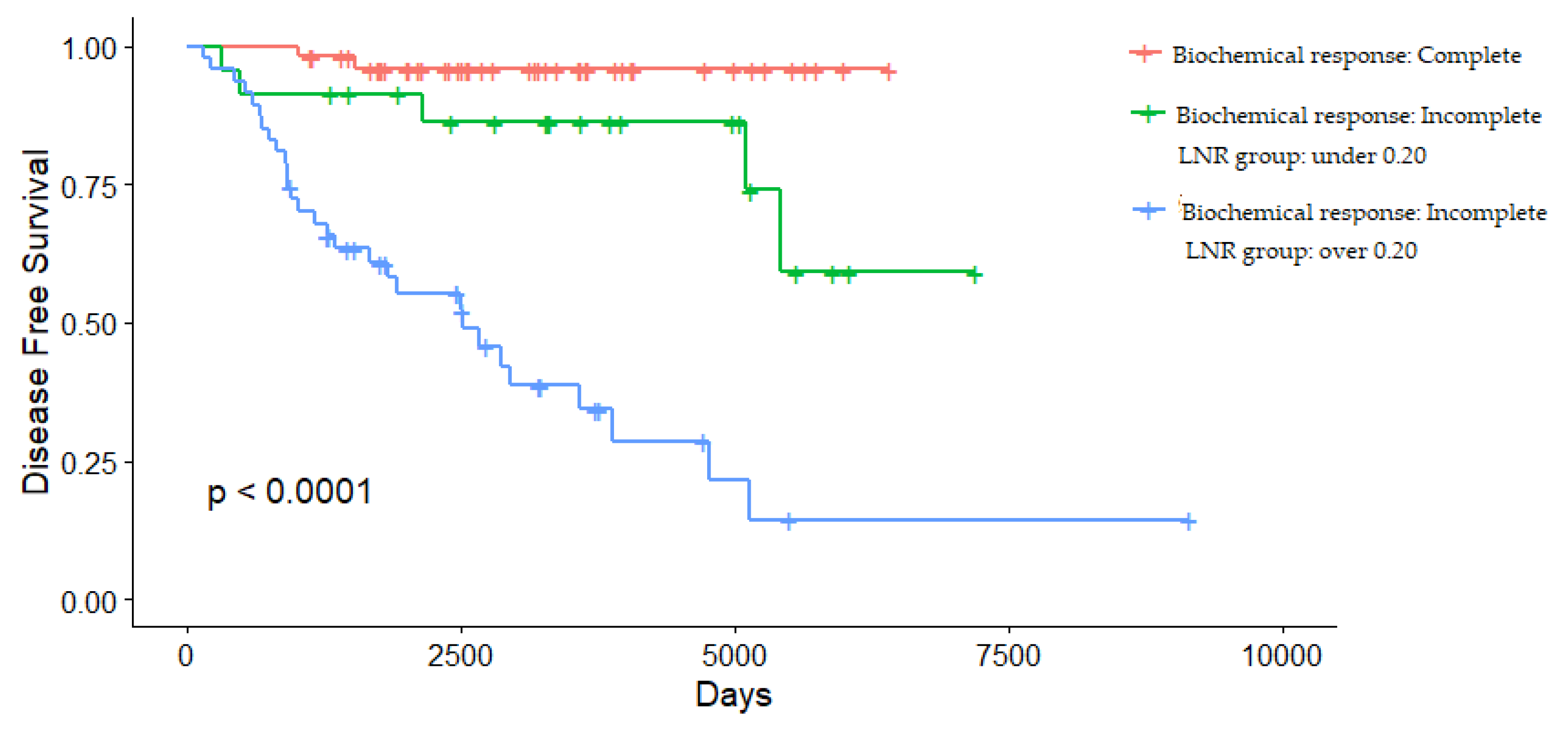
3.4. Determining the Cut-Off Value of LNR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fagin, J.A.; Wells, S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.A.; Asa, S.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.A.A.; Bakker, L.E.H.; Valk, G.D.; De Herder, W.W.; De Wilt, J.H.W.; Netea-Maier, R.T.; Schaper, N.; Fliers, E.; Lips, P.; Plukker, J.T.; et al. Radioactive iodine in the treatment of medullary thyroid carcinoma: A controlled multicenter study. Eur. J. Endocrinol. 2013, 168, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; Grunwell, J. Extent of disease and practice patterns for medullary thyroid cancer. J. Am. Coll. Surg. 2005, 200, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Prognostic impact of N staging in 715 medullary thyroid cancer patients: Proposal for a revised staging system. Ann. Surg. 2013, 257, 323–329. [Google Scholar] [CrossRef]
- Esfandiari, N.H.; Hughes, D.T.; Yin, H.; Banerjee, M.; Haymart, M.R. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, 448–454. [Google Scholar] [CrossRef]
- Meng, K.; Luo, H.; Chen, H.; Guo, H.; Xia, W. Prognostic value of numbers of metastatic lymph node in medullary thyroid carcinoma: A population-based study using the SEER 18 database. Medicine 2019, 98, e13884. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jiang, S.; Gao, L.; Xi, X.; Zhao, R.; Lai, X.; Zhang, B.; Jiang, Y. Construction and validation of a nomogram based on the log odds of positive lymph nodes to predict the prognosis of medullary thyroid carcinoma after surgery. Ann. Surg. Oncol. 2021, 28, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Leggett, M.D.; Chen, S.L.; Schneider, P.D.; Martinez, S.R. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Ann. Surg. Oncol. 2008, 15, 2493–2499. [Google Scholar] [CrossRef]
- Rozenblat, T.; Hirsch, D.; Robenshtok, E.; Grozinsky-Glasberg, S.; Gross, D.J.; Mazeh, H.; Benbassat, C.; Twito, O.; Levy, S.; Mizrachi, A.; et al. The prognostic value of lymph node ratio in Medullary thyroid carcinoma: A multi-center study. Eur. J. Surg. Oncol. 2020, 46, 2023–2028. [Google Scholar] [CrossRef]
- Qu, N.; Shi, R.-L.; Lu, Z.-W.; Liao, T.; Wen, D.; Sun, G.-H.; Li, D.-S.; Ji, Q.-H. Metastatic lymph node ratio can further stratify risk for mortality in medullary thyroid cancer patients: A population-based analysis. Oncotarget 2016, 7, 65937–65945. [Google Scholar] [CrossRef]
