Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting
Abstract
:Simple Summary
Abstract
1. Introduction
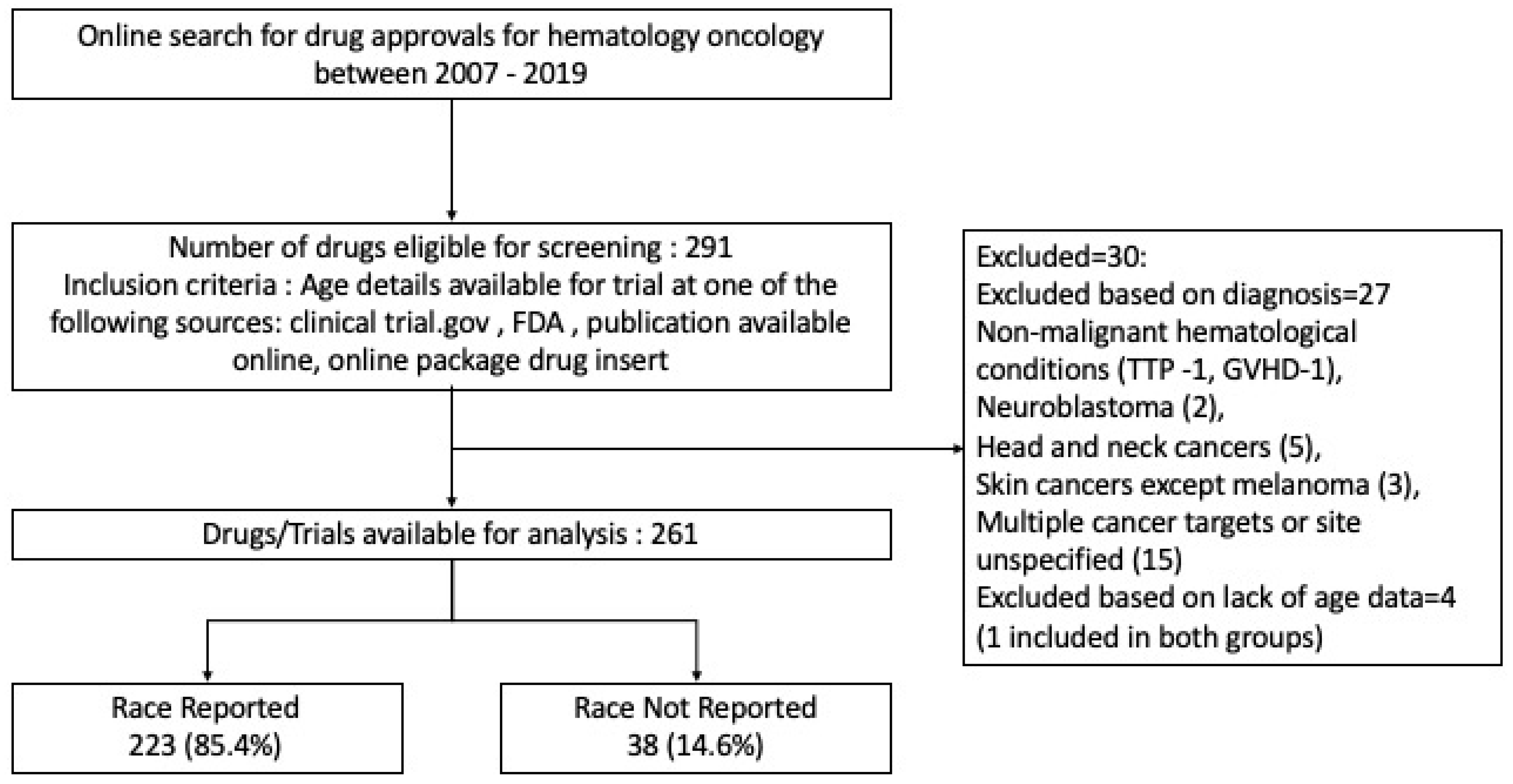
2. Methods
2.1. Data Collection and Outcomes
- Inclusion criteria: age details available for the trial at any of the above sources.
- Exclusion criteria: age details not available, non-malignant hematological conditions, drug approvals for cancer of multiple sites, or pediatric cancers.
