Expression of the Chemokine Receptor CCR7 in the Normal Adrenal Gland and Adrenal Tumors and Its Correlation with Clinical Outcome in Adrenocortical Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Gene Expression Analysis
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
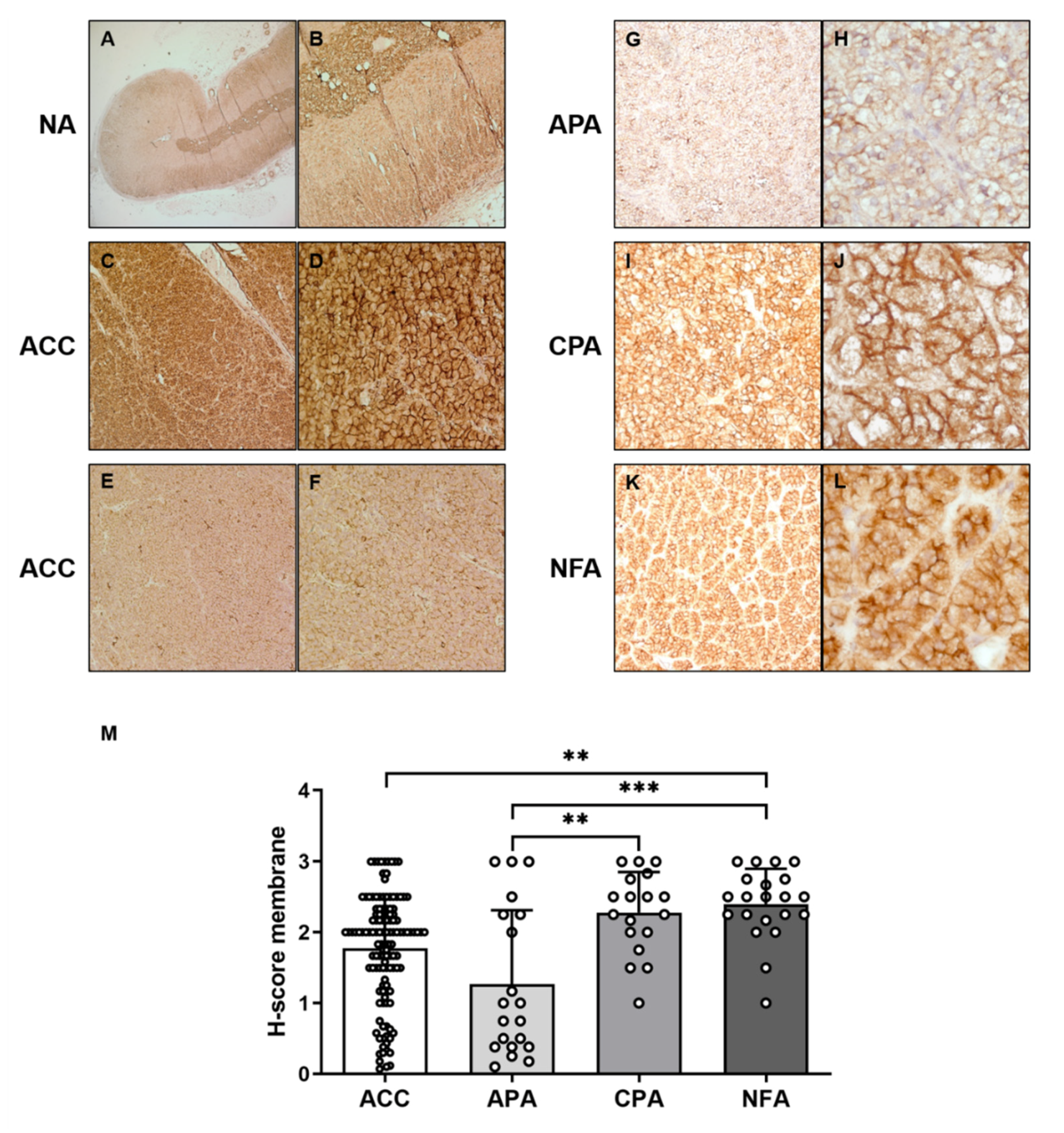
3.1. Protein Expression of CCR7 in the Normal Adrenal, Adrenocortical Adenoma, and Adrenocortical Carcinoma
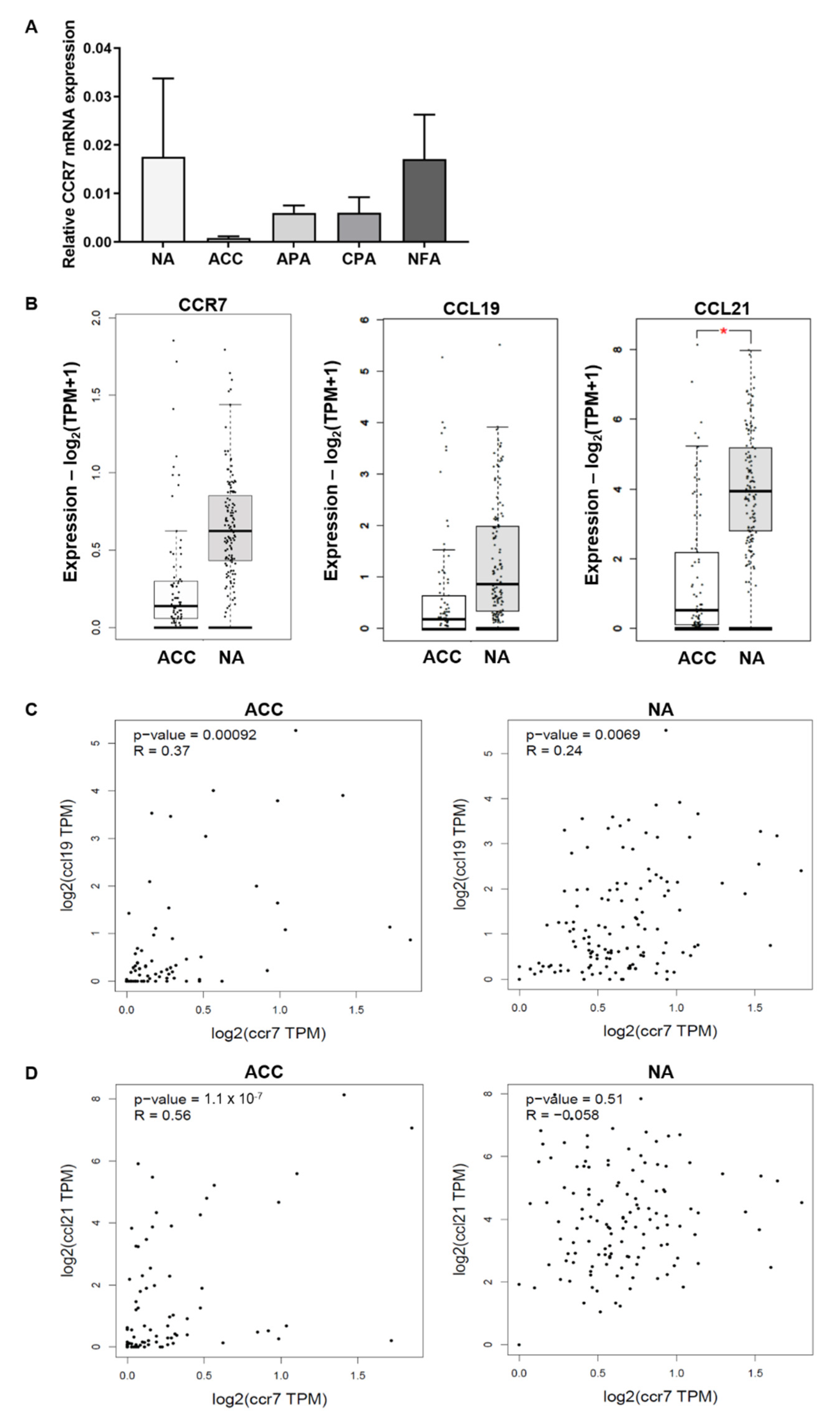
3.2. mRNA Expression of CCR7 in the Normal Adrenal, Adrenocortical Tumors, and Adrenocortical Carcinoma
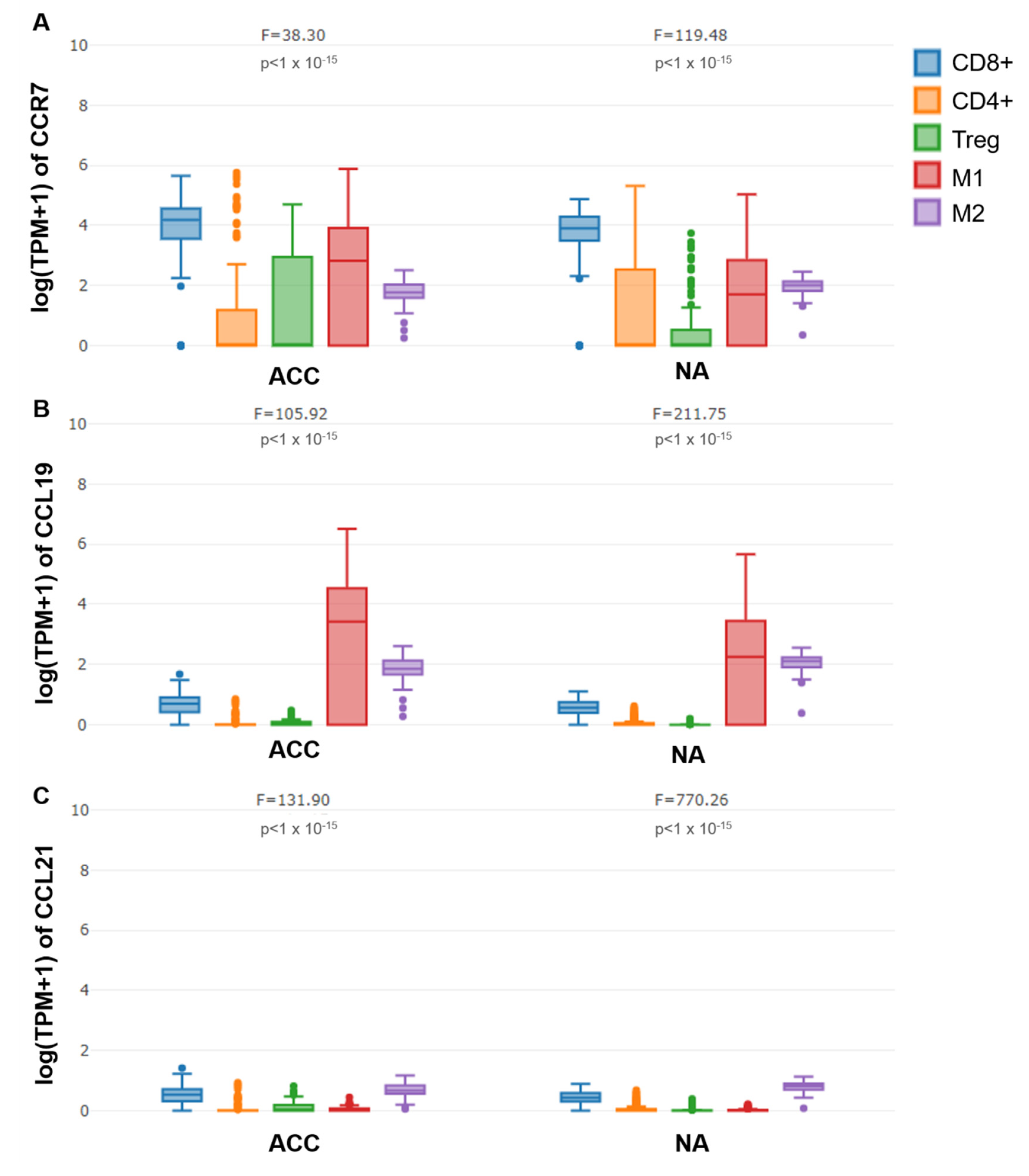
3.3. mRNA Expression of CCR7, CCL19 and CCL21 in Immune Cells of ACC and Normal Adrenal Glands
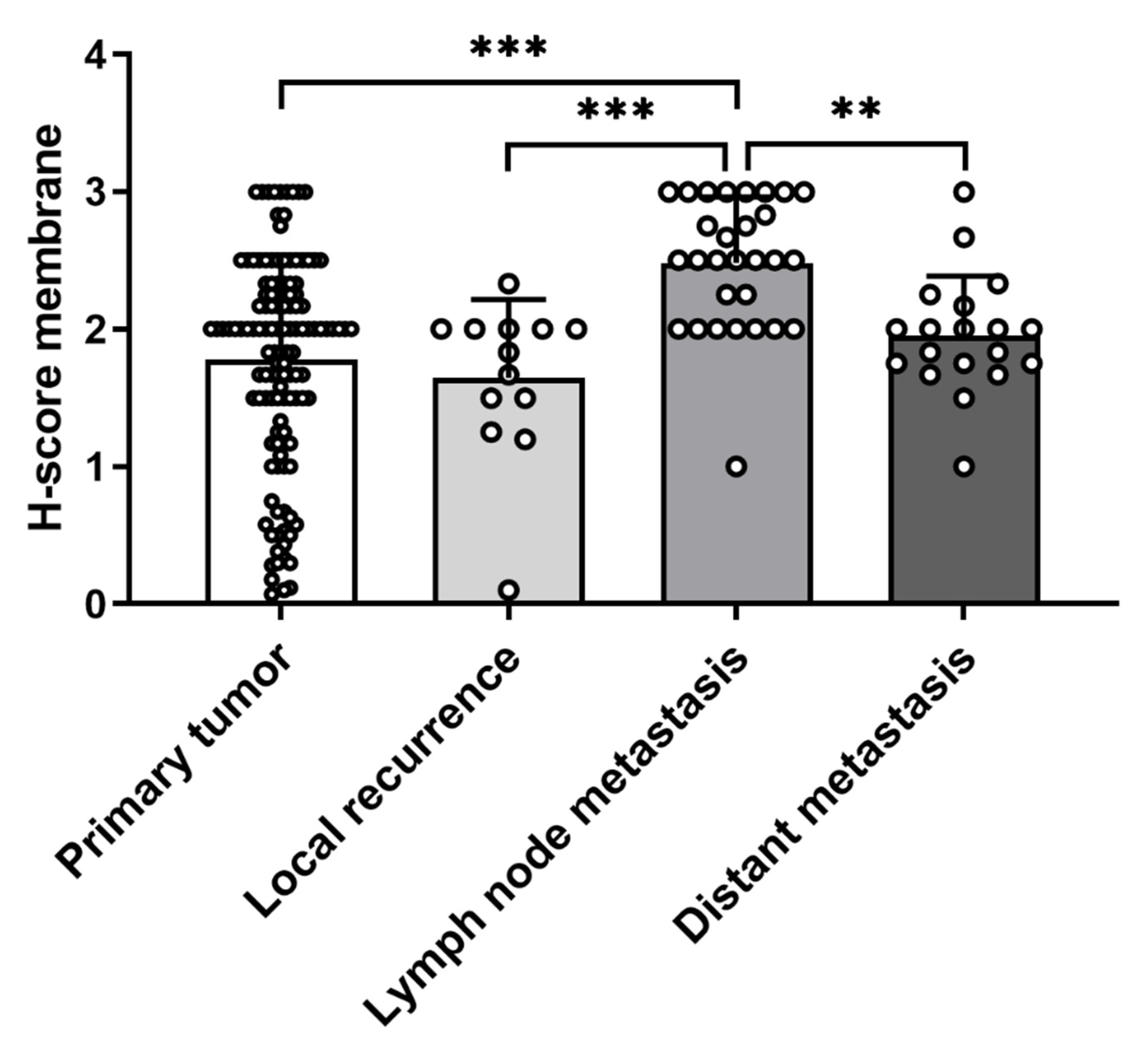
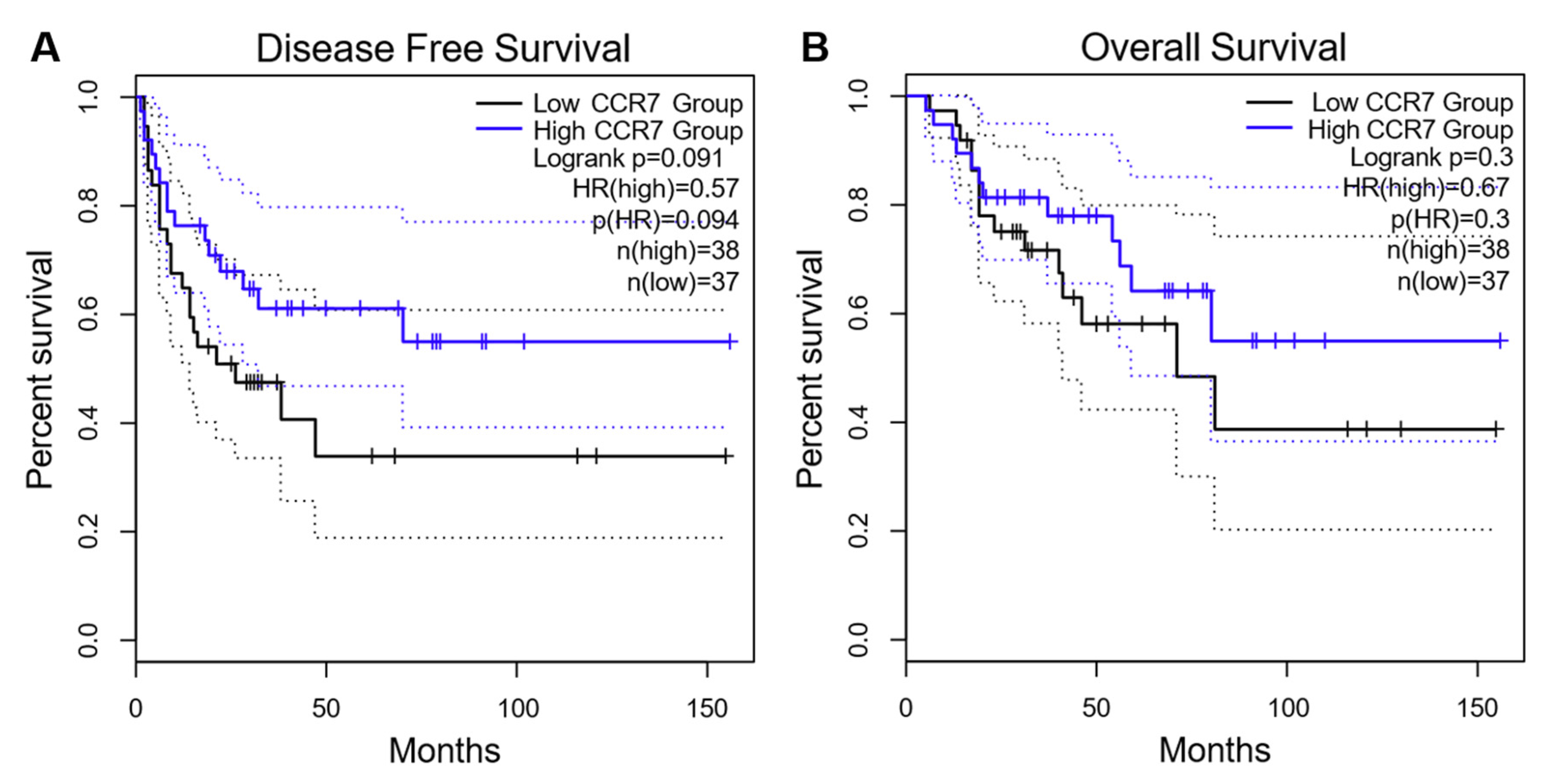
3.4. Predictive Role of CCR7 Staining Intensity and mRNA Expression on Clinical Outcome in Adrenocortical Carcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerkhofs, T.M.; Verhoeven, R.H.; Van der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van de Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 2013, 49, 2579–2586. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Bancos, I.; Hamrahian, A.; Habra, M.A.; Vaidya, A.; Levine, A.C.; Else, T. American Association of Clinical Endocrinology Disease State Clinical Review on the Evaluation and Management of Adrenocortical Carcinoma in an Adult: A Practical Approach. Endocr. Pr. 2020, 26, 1366–1383. [Google Scholar] [CrossRef]
