Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Radiopharmaceutical Development
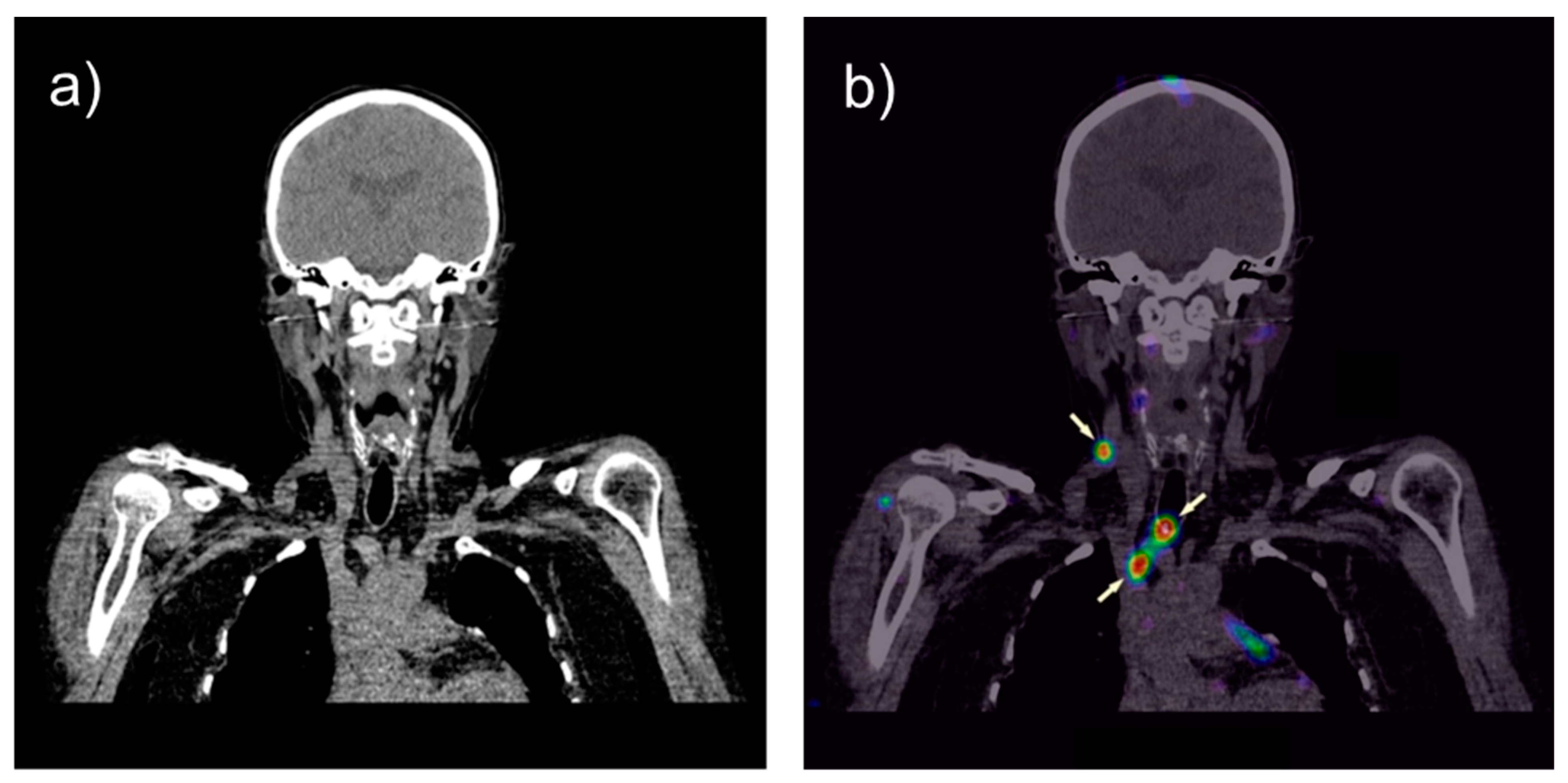
3. Tumor Imaging with Radiolabeled Gastrin Analogs
4. Tumor Therapy with Radiolabeled Gastrin Analogs
5. Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reubi, J.C.; Schaer, J.C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar] [PubMed]
- Reubi, J.C. Targeting CCK receptors in human cancers. Curr. Top. Med. Chem. 2007, 7, 1239–1242. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J. Cancer 1996, 67, 644–647. [Google Scholar] [CrossRef]
- Behr, T.M.; Jenner, N.; Radetzky, S.; Behe, M.; Gratz, S.; Yücekent, S.; Raue, F.; Becker, W. Targeting of cholecystokinin-B/gastrin receptors in vivo: Preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur. J. Nucl. Med. 1998, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Jenner, N.; Behe, M.; Angerstein, C.; Gratz, S.; Raue, F.; Becker, W. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J. Nucl. Med. 1999, 40, 1029–1044. [Google Scholar] [PubMed]
- de Jong, M.; Bakker, W.H.; Bernard, B.F.; Valkema, R.; Kwekkeboom, D.J.; Reubi, J.C.; Srinivasan, A.; Schmidt, M.; Krenning, E.P. Preclinical and initial clinical evaluation of 111In-labeled nonsulfated CCK8 analog: A peptide for CCK-B receptor-targeted scintigraphy and radionuclide therapy. J. Nucl. Med. 1999, 40, 2081–2087. [Google Scholar]
- Behe, M.; Behr, T.M. Cholecystokinin-B (CCK-B)/gastrin receptor targeting peptides for staging andtherapy of medullary thyroidcancer and other CCK-B receptor expressing malignancies. Biopolymers 2002, 66, 399–418. [Google Scholar] [CrossRef]
- Breeman, W.A.; Froberg, A.C.; de Blois, E.; van Gameren, A.; Melis, M.; de Jong, M.; Maina, T.; Nock, B.A.; Erion, J.L.; Macke, H.R.; et al. Optimised labeling, preclinical and initial clinical aspects of CCK-2 receptor-targeting with 3 radiolabeled peptides. Nucl. Med. Biol. 2008, 35, 839–849. [Google Scholar] [CrossRef]
- Fröberg, A.C.; de Jong, M.; Nock, B.A.; Breeman, W.A.; Erion, J.L.; Maina, T.; Verdijsseldonck, M.; de Herder, W.W.; van der Lugt, A.; Kooij, P.P.; et al. Comparison of three radiolabelled peptide analogues for CCK-2 receptor scintigraphy in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1265–1272. [Google Scholar] [CrossRef]
- Aloj, L.; Aurilio, M.; Rinaldi, V.; D’Ambrosio, L.; Tesauro, D.; Peitl, P.K.; Maina, T.; Mansi, R.; von Guggenberg, E.; Joosten, L.; et al. Comparison of the binding and internalization properties of 12 DOTA-coupled and 111In-labelled CCK2/gastrin receptor binding peptides: A collaborative project under COST Action BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1417–1425. [Google Scholar] [CrossRef]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Macke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 111In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef]
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.K.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.; Reubi, J.C.; et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435. [Google Scholar] [CrossRef]