2.2. Statistical Analysis
3. Results
3.1. Race Disparity
3.2. Age Disparity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loree, J.M.; Anand, S.; Dasari, A.; Unger, J.M.; Gothwal, A.; Ellis, L.M.; Varadhachary, G.; Kopetz, S.; Overman, M.J.; Raghav, K. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. 2019, 5, e191870. [Google Scholar] [CrossRef]
- Gopishetty, S.; Kota, V.; Guddati, A.K. Age and Race Distribution in Patients in Phase III Oncology Clinical Trials. Am. J. Transl. Res. 2020, 12, 5977–5983. [Google Scholar]
- Lee, E.; Wen, P. Gender and Sex Disparity in Cancer Trials. ESMO Open 2020, 5, e000773. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Mishra, M.; Pentz, R.; Owonikoko, T.K. Enrollment of Racial Minorities in Clinical Trials: Old Problem Assumes New Urgency in the Age of Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 3–10. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in Cancer Clinical Trials: Race-, Sex-, and Age-Based Disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Dotto, G.-P. Racial Differences in Cancer Susceptibility and Survival: More Than the Color of the Skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duma, N.; Azam, T.; Riaz, I.B.; Gonzalez-Velez, M.; Ailawadhi, S.; Go, R.S. Representation of Minorities and Elderly Patients in Multiple Myeloma Clinical Trials. Oncologist 2018, 23, 1076–1078. [Google Scholar] [CrossRef] [Green Version]
- SEER Cancer Statistics Review, 1975–2010—Previous Version—SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/archive/csr/1975_2010/index.html (accessed on 10 April 2021).
- Cancer Statistics—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 10 April 2021).
- Key Statistics for Acute Myeloid Leukemia (AML). Available online: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html (accessed on 10 April 2021).
- Available online: Topic_med_age.Pdf (accessed on 1 August 2020).
- Epperla, N.; Vaughn, J.L.; Othus, M.; Hallack, A.; Costa, L.J. Recent Survival Trends in Diffuse Large B-Cell Lymphoma––Have We Made Any Progress beyond Rituximab? Cancer Med. 2020, 9, 5519–5525. [Google Scholar] [CrossRef]
- Multiple Myeloma—Risk Factors. Available online: https://www.cancer.net/cancer-types/multiple-myeloma/risk-factors (accessed on 10 April 2021).
- Karkera, A.C.; Parsons, B.M.; Borgert, A.; Go, R.S. NK/T Cell Lymphoma in the U.S: A Population-Based Study Using the National Cancer Database from 1998–2012. J. Clin. Oncol. 2016, 34, e19038. [Google Scholar] [CrossRef]
- Marginal Zone Lymphoma—Lymphoma Research Foundation. Available online: https://lymphoma.org/aboutlymphoma/nhl/mzl/ (accessed on 10 April 2021).
- Melanoma Skin Cancer Statistics. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Lung Cancer Statistics|How Common Is Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Stomach (Gastric) Cancer Key Statistics. Available online: https://www.cancer.org/cancer/stomach-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Goodman, M.T.; Shvetsov, Y.B. Incidence of Ovarian, Peritoneal, and Fallopian Tube Carcinomas in the United States, 1995–2004. Cancer Epidemiol. Biomark. Prev. 2009, 18, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Key Statistics About Kidney Cancer. Available online: https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Zeng, Y.; Ruan, W.; Liu, J.; Liang, W.; He, J.; Cui, F.; Pan, H.; He, J. Esophageal Cancer in Patients under 50: A SEER Analysis. J. Thorac. Dis. 2018, 10, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, P.E.; O’Neil, D.S.; Neugut, A.I.; Chabot, J.; Fojo, T. Epidemiologic Trends in Neuroendocrine Tumors: An Examination of Incidence Rates and Survival of Specific Patient Subgroups over the Past 20 Years. Semin. Oncol. 2018, 45, 249–258. [Google Scholar] [CrossRef]
- Yang, J.D.; Alterkruse, S.; Nguyen, M.H.; Gores, G.J.; Roberts, L.R. Impact of Country of Birth on the Age of Diagnosis of Hepatocellular Carcinoma in the United States. Cancer 2017, 123, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key Statistics for Endometrial Cancer. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Key Statistics for Prostate Cancer|Prostate Cancer Facts. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (accessed on 10 April 2021).
- Unger, J.M.; Hershman, D.L.; Osarogiagbon, R.U.; Gothwal, A.; Anand, S.; Dasari, A.; Overman, M.; Loree, J.M.; Raghav, K. Representativeness of Black Patients in Cancer Clinical Trials Sponsored by the National Cancer Institute Compared with Pharmaceutical Companies. JNCI Cancer Spectr. 2020, 4, pkaa034. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Lara, P.N.; Dang, J.H.T.; Paterniti, D.A.; Kelly, K. Twenty Years Post-NIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): Laying the Groundwork for Improving Minority Clinical Trial Accrual: Renewing the Case for Enhancing Minority Participation in Cancer Clinical Trials. Cancer 2014, 120 (Suppl. 7), 1091–1096. [Google Scholar] [CrossRef]
- Parekh, T.; Desai, A. Demographic and Socioeconomic Disparities Among Cancer Survivors in Clinical Trials Participation, USA, 2016–2018. J. Cancer Educ. 2020. [Google Scholar] [CrossRef]
- Gerber, D.E.; Lakoduk, A.M.; Priddy, L.L.; Yan, J.; Xie, X.-J. Temporal Trends and Predictors for Cancer Clinical Trial Availability for Medically Underserved Populations. Oncologist 2015, 20, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research—Updated 2 August 2000. Available online: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-048.html (accessed on 9 April 2021).