- Bedrose, S.; Daher, M.; Altameemi, L.; Habra, M.A. Adjuvant Therapy in Adrenocortical Carcinoma: Reflections and Future Directions. Cancers 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Laganà, M.; Grisanti, S.; Cosentini, D.; Ferrari, V.D.; Lazzari, B.; Ambrosini, R.; Sardini, C.; Volta, A.D.; Palumbo, C.; Poliani, P.L.; et al. Efficacy of the EDP-M Scheme Plus Adjunctive Surgery in the Management of Patients with Advanced Adrenocortical Carcinoma: The Brescia Experience. Cancers 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.; Baudin, E.; Terzolo, M.; Allolio, B.; Chadarevian, R.; Leboulleux, S.; Mantero, F.; Haak, H.; Fassnacht, M. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. Endocr. Abstr. 2013, 98, 4759–4767. [Google Scholar] [CrossRef]
- Vaidya, A.; Nehs, M.; Kilbridge, K. Treatment of Adrenocortical Carcinoma. Surg. Pathol. Clin. 2019, 12, 997–1006. [Google Scholar] [CrossRef]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libe, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major Prognostic Role of Ki67 in Localized Adrenocortical Carcinoma After Complete Resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef]
- Libe, R.; Borget, I.; Ronchi, C.; Zaggia, B.; Kroiss, M.; Kerkhofs, T.; Bertherat, J.; Volante, M.; Quinkler, M.; Chabre, O.; et al. Prognostic factors in stage III–IV adrenocortical carcinomas (ACC): An European Network for the Study of Adrenal Tumor (ENSAT) study. Ann. Oncol. 2015, 26, 2119–2125. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Chifu, I.; Heinze, B.; Fuss, C.T.; Lang, K.; Kroiss, M.; Kircher, S.; Ronchi, C.L.; Altieri, B.; Schirbel, A.; Fassnacht, M.; et al. Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 597878. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Hahner, S.; Heinze, B.; Fassnacht, M.; Kroiss, M.; Bley, T.A.; Wester, H.-J.; Kropf, S.; Lapa, C.; Schirbel, A.; et al. Investigating the Chemokine Receptor 4 as Potential Theranostic Target in Adrenocortical Cancer Patients. Clin. Nucl. Med. 2017, 42, e29–e34. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.D.; Huff, L.M.; Evbuomwan, M.O.; Xu, X.; Dang, H.D.; Velez, D.S.; Singh, S.P.; Zhang, H.H.; Gardina, P.J.; Lee, J.-H.; et al. Screening of cancer tissue arrays identifies CXCR4 on adrenocortical carcinoma: Correlates with expression and quantification on metastases using 64Cu-plerixafor PET. Oncotarget 2017, 8, 73387–73406. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Fan, L.; Reilly, C.R.; Luo, Y.; Dorf, M.E.; Lo, D. Cutting Edge: Ectopic Expression of the Chemokine TCA4/SLC Is Sufficient to Trigger Lymphoid Neogenesis. J. Immunol. 2000, 164, 3955–3959. [Google Scholar] [CrossRef]
- Müller, G.; Höpken, U.E.; Lipp, M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 2003, 195, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Till, K.J.; Lin, K.; Zuzel, M.; Cawley, J.C. The chemokine receptor CCR7 and α4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 2002, 99, 2977–2984. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Cunningham, H.D.; Shannon, L.A.; Calloway, P.A.; Fassold, B.C.; Dunwiddie, I.; Vielhauer, G.; Zhang, M.; Vines, C.M. Expression of the C-C Chemokine Receptor 7 Mediates Metastasis of Breast Cancer to the Lymph Nodes in Mice. Transl. Oncol. 2010, 3, 354–361. [Google Scholar] [CrossRef]
- Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers 2020, 12, 1036. [Google Scholar] [CrossRef]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.-T.; Hwang, S.T. Expression of CC Chemokine Receptor-7 and Regional Lymph Node Metastasis of B16 Murine Melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Sancho, M.; Vieira, J.M.; Casalou, C.; Mesquita, M.; Pereira, T.; Cavaco, B.M.; Dias, S.; Leite, V. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. J. Endocrinol. 2006, 191, 229–238. [Google Scholar] [CrossRef]
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.-F.; Chen, Z.; Zu, X.-B. CCL21/CCR7 Enhances the Proliferation, Migration, and Invasion of Human Bladder Cancer T24 Cells. PLoS ONE 2015, 10, e0119506. [Google Scholar] [CrossRef]
- Boyle, S.; Ingman, W.; Poltavets, V.; Faulkner, J.W.; Whitfield, R.J.; McColl, S.R.; Kochetkova, M. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene 2016, 35, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.T.; Gieniec, K.A.; Gregor, C.E.; Faulkner, J.W.; McColl, S.R.; Kochetkova, M. Interplay between CCR7 and Notch1 axes promotes stemness in MMTV-PyMT mammary cancer cells. Mol. Cancer 2017, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Poeta, V.M.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.-H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma. Cancer 2008, 115, 243–250. [Google Scholar] [CrossRef]