- Le Voyer, T.E.; Sigurdson, E.R.; Hanlon, A.L.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J. Clin. Oncol. 2003, 21, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Stokes, W.; Eguchi, M.; Hararah, M.; Sumner, W.; Amini, A.; Goddard, J.; Somerset, H.; Bradley, C.; McDermott, J.; et al. Association between lymph node ratio and recurrence and survival outcomes in patients with oral cavity cancer. JAMA Otolaryngol. Neck Surg. 2019, 145, 53. [Google Scholar] [CrossRef]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann. Thorac. Surg. 2012, 93, 1614–1620. [Google Scholar] [CrossRef] [Green Version]
- Ataseven, B.; Grimm, C.; Harter, P.; Prader, S.; Traut, A.; Heitz, F.; du Bois, A. Prognostic value of lymph node ratio in patients with advanced epithelial ovarian cancer. Gynecol. Oncol. 2014, 135, 435–440. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, H.; Shim, H.-J.; Bae, W.-K.; Hwang, E.-C.; Jeong, O.; Ryu, S.Y.; Park, Y.K.; Cho, S.-H.; Chung, I.-J. Lymph-node ratio is an important clinical determinant for selecting the appropriate adjuvant chemotherapy regimen for curative D2-resected gastric cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.G.; Kim, K.; Yim, S.H.; Ryu, H.; Lee, C.R.; Kang, S.W.; Jeong, J.J.; Nam, K.-H.; Chung, W.Y.; et al. Clinical value of lymph node ratio integration with the 8th edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci. Rep. 2019, 9, 13361. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-L.; Gong, Y.; Pei, Y.-C.; Ji, P.; Hu, X.; Shao, Z.-M. Modified lymph node ratio improves the prognostic predictive ability for breast cancer patients compared with other lymph node staging systems. Breast 2020, 49, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.J.; Thomas, S.; Dinan, M.A.; Roman, S.; Sosa, J.A.; Hyslop, T. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J. Clin. Oncol. 2016, 34, 3434–3439. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Califf, R.M.; Pryor, D.B.; Rosati, R.A. Regression modelling strategies for improved prognostic prediction. Stat. Med. 1984, 3, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Chae, J.E.; Moon, H.; Noh, J.; Park, Y.J.; Kim, S.G. Trends in the diagnosis and treatment of patients with medullary thyroid carcinoma in Korea. Endocrinol. Metab. 2020, 35, 811–819. [Google Scholar] [CrossRef]
- Kebebew, E.; Ituarte, P.H.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000, 88, 1139–1148. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, B.; Song, X.-N.; Liu, N.; Zhang, R.-P.; Wang, C.-L.; Liang, H. Prognostic value of the lymph node ratio in stage III gastric cancer patients undergoing radical resection. PLoS ONE 2014, 9, e96455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Xu, Y.; Li, D.M.; Wang, Z.N.; Zhu, G.L.; Huang, B.J.; Li, K.; Xu, H.M. Log odds of positive lymph nodes: A novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010, 116, 2571–2580. [Google Scholar] [CrossRef]