- Kolenc-Peitl, P.; Mansi, R.; Tamma, M.; Gmeiner-Stopar, T.; Sollner-Dolenc, M.; Waser, B.; Baum, R.P.; Reubi, J.C.; Maecke, H.R. Highly improved metabolic stability and pharmacokinetics of indium-111-DOTA-gastrin conjugates for targeting of the gastrin receptor. J. Med. Chem. 2011, 54, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.J.; McKenzie, A.J.; Sosabowski, J.K.; Morris, T.M.; Ellison, D.; Watson, S.A. Selection of radiolabeled gastrin analogs for peptide receptor–targeted radionuclide therapy. J. Nucl. Med. 2007, 48, 615–622. [Google Scholar] [CrossRef]
- Sosabowski, J.K.; Matzow, T.; Foster, J.M.; Finucane, C.; Ellison, D.; Watson, S.A.; Mather, S.J. Targeting of CCK-2 receptor–expressing tumors using a radiolabeled divalent gastrin peptide. J. Nucl. Med. 2009, 50, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Kroess, A.; Woerndle, R.; Rangger, C.; Klingler, M.; Haas, H.; Kremser, L.; Lindner, H.H.; von Guggenberg, E.; Decristoforo, C. Multimerization results in formation of re-bindable metabolites: A proof of concept study with FSC-based minigastrin imaging probes targeting CCK2R expression. PLoS ONE 2018, 13, e0201224. [Google Scholar] [CrossRef] [PubMed]
- von Guggenberg, E.; Sallegger, W.; Helbok, A.; Ocak, M.; King, R.; Mather, S.J.; Decristoforo, C. Cyclic minigastrin analogues for gastrin receptor scintigraphy with technetium-99m: Preclinical evaluation. J. Med. Chem. 2009, 52, 4786–4793. [Google Scholar] [CrossRef]
- Erba, P.A.; Maecke, H.; Mikolajczak, R.; Decristoforo, C.; Zaletel, K.; Maina-Nock, T.; Peitl, P.K.; Garnuszek, P.; Fröberg, A.; Goebel, G.; et al. A novel CCK2/gastrin receptor-localizing radiolabeled peptide probe for personalized diagnosis and therapy of patients with progressive or metastatic medullary thyroid carcinoma: A multicenter phase I GRAN-T-MTC study. Pol. Arch. Intern. Med. 2018, 128, 791–795. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 receptor agonist 177Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: Results of the lumed phase 0a study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Valkema, R.; Krenning, E.P.; de Jong, M.; Maina, T. Impact of clinically tested NEP/ACE inhibitors on tumor uptake of [111In-DOTA]MG11—first estimates for clinical translation. EJNMMI Res. 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Improving the in vivo profile of minigastrin radiotracers: A comparative study involving the neutral endopeptidase inhibitor phosphoramidon. Cancer Biother. Radiopharm. 2016, 31, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.M.; Geistlich, S.; Behe, M.; Rottenburger, C.; et al. Targeting of the cholecystokinin-2 receptor with the minigastrin analog 177Lu-DOTA-PP-F11N: Does the use of protease inhibitors further improve in vivo distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; von Guggenberg, E. DOTA-MGS5, a new cholecystokinin-2 receptor-targeting peptide analog with an optimized targeting profile for theranostic use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Krenning, E.P.; Maina, T.; de Jong, M. Radiolabeled gastrin/CCK analogs in tumor diagnosis: Towards higher stability and improved tumor targeting. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 287–302. [Google Scholar] [PubMed]