- Altieri, B.; Sbiera, S.; Della Casa, S.; Weigand, I.; Wild, V.; Steinhauer, S.; Fadda, G.; Kocot, A.; Bekteshi, M.; Mambretti, E.M.; et al. Livin/BIRC7 expression as malignancy marker in adrenocortical tumors. Oncotarget 2016, 8, 9323–9338. [Google Scholar] [CrossRef]
- Ronchi, C.L.; Sbiera, S.; Altieri, B.; Steinhauer, S.; Wild, V.; Bekteshi, M.; Kroiss, M.; Fassnacht, M.; Allolio, B. Notch1 pathway in adrenocortical carcinomas: Correlations with clinical outcome. Endocr.-Relat. Cancer 2015, 22, 531–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Laufs, V.; Altieri, B.; Sbiera, S.; Kircher, S.; Steinhauer, S.; Beuschlein, F.; Quinkler, M.; Willenberg, H.S.; Rosenwald, A.; Fassnacht, M.; et al. ERCC1 as predictive biomarker to platinum-based chemotherapy in adrenocortical carcinomas. Eur. J. Endocrinol. 2018, 178, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Heinze, B.; Fuss, C.T.; Mulatero, P.; Beuschlein, F.; Reincke, M.; Mustafa, M.; Schirbel, A.; Deutschbein, T.; Williams, T.A.; Rhayem, Y.; et al. Targeting CXCR4 (CXC Chemokine Receptor Type 4) for Molecular Imaging of Aldosterone-Producing Adenoma. Hypertension 2018, 71, 317–325. [Google Scholar] [CrossRef]
- Larangé, A.; Antonios, D.; Pallardy, M.; Kerdine-Römer, S. Glucocorticoids inhibit dendritic cell maturation induced by Toll-like receptor 7 and Toll-like receptor 8. J. Leukoc. Biol. 2011, 91, 105–117. [Google Scholar] [CrossRef]
- Besedovsky, L.; Linz, B.; Dimitrov, S.; Groch, S.; Born, J.; Lange, T. Cortisol increases CXCR4 expression but does not affect CD62L and CCR7 levels on specific T cell subsets in humans. Am. J. Physiol. Metab. 2014, 306, E1322–E1329. [Google Scholar] [CrossRef]