- Ramacciato, G.; Nigri, G.; Petrucciani, N.; Pinna, A.D.; Ravaioli, M.; Jovine, E.; Minni, F.; Grazi, G.L.; Chirletti, P.; Tisone, G.; et al. Prognostic role of nodal ratio, LODDS, pN in patients with pancreatic cancer with venous involvement. BMC Surg. 2017, 17, 109. [Google Scholar] [CrossRef] [Green Version]
- Baqar, A.R.; Wilkins, S.; Wang, W.; Oliva, K.; McMurrick, P. Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer 2020, 20, 762. [Google Scholar] [CrossRef]
- Jin, L.X.; Moley, J.F. Surgery for lymph node metastases of medullary thyroid carcinoma: A review. Cancer 2016, 122, 358–366. [Google Scholar] [CrossRef]
- Papaleontiou, M.; Hughes, D.T.; Guo, C.; Banerjee, M.; Haymart, M.R. Population-based assessment of complications following surgery for thyroid cancer. J. Clin. Endocrinol. Metab. 2017, 102, 2543–2551. [Google Scholar] [CrossRef]
- Schneider, D.F.; Chen, H.; Sippel, R.S. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2012, 20, 1906–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grozinsky-Glasberg, S.; Benbassat, C.A.; Tsvetov, G.; Feinmesser, R.; Peretz, H.; Shimon, I.; Lapidot, M. Medullary thyroid cancer: A retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid 2007, 17, 549–556. [Google Scholar] [CrossRef]
- Weber, T.; Schilling, T.; Frank-Raue, K.; Colombo-Benkmann, M.; Hinz, U.; Ziegler, R.; Klar, E. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery 2001, 130, 1044–1049. [Google Scholar] [CrossRef]
- Yang, J.H.; Lindsey, S.C.; Camacho, C.P.; Valente, F.O.F.; Germano-Neto, F.; Machado, A.L.; Mamone, M.C.O.C.; Brodskyn, F.; Biscolla, R.P.M.; Tuttle, R.M.; et al. Integration of a postoperative calcitonin measurement into an anatomical staging system improves initial risk stratification in medullary thyroid cancer. Clin. Endocrinol. 2015, 83, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Gimm, O.; Ukkat, J.; Hinze, R.; Schneyer, U.; Dralle, H. Improved prediction of calcitonin normalization in medullary thyroid carcinoma patients by quantitative lymph node analysis. Cancer 2000, 88, 1909–1915. [Google Scholar] [CrossRef]