- Grob, N.M.; Haussinger, D.; Deupi, X.; Schibli, R.; Behe, M.; Mindt, T.L. Triazolo-peptidomimetics: Novel radiolabeled minigastrin analogs for improved tumor targeting. J. Med. Chem. 2020, 63, 4484–4495. [Google Scholar] [CrossRef]
- Grob, N.M.; Schibli, R.; Behe, M.; Mindt, T.L. Improved tumor-targeting with peptidomimetic analogs of minigastrin 177Lu-PP-F11N. Cancers 2021, 13, 2629. [Google Scholar] [CrossRef]
- Grob, N.M.; Schmid, S.; Schibli, R.; Behe, M.; Mindt, T.L. Design of radiolabeled analogs of minigastrin by multiple amide-to-triazole substitutions. J. Med. Chem. 2020, 63, 4496–4505. [Google Scholar] [CrossRef]
- Klingler, M.; Hörmann, A.A.; Rangger, C.; Desrues, L.; Castel, H.; Gandolfo, P.; von Guggenberg, E. Stabilization strategies for linear minigastrin analogues: Further improvements via the inclusion of proline into the peptide sequence. J. Med. Chem. 2020, 63, 14668–14679. [Google Scholar] [CrossRef]
- Novak, D.; Tomasic, T.; Kroselj, M.; Javornik, U.; Plavec, J.; Anderluh, M.; Kolenc Peitl, P. Radiolabelled CCK2 R antagonists containing peg linkers: Design, synthesis and evaluation. Chem. Med. Chem. 2021, 16, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Ritler, A.; Shoshan, M.S.; Deupi, X.; Wilhelm, P.; Schibli, R.; Wennemers, H.; Behe, M. Elucidating the structure-activity relationship of the pentaglutamic acid sequence of minigastrin with cholecystokinin receptor subtype 2. Bioconjug. Chem. 2019, 30, 657–666. [Google Scholar] [CrossRef]
- Abiraj, K.; Mansi, R.; Tamma, M.L.; Fani, M.; Forrer, F.; Nicolas, G.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Bombesin antagonist–based radioligands for translational nuclear imaging of gastrin-releasing peptide receptor–positive tumors. J. Nucl. Med. 2011, 52, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid–conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef]
- Ueno, M.; Li, C.P.; Ikeda, M.; Ishii, H.; Mizuno, N.; Yamaguchi, T.; Ioka, T.; Oh, D.Y.; Ichikawa, W.; Okusaka, T.; et al. A randomized phase II study of gemcitabine plus Z-360, a CCK2 receptor-selective antagonist, in patients with metastatic pancreatic cancer as compared with gemcitabine plus placebo. Cancer Chemother. Pharmacol. 2017, 80, 307–315. [Google Scholar] [CrossRef]
- Good, S.; Walter, M.A.; Waser, B.; Wang, X.; Muller-Brand, J.; Behe, M.P.; Reubi, J.C.; Maecke, H.R. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Decristoforo, C.; Rangger, C.; Summer, D.; Foster, J.; Sosabowski, J.K.; von Guggenberg, E. Site-specific stabilization of minigastrin analogs against enzymatic degradation for enhanced cholecystokinin-2 receptor targeting. Theranostics 2018, 8, 2896–2908. [Google Scholar] [CrossRef]
- Klingler, M.; Hörmann, A.A.; Guggenberg, E.V. Cholecystokinin-2 receptor targeting with radiolabeled peptides: Current status and future directions. Curr. Med. Chem. 2020, 27, 7112–7132. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Decristoforo, C.; Uprimny, C.; Virgolini, I.J.; von Guggenberg, E. Radiopharmaceutical formulation and preclinical testing of 68Ga-labeled DOTA-MGS5 for the regulatory approval of a first exploratory clinical trial. Pharmaceuticals 2021, 14, 575. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rezaeianpour, M.; Hormann, N.; Gust, R.; Shahhosseini, S.; Guggenberg, E.V. Initial in vitro and in vivo evaluation of a novel CCK2R targeting peptide analog labeled with lutetium-177. Molecules 2020, 25, 4585. [Google Scholar] [CrossRef] [PubMed]