- Guo, J.; Lou, W.; Ji, Y.; Zhang, S. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncol. Lett. 2013, 5, 1572–1578. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Li, N.; Gao, J.; Yu, J.; Zhao, J.; Li, M.; Zhao, Z. High Expression of CCR7 Predicts Lymph Node Metastasis and Good Prognosis in Triple Negative Breast Cancer. Cell. Physiol. Biochem. 2017, 43, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, L.; Li, S.; Qin, J.; Chen, L.; Liu, X.; Xu, P.; Wang, F.; Xiao, H.; Zhou, S.; et al. CCR7 enhances TGF-beta1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 2015, 6, 24348–24360. [Google Scholar] [CrossRef]
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as Novel Biomarkers Predicting Axillary Lymph Node Metastasis in T1Breast Cancer. Clin. Cancer Res. 2005, 11, 5686–5693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xu, S.; Tao, H.; Zhen, Z.; Chen, G.; Zhang, Z.; Yang, Y. CCR7 Expression and Intratumoral FOXP3+ Regulatory T Cells are Correlated with Overall Survival and Lymph Node Metastasis in Gastric Cancer. PLoS ONE 2013, 8, e74430. [Google Scholar] [CrossRef][Green Version]
- Irino, T.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T.; et al. CC-Chemokine receptor CCR7: A key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 291. [Google Scholar] [CrossRef] [PubMed]

| Total Cohort n = 120 | H-Score < 2 n = 55 | H-Score ≥ 2 n = 65 | p-Value * | |
|---|---|---|---|---|