- Yip, D.T.; Hassan, M.; Pazaitou-Panayiotou, K.; Ruan, D.T.; Gawande, A.; Gaz, R.D.; Moore, F.D.; Hodin, R.A.; Stephen, A.E.; Sadow, P.; et al. Preoperative basal calcitonin and tumor stage correlate with postoperative calcitonin normalization in patients undergoing initial surgical management of medullary thyroid carcinoma. Surgery 2011, 150, 1168–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, S.C.; Ganly, I.; Palmer, F.; Tuttle, R.M. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid 2015, 25, 242–249. [Google Scholar] [CrossRef]
| Number | 132 |
|---|---|
| Age, years (mean ± SD) Sex, women (%) | 49.7 ± 13.9 86 (65) |
| Hereditary type, number (%) | 18 (13.7) |
| Size, cm (mean ± SD) Gross extra thyroidal extension, number (%) | 1.98 ± 1.39 25 (19) |
| Bilateral tumors, number (%) | 22 (17) |
| T stage, number (%) | |
| 1a | 35 (26.5) |
| 1b | 35 (26.5) |
| 2 | 27 (20.5) |
| 3a | 7 (5.3) |
| 3b | 17 (12.9) |
| 4a | 11 (8.3) |
| N stage, number (%) | |
| 0 | 50 (37.9) |
| 1a | 23 (17.4) |
| 1b | 59 (44.7) |
| AJCC 8th stage, number (%) | |
| I | 37 (28.0) |
| II | 13 (9.8) |
| III | 23 (17.4) |
| IVA | 59 (44.7) |
| The surgical extent of neck dissection, number (%) Central neck dissection Central + Ipsilateral neck dissection Central + Bilateral neck dissection | 37 (28.0) 69 (52.3) 26 (19.7) |
| Lymph node status Metastasized lymph nodes, number (median (IQR)) Resected lymph nodes, number (median (IQR)) Lymph node ratio, number (median (IQR)) | 2.5 (0–10) 29 (13–44) 0.122 (0–0.311) |
| Follow-up duration, years (median (IQR)) | 8.7 (5.5–12.9) |
| N Stage | LNM | LNR | |
|---|---|---|---|
| C-index (SD) | 0.704 (0.034) | 0.760 (0.039) | 0.799 (0.036) |
| p | (reference) | 0.023 | 0.002 |
| N Stage | Median (IQR) | HR (95% CI) | p | |
|---|---|---|---|---|
| LNM | N1a | 2 (1–4) | 1.28 (0.99–1.64) | 0.056 |
| (number) | N1b | 10 (6.5–16.5) | 1.02 (1.01–1.04) | 0.001 |
| LNR | N1a | 15.4 (4.6–38.2) | 1.04 (1.01–1.08) | 0.010 |
| (%) | N1b | 28.8 (20.0–43.6) | 1.05 (1.03–1.07) | <0.001 |
| Univariate Analysis | Multivariate Analysis (Model 1) | Multivariate Analysis (Model 2) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.98 (0.95–1.00) | 0.057 | ||||
| Sex, men | 1.39 (0.73–2.64) | 0.314 | ||||
| Size | 1.19 (0.99–1.42) | 0.062 | ||||
| Gross ETE, positive | 5.17 (2.74–9.77) | <0.001 | 2.89 (1.47–5.69) | 0.002 | 1.56 (0.75–3.21) | 0.232 |
| N stage 0 1a 1b | 1.00 (reference) 12.96 (2.78–60.4) 15.42 (3.66–65.2) | 0.001 <0.001 | 8.94 (1.79–44.8) 11.0 (2.52–48.1) | 0.008 0.001 | 4.76 (0.91–24.7) 1.16 (0.20–6.75) | 0.064 0.868 |
| LNR (%) | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.02–1.06) | <0.001 | ||
| Postoperative calcitonin ≥ 5 pg/mL | 15.76 (3.80–65.40) | <0.001 | 5.43 (1.13–26.1) | 0.035 | ||