- Carthon, B.; Sibold, H.C.; Blee, S.; Pentz, R.D. Prostate Cancer: Community Education and Disparities in Diagnosis and Treatment. Oncologist 2021, 26, 537–548. [Google Scholar] [CrossRef]
- Robinson, B.N.; Newman, A.F.; Tefera, E.; Herbolsheimer, P.; Nunes, R.; Gallagher, C.; Randolph-Jackson, P.; Omogbehin, A.; Dilawari, A.; Pohlmann, P.R.; et al. Video Intervention Increases Participation of Black Breast Cancer Patients in Therapeutic Trials. NPJ Breast Cancer 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Hamel, L.M.; Penner, L.A.; Albrecht, T.L.; Heath, E.; Gwede, C.K.; Eggly, S. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients with Cancer. Cancer Control 2016, 23, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Tharakan, S.; Zhong, X.; Galsky, M.D. The Impact of the Globalization of Cancer Clinical Trials on the Enrollment of Black Patients. Cancer 2021, 127, 2294–2301. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Number of Trials (%) or Median (IQR Interquartile Range) |
|---|---|
| Age (years) | 61 (55–64) |
| Sample size | 405 (185–707) |
| Year of approval | |
| 2007–2010 | 34 (13.0%) |
| 2011–2014 | 61 (23.4%) |
| 2015–2019 | 166 (63.6%) |
| Type of Approval | |
| New Indication | 208 (79.7%) |
| Change of Label | 53 (20.3%) |
| Phase of Trial | |
| Phase 1/2 | 91 (34.8%) |
| Phase 3 | 168 (64.4%) |
| Phase 4 | 2 (0.8%) |
| Randomized | |
| Yes | 200 (76.6%) |
| No | 61 (23.4%) |
| Disease Type | |
| Hematological | |
| Acute | |
| Acute Myeloid Leukemia | 8 (3.1%) |
| Acute Lymphoblastic Leukemia | 4 (1.5%) |
| Chronic | |
| Chronic Lymphocytic Leukemia | 16 (6.1%) |
| Chronic Myeloid Leukemia | 11 (4.2%) |
| Myelofibrosis | 2 (0.8%) |
| Polycythemia Vera | 1 (0.4%) |
| Other | |
| Diffuse Large B-Cell Lymphoma | 8 (3.1%) |
| Follicular Lymphoma | 6 (2.3%) |
| T-Cell Lymphoma | 7 (2.7%) |
| Marginal Zone Lymphoma | 5 (1.9%) |
| Hodgkin Lymphoma | 5 (1.9%) |
| Multiple Myeloma | 17 (6.5%) |
| Hairy Cell Leukemia | 1 (0.4%) |
| Solid | |
| Lung | 37 (14.2%) |
| Breast | 26 (10.0%) |
| Melanoma | 17 (6.5%) |
| Kidney | 15 (5.8%) |
| Prostate | 11 (4.2%) |
| Colorectal | 9 (3.5%) |
| Primary Peritoneal | 9 (3.5%) |
| Hepatocellular carcinoma | 6 (2.3%) |
| Bladder | 6 (2.3%) |
| Soft Tissue Sarcoma | 6 (2.3%) |
| Gastric | 5 (1.9%) |
| Pancreatic | 5 (1.9%) |
| Thyroid | 5 (1.9%) |
| Ovarian | 4 (1.5%) |
| Central Nervous System | 3 (1.2%) |
| Esophageal | 2 (0.8%) |
| Neuroendocrine Tumor | 2 (0.8%) |
| Endometrial | 1 (0.4%) |
| Cervical cancer | 1 (0.4%) |
| Disease Type | Average Age in Years (Population) | Sample Size (Combined for All Trials) | Weighted Mean Difference of Ages a; Mean (IQR) | p-Value | Reference |
|---|---|---|---|---|---|
| Hematological | |||||
| Acute | |||||
| Acute Myeloid Leukemia | 68 | 2361 | −10.8 (−21.0 to −1.0) | 0.156 | [10] |
| Acute Lymphoblastic Leukemia | 14 | 954 | 24.3 (23.0 to 33.0) | 0.039 | [11] |
| Chronic | |||||
| Chronic Myeloid Leukemia | 64 | 4512 | −11.2 (−11.0 to −5.0) | 0.006 | [11] |