| Age, years | 50 ± 16 | 50 ± 16 | 50 ± 17 | 0.917 |
| Female, n (%) | 78 (65) | 35 (64) | 43 (66) | 0.773 |
| ENSAT stage, n (%) | 0.477 | |||
| I | 5 (4) | 2 (4) | 3 (5) | |
| II | 48 (42) | 26 (48) | 22 (35) | |
| III | 35 (30) | 13 (25) | 22 (35) | |
| IV | 27 (24) | 12 (23) | 15 (25) | |
| Resection status, n (%) | 0.547 | |||
| R0 | 59 (49) | 27 (49) | 32 (49) | |
| R1 | 6 (5) | 4 (7) | 2 (3) | |
| R2 | 23 (19) | 12 (22) | 11 (17) | |
| RX | 9 (8) | 6 (11) | 3 (5) | |
| Unknown | 23 (19) | 6 (11) | 17 (26) | |
| Autonomous hormone secretion, n (%) | 0.646 | |||
| GC | 18 (15) | 8 (14) | 10 (15) | |
| GC + other | 30 (25) | 11 (20) | 19 (29) | |
| MC | 4 (3) | 3 (6) | 1 (2) | |
| A | 8 (7) | 3 (6) | 5 (8) | |
| A + MC | 1 (1) | 0 | 1 (2) | |
| None | 16 (13) | 8 (14) | 8 (12) | |
| No preoperative endocrine work-up | 43 (36) | 22 (40) | 21 (32) | |
| Tumor size, cm | 11.4 ± 4.8 | 11.3 ± 5.0 | 11.5 ± 4.6 | 0.881 |
| Ki67 index, % | 15 ± 12 | 14 ± 13 | 16 ± 11 | 0.340 |
| Weiss-Score | 5.7 ± 1.7 | 5.8 ± 1.6 | 5.6 ± 1.7 | 0.568 |
| Lymph node metastasis, n (%) | ||||
| Overall | 51 (43) | 21 (38) | 30 (46) | 0.352 |
| At primary diagnosis | 19 (16) | 6 (11) | 13 (20) | 0.091 |
| At follow-up | 36 (30) | 17 (31) | 19 (29) | 0.722 |
| Distant metastasis, n (%) | 91 (76) | 40 (73) | 51 (78) | 0.609 |
| Disease progression, n (%) | 95 (79) | 40 (73) | 55 (85) | 0.068 |
| Death overall, n (%) | 78 (65) | 34 (62) | 44 (68) | 0.431 |
| Death due to ACC, n (%) | 68 (57) | 30 (55) | 38 (59) | 0.463 |
| Median overall survival, months (±SD) | 63 ± 53 | 65 ± 48 | 61 ± 59 | 0.632 |