- Corlett, A.; Sani, M.A.; Van Zuylekom, J.; Ang, C.S.; von Guggenberg, E.; Cullinane, C.; Blyth, B.; Hicks, R.J.; Roselt, P.D.; Thompson, P.E.; et al. A new turn in peptide-based imaging agents: Foldamers afford improved theranostics targeting cholecystokinin-2 receptor-positive cancer. J. Med. Chem. 2021, 64, 4841–4856. [Google Scholar] [CrossRef] [PubMed]
- Grzmil, M.; Qin, Y.; Schleuniger, C.; Frank, S.; Imobersteg, S.; Blanc, A.; Spillmann, M.; Berger, P.; Schibli, R.; Behe, M. Pharmacological inhibition of mTORC1 increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue [177Lu]Lu-PP-F11N. Theranostics 2020, 10, 10861–10873. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Imobersteg, S.; Blanc, A.; Frank, S.; Schibli, R.; Behe, M.P.; Grzmil, M. Evaluation of actinium-225 labeled minigastrin analogue [225Ac]Ac-DOTA-PP-F11N for targeted alpha particle therapy. Pharmaceutics 2020, 12, 1088. [Google Scholar] [CrossRef]
- Roosenburg, S.; Laverman, P.; Joosten, L.; Cooper, M.S.; Kolenc-Peitl, P.K.; Foster, J.M.; Hudson, C.; Leyton, J.; Burnet, J.; Oyen, W.J.; et al. PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with 64Cu, 68Ga, and 111In. Mol. Pharm. 2014, 11, 3930–3937. [Google Scholar] [CrossRef]
- Müller, C.; Fischer, E.; Behe, M.; Köster, U.; Dorrer, H.; Reber, J.; Haller, S.; Cohrs, S.; Blanc, A.; Grünberg, J.; et al. Future prospects for SPECT imaging using the radiolanthanide terbium-155—production and preclinical evaluation in tumor-bearing mice. Nucl. Med. Biol. 2014, 41, e58–e65. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med. 2017, 58, 61S–66S. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef]
- Wayua, C.; Low, P.S. Evaluation of a nonpeptidic ligand for imaging of cholecystokinin 2 receptor-expressing cancers. J. Nucl. Med. 2015, 56, 113–119. [Google Scholar] [CrossRef]
- Kaloudi, A.; Kanellopoulos, P.; Radolf, T.; Chepurny, O.G.; Rouchota, M.; Loudos, G.; Andreae, F.; Holz, G.G.; Nock, B.A.; Maina, T. [99mTc]Tc-DGA1, a promising cck2r-antagonist-based tracer for tumor diagnosis with single-photon emission computed tomography. Mol. Pharm. 2020, 17, 3116–3128. [Google Scholar] [CrossRef]
- Verona, M.; Rubagotti, S.; Croci, S.; Sarpaki, S.; Borgna, F.; Tosato, M.; Vettorato, E.; Marzaro, G.; Mastrotto, F.; Asti, M. Preliminary study of a 1,5-benzodiazepine-derivative labelled with indium-111 for CCK-2 receptor targeting. Molecules 2021, 26, 918. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Anderluh, M.; Kolenc Peitl, P. CCK2R antagonists: From SAR to clinical trials. Drug Discov. Today 2020, 25, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Costante, G.; Meringolo, D. Calcitonin as a biomarker of C cell disease: Recent achievements and current challenges. Endocrine 2020, 67, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Vardarli, I.; Weber, M.; Weidemann, F.; Führer, D.; Herrmann, K.; Görges, R. Diagnostic accuracy of routine calcitonin measurement for the detection of medullary thyroid carcinoma in the management of patients with nodular thyroid disease: A meta-analysis. Endocr. Connect. 2021, 10, 358–370. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Network, N.C.C. Thyroid Carcinoman (Version 2). Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 22 September 2021).