| Chronic Lymphocytic Leukemia | 71 | 5152 | −2.9 (−1.0 to −6.0) | 0.039 | [11] |
| Other | |||||
| Hodgkin Lymphoma | 38 | 2286 | −2.0 (−2.0 to −1.7) | 0.935 | [11] |
| Follicular Lymphoma | 60 | 3770 | 1.1 (−1.0 to 3.0) | 0.019 | [8] |
| Diffuse Large B-Cell Lymphoma | 70 | 1473 | −13.2 (−14.0 to −4.0) | 0.002 | [12] |
| Hairy Cell Leukemia | 67 | 80 | −7.0 (no IQR) | [11] | |
| Multiple Myeloma | 70 | 8683 | −6.7 (−8.6 to −4.0) | 0.0005 | [13] |
| T-Cell Lymphoma | 53 | 1421 | 7.2 (5.0 to 11.0) | 0.0001 | [14] |
| Marginal Zone Lymphoma | 60 | 543 | 7.4 (7.0 to 8.0) | 0.007 | [15] |
| Myelofibrosis | 64 | 420 | −0.6 (−4.0 to 1.0) | 0.609 | [11] |
| Solid | |||||
| Pancreatic | 71 | 1454 | −10.8 (−13.0 to −8.0) | <0.005 | [8] |
| Central Nervous System | 57 | 169 | −10.1 (no IQR) | 0.293 | [11] |
| Melanoma | 65 | 10,650 | −9.5 (−14.0 to −4.0) | <0.005 | [16] |
| Lung | 70 | 19,804 | −8.7 (−9.0 to −6.0) | <0.005 | [17] |
| Ovarian | 63 | 687 | −6.4 (−5.0 to −3.0) | 0.130 | [11] |
| Breast | 62 | 24,739 | −8.0 (−11.0 to −6.5) | <0.005 | [8] |
| Colorectal | 69 | 6145 | −7.5 (−8.0 to −6.0) | <0.005 | [11] |
| Gastric | 68 | 2380 | −6.9 (−8.0 to −6.0) | <0.005 | [18] |
| Primary Peritoneal Carcinoma | 67 | 4182 | −7.5 (−11.0 to −6.0) | <0.005 | [19] |
| Bladder | 73 | 1440 | −5.8 (−7.0 to −5.0) | <0.005 | [20] |
| Cervical | 49 | 98 | −4.0 (no IQR) | n/a | [11] |
| Kidney | 64 | 9099 | −4.2 (−5.0 to −2.0) | 0.009 | [21] |
| Soft Tissue Sarcoma | 58 | 2892 | −1.1 (−3.0 to 0.5) | 0.301 | [11] |
| Esophageal | 65 | 749 | 0 (IQR) | n/a | [22] |
| Neuroendocrine Tumor | 63 | 433 | 0.5 (0.0 to 1.0) | 0.423 | [23] |
| Hepatocellular Carcinoma | 62 | 3232 | 1.2 (0.0 to 2.0) | 0.053 | [24] |
| Endometrial | 60 | 54 | 4.0 (IQR) | n/a | [25] |
| Prostate | 66 | 12,375 | 4.6 (3.0 to 8.0) | <0.005 | [26] |
| Thyroid | 50 | 1496 | 9.0 (5.0 to 13.0) | 0.014 | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakrishnan, T.; Aulakh, S.; Baksh, M.; Nguyen, K.; Ailawadhi, M.; Samreen, A.; Parrondo, R.; Sher, T.; Roy, V.; Manochakian, R.; et al. Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting. Cancers 2021, 13, 5770. https://doi.org/10.3390/cancers13225770
Jayakrishnan T, Aulakh S, Baksh M, Nguyen K, Ailawadhi M, Samreen A, Parrondo R, Sher T, Roy V, Manochakian R, et al. Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting. Cancers. 2021; 13(22):5770. https://doi.org/10.3390/cancers13225770
Chicago/Turabian StyleJayakrishnan, Thejus, Sonikpreet Aulakh, Mizba Baksh, Kianna Nguyen, Meghna Ailawadhi, Ayesha Samreen, Ricardo Parrondo, Taimur Sher, Vivek Roy, Rami Manochakian, and et al. 2021. "Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting" Cancers 13, no. 22: 5770. https://doi.org/10.3390/cancers13225770
APA StyleJayakrishnan, T., Aulakh, S., Baksh, M., Nguyen, K., Ailawadhi, M., Samreen, A., Parrondo, R., Sher, T., Roy, V., Manochakian, R., Paulus, A., Chanan-Khan, A., & Ailawadhi, S. (2021). Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting. Cancers, 13(22), 5770. https://doi.org/10.3390/cancers13225770