| Median progression-free survival, months (±SD) | 28 ± 38 | 32 ± 37 | 25 ± 39 | 0.318 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuss, C.T.; Other, K.; Heinze, B.; Landwehr, L.-S.; Wiegering, A.; Kalogirou, C.; Hahner, S.; Fassnacht, M. Expression of the Chemokine Receptor CCR7 in the Normal Adrenal Gland and Adrenal Tumors and Its Correlation with Clinical Outcome in Adrenocortical Carcinoma. Cancers 2021, 13, 5693. https://doi.org/10.3390/cancers13225693
Fuss CT, Other K, Heinze B, Landwehr L-S, Wiegering A, Kalogirou C, Hahner S, Fassnacht M. Expression of the Chemokine Receptor CCR7 in the Normal Adrenal Gland and Adrenal Tumors and Its Correlation with Clinical Outcome in Adrenocortical Carcinoma. Cancers. 2021; 13(22):5693. https://doi.org/10.3390/cancers13225693
Chicago/Turabian StyleFuss, Carmina Teresa, Katharina Other, Britta Heinze, Laura-Sophie Landwehr, Armin Wiegering, Charis Kalogirou, Stefanie Hahner, and Martin Fassnacht. 2021. "Expression of the Chemokine Receptor CCR7 in the Normal Adrenal Gland and Adrenal Tumors and Its Correlation with Clinical Outcome in Adrenocortical Carcinoma" Cancers 13, no. 22: 5693. https://doi.org/10.3390/cancers13225693
APA StyleFuss, C. T., Other, K., Heinze, B., Landwehr, L.-S., Wiegering, A., Kalogirou, C., Hahner, S., & Fassnacht, M. (2021). Expression of the Chemokine Receptor CCR7 in the Normal Adrenal Gland and Adrenal Tumors and Its Correlation with Clinical Outcome in Adrenocortical Carcinoma. Cancers, 13(22), 5693. https://doi.org/10.3390/cancers13225693