- Treglia, G.; Aktolun, C.; Chiti, A.; Frangos, S.; Giovanella, L.; Hoffmann, M.; Iakovou, I.; Mihailovic, J.; Krause, B.J.; Langsteger, W.; et al. The 2015 Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: The “evidence-based” refusal to endorse them by EANM due to the “not evidence-based” marginalization of the role of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1486–1490. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; Iakovou, I.; Mihailovic, J.; Verburg, F.A.; Luster, M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 61–77. [Google Scholar] [CrossRef]
- Lee, S.W.; Shim, S.R.; Jeong, S.Y.; Kim, S.J. Comparison of 5 different pet radiopharmaceuticals for the detection of recurrent medullary thyroid carcinoma: A network meta-analysis. Clin. Nucl. Med. 2020, 45, 341–348. [Google Scholar] [CrossRef]
- Kolenc Peitl, P.; Tamma, M.; Kroselj, M.; Braun, F.; Waser, B.; Reubi, J.C.; Sollner Dolenc, M.; Maecke, H.R.; Mansi, R. Stereochemistry of amino acid spacers determines the pharmacokinetics of 111In−DOTA−minigastrin analogues for targeting the CCK2/gastrin receptorr. Bioconjug. Chem. 2015, 26, 1113–1119. [Google Scholar] [CrossRef]
- Schwarzenböck, S.M.; Garibotto, V. Highlights of the 32th annual congress of the EANM, Barcelona 2019: The nucleolympic games of nuclear medicine-a global competition for excellence. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1808–1819. [Google Scholar] [CrossRef]
- Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Decristoforo, C.; Kolenc-Peitl, P.; Erba, P.; Zaletel, K.; Maecke, H.; Maina, T.; Konijnenberg, M.; Garnuszek, P. Phase I clinical trial using a novel CCK 2 receptor-localizing radiolabelled peptide probe for personalized diagnosis and therapy of patients with progressive or metastatic medullary thyroid carcinoma-final results. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S339. [Google Scholar]
- Kunikowska, J.; Ziemnicka, K.; Pawlak, D.; Ruchala, M.; Kolasa, A.; Janicka-Jedynska, M.; Wozniak, A.; Mikolajczak, R.; Krolicki, L. Medullary thyroid carcinoma—PET/CT imaging with 68Ga-labelled gastrin and somatostatin analogues. Endokrynol. Pol. 2016, 67, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Uprimny, C.; von Guggenberg, E.; Svirydenka, A.; Mikolajczak, R.; Hubalewska-Dydejczyk, A.; Virgolini, I.J. Comparison of PET/CT imaging with [18F]FDOPA and cholecystokinin-2 receptor targeting [68Ga]Ga-DOTA-MGS5 in a patient with advanced medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Tacito, A.; Ramone, T.; Ciampi, R.; Bottici, V.; Cappagli, V.; Viola, D.; Matrone, A.; Lorusso, L.; Valerio, L.; et al. Twenty-five years experience on ret genetic screening on hereditary mtc: An update on the prevalence of germline ret mutations. Genes 2019, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Raue, F.; Frank-Raue, K. Update on multiple endocrine neoplasia type 2: Focus on medullary thyroid carcinoma. J. Endocr. Soc. 2018, 2, 933–943. [Google Scholar] [CrossRef]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef]