| Postoperative CEA ≥5 ng/mL | 7.79 (4.11–14.77) | <0.001 | 2.83 (1.39–5.78) | 0.004 | ||
| Model 1 | Model 1 +Postoperative Calcitonin | Model 1 +Lymph Node Ratio | Model 2 | |
|---|---|---|---|---|
| C-index (SD) | 0.762 (0.036) | 0.797 (0.034) | 0.810 (0.036) | 0.850 (0.034) |
| p | (reference) | 0.100 | 0.089 | 0.002 |
| LNR Cut-Off | HR | 95% CI | p | C-Index (Standard Error) |
|---|---|---|---|---|
| 0.05 | 16.89 | 4.05–70.38 | <0.001 | 0.696 (0.028) |
| 0.10 | 22.45 | 5.38–93.64 | <0.001 | 0.728 (0.027) |
| 0.15 | 11.57 | 4.50–29.74 | <0.001 | 0.741 (0.031) |
| 0.20 | 10.29 | 4.50–23.53 | <0.001 | 0.750 (0.034) |
| 0.25 | 9.07 | 4.36–18.87 | <0.001 | 0.733 (0.039) |
| 0.30 | 5.71 | 2.99–10.91 | <0.001 | 0.689 (0.040) |
| 0.35 | 5.82 | 3.09–10.96 | <0.001 | 0.687 (0.040) |
| 0.40 | 6.12 | 3.22–11.62 | <0.001 | 0.668 (0.040) |
| 0.45 | 5.08 | 2.64–9.77 | <0.001 | 0.629 (0.038) |
| 0.50 | 5.44 | 2.81–10.54 | <0.001 | 0.623 (0.037) |
| Variables | LNR < 0.20 (n = 79) | LNR ≥ 0.20 (n = 53) | p |
|---|---|---|---|
| Age (mean ± SD) | 50.67 (12.70) | 48.30 (15.60) | 0.340 |
| Sex, men (%) | 23 (29.1) | 23 (43.4) | 0.133 |
| Size, cm (mean ± SD) | 1.72 (1.08) | 2.38 (1.68) | <0.001 |
| Gross extrathyroidal extension, number (%) | 4 (5.1) | 21 (39.6) | 0.007 |
| Bilaterality, number (%) | 13 (16.5) | 9 (17.0) | 1.000 |
| N stage | <0.001 | ||
| 0 | 49 (62.0) | 0 (0.00 | |
| 1a | 14 (17.7) | 9 (17.0) | |
| 1b | 16 (20.3) | 44 (83.0) | |
| Postoperative calcitonin ≥ 5pg/mL | 23 (29.1) | 51 (96.2) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, J.; Park, H.; Choi, M.S.; Jang, H.W.; Kim, T.H.; Kim, S.W.; Chung, J.H. Metastatic Lymph Node Ratio for Predicting Recurrence in Medullary Thyroid Cancer. Cancers 2021, 13, 5842. https://doi.org/10.3390/cancers13225842
Kim J, Park J, Park H, Choi MS, Jang HW, Kim TH, Kim SW, Chung JH. Metastatic Lymph Node Ratio for Predicting Recurrence in Medullary Thyroid Cancer. Cancers. 2021; 13(22):5842. https://doi.org/10.3390/cancers13225842
Chicago/Turabian StyleKim, Jinyoung, Jun Park, Hyunju Park, Min Sun Choi, Hye Won Jang, Tae Hyuk Kim, Sun Wook Kim, and Jae Hoon Chung. 2021. "Metastatic Lymph Node Ratio for Predicting Recurrence in Medullary Thyroid Cancer" Cancers 13, no. 22: 5842. https://doi.org/10.3390/cancers13225842
APA StyleKim, J., Park, J., Park, H., Choi, M. S., Jang, H. W., Kim, T. H., Kim, S. W., & Chung, J. H. (2021). Metastatic Lymph Node Ratio for Predicting Recurrence in Medullary Thyroid Cancer. Cancers, 13(22), 5842. https://doi.org/10.3390/cancers13225842