- Kushchayev, S.V.; Kushchayeva, Y.S.; Tella, S.H.; Glushko, T.; Pacak, K.; Teytelboym, O.M. Medullary thyroid carcinoma: An update on imaging. J. Thyroid Res. 2019, 2019, 1893047. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, A.K.; Feldt-Rasmussen, U.; Schytte, S.; Londero, S.C.; Pedersen, H.B.; et al. Survival and long-term biochemical cure in medullary thyroid carcinoma in denmark 1997–2014: A nationwide study. Thyroid 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.; McDougall, L.; Fürstner, M.; Hentschel, M.; Kaul, F.; Christ, E.; Cachovan, M.; Vija, H.; Schibli, R. The CCK-2 receptor agonist Lu-177-PP-F11N for PRRT of medullary thyroid cancer–Recent results of the phase 1 “LUMED” Study. Nuklearmedizin 2021, 60, L15. [Google Scholar]
- Parghane, R.V.; Naik, C.; Talole, S.; Desmukh, A.; Chaukar, D.; Banerjee, S.; Basu, S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck 2020, 42, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Salaun, P.Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: Biomarker response and survival improvement. J. Nucl. Med. 2012, 53, 1185–1192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uccelli, L.; Martini, P.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic radiometals: Worldwide scientific literature trend analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, R.; van der Meulen, N.P.; Lapi, S.E. Radiometals for imaging and theranostics, current production, and future perspectives. J. Label. Comp. Radiopharm. 2019, 62, 615–634. [Google Scholar] [CrossRef]
- Sandrine Huclier-Markai, C.A. Rabha Kerdjoudj, Marie Mougin-Degraef, Nicolas Chouin, and Ferid Haddad. Promising scandium radionuclides for nuclear medicine: A review on the production and chemistry up to in vivo proofs of concept. Cancer Biother. Radiopharm. 2018, 33, 316–329. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Kulkarni, H.R.; Bernhardt, P.; Rydén, T.; Schuchardt, C.; Gracheva, N.; Grundler, P.V.; Köster, U.; Müller, D.; et al. First-in-humans application of 161Tb: A feasibility study using 161Tb-DOTATOC. J. Nucl. Med. 2021, 62, 1391–1397. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R. Radiopharmaceutical development of radiolabelled peptides. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, S11–S30. [Google Scholar] [CrossRef]



| CCK2R Targeting Peptide | Radiopeptide Injected | Tumor Xenograft/ Mouse Model | Tumor Uptake 1 | Tumor Uptake 1 + PA Co-Injection 2 | Ref. |
|---|---|---|---|---|---|
| DOTA-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 | [111In]In-DOTA-MG11 | AR42J/SCID A431-CCK2R/SCID | <2% IA/g 2.5% IA/g | 15% IA/g 16% IA/g | [21] |
| DOTA-DGlu-(Glu)5-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 | [111In]In-DOTA-MG0 | A431-CCK2R/SCID | 12% IA/g | 17% IA/g | [22] |
| DOTA-DGlu-(DGlu)5-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 | [111In]In-CP04 (PP-F11, MG48) | A431-CCK2R/SCID | 9% IA/g | 16% IA/g | [22] |
| DOTA-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 | [177Lu]Lu-DOTA-MG11 | A431-CCK2R/athymic nude | 1.5% IA/g | 7% IA/g | [23] |
| DOTA-DGlu-(DGlu)5-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-PP-F11N | A431-CCK2R/athymic nude | 7% IA/g | 9% IA/g | [23] |
| DOTA-DGlu-Ala-Tyr-Gly-Trp-(N-Me)Nle-Asp-1-Nal-NH2 | [111In]In/[177Lu]Lu/[68Ga]Ga-DOTA-MGS5 | A431-CCK2R/athymic nude | 23–24% IA/g | Not determined | [24] |
| CCK2R Targeting Peptide | Radiopeptide Injected | Cell Internalization 4 h Incubation 1 | In Vitro Stability 2 | Tumor Uptake 4 h p.i. 1 | Ref. |
|---|---|---|---|---|---|
| DOTA-DGlu-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-TZMG 6 | >50% | <10% | 3.9% IA/g | [27,29] |
| DOTA-DGlu-Ψ[Tz]-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-TZMG 86 | >50% | >30% | 6.0% IA/g | [29] |
| DOTA-DGlu-Ψ[Tz]-Ala-Ψ[Tz]-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-TZMG 876 | ~50% | ~10% | 6.0% IA/g | [29] |
| DOTA-(DGlu)6-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-NMG 2 | >70% | >95% | 7.2% IA/g | [28] |
| DOTA-DGlu-Ψ[Tz]-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | [177Lu]Lu-NMG 3 | >70% | >95% | 6.9% IA/g | [28] |
| CCK2R Targeting Peptide | Radiopeptide Injected | Cell Internalization 2 h Incubation 1 | In Vitro Stability 2 | Tumor Uptake 1 | Ref. |
|---|---|---|---|---|---|
| DOTA-DGlu-Ala-Tyr-Gly-Trp-Nle-Asp-1-Nal-NH2 | [111In]In-MGS1 | ~25% | ~60% | 1.2% IA/g | [38] |
| DOTA-DGlu-Ala-Tyr-Gly-Trp-(N-Me)Nle-Asp-(N-Me)Phe -NH2 | [111In]In-MGS4 | ~25% | >95% | 10% IA/g | [38] |
| DOTA-DGlu-Ala-Tyr-Gly-Trp-(N-Me)Nle-Asp-1-Nal-NH2 | [111In]In/[177Lu]Lu/[68Ga]Ga-DOTA-MGS5 | ~50% | >95% | 23–24% IA/g | [24] |
| Study Acronym | Sponsor | Intervention/Treatment | Identifier | Recruitment Status |
|---|---|---|---|---|
| GRAN-T-MTC | Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy | [111In]In-CP04, Gelofusine | ClinicalTrials.gov (accessed on 22 September 2021): NCT03246659 | Completed |
| Ga-68-CCK2R PET/CT in NET | Medical University of Innsbruck, Austria | [68Ga]Ga-DOTA-MGS5 | EudraCT: 2020-003932-26 | Recruiting |
| Lumed phase 0/A and phase I | University Hospital Basel, Switzerland | [177Lu]Lu-PP-F11N, Gelofusine (phase 0/A) | ClinicalTrials.gov (accessed on 22 September 2021): NCT02088645 | Completed (phase 0/A) Recruiting (phase I) |
| Lumed phase 0/B | University Hospital Basel, Switzerland | [177Lu]Lu-PP-F11N, Sacuitril | ClinicalTrials.gov (accessed on 22 September 2021): NCT03647657 | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Guggenberg, E.; Kolenc, P.; Rottenburger, C.; Mikołajczak, R.; Hubalewska-Dydejczyk, A. Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals. Cancers 2021, 13, 5776. https://doi.org/10.3390/cancers13225776
von Guggenberg E, Kolenc P, Rottenburger C, Mikołajczak R, Hubalewska-Dydejczyk A. Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals. Cancers. 2021; 13(22):5776. https://doi.org/10.3390/cancers13225776
Chicago/Turabian Stylevon Guggenberg, Elisabeth, Petra Kolenc, Christof Rottenburger, Renata Mikołajczak, and Alicja Hubalewska-Dydejczyk. 2021. "Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals" Cancers 13, no. 22: 5776. https://doi.org/10.3390/cancers13225776
APA Stylevon Guggenberg, E., Kolenc, P., Rottenburger, C., Mikołajczak, R., & Hubalewska-Dydejczyk, A. (2021). Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals. Cancers, 13(22), 5776. https://doi.org/10.3390/cancers13225776