Prognostic Effect of Preoperative Psoas Muscle Hounsfield Unit at Radical Cystectomy for Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
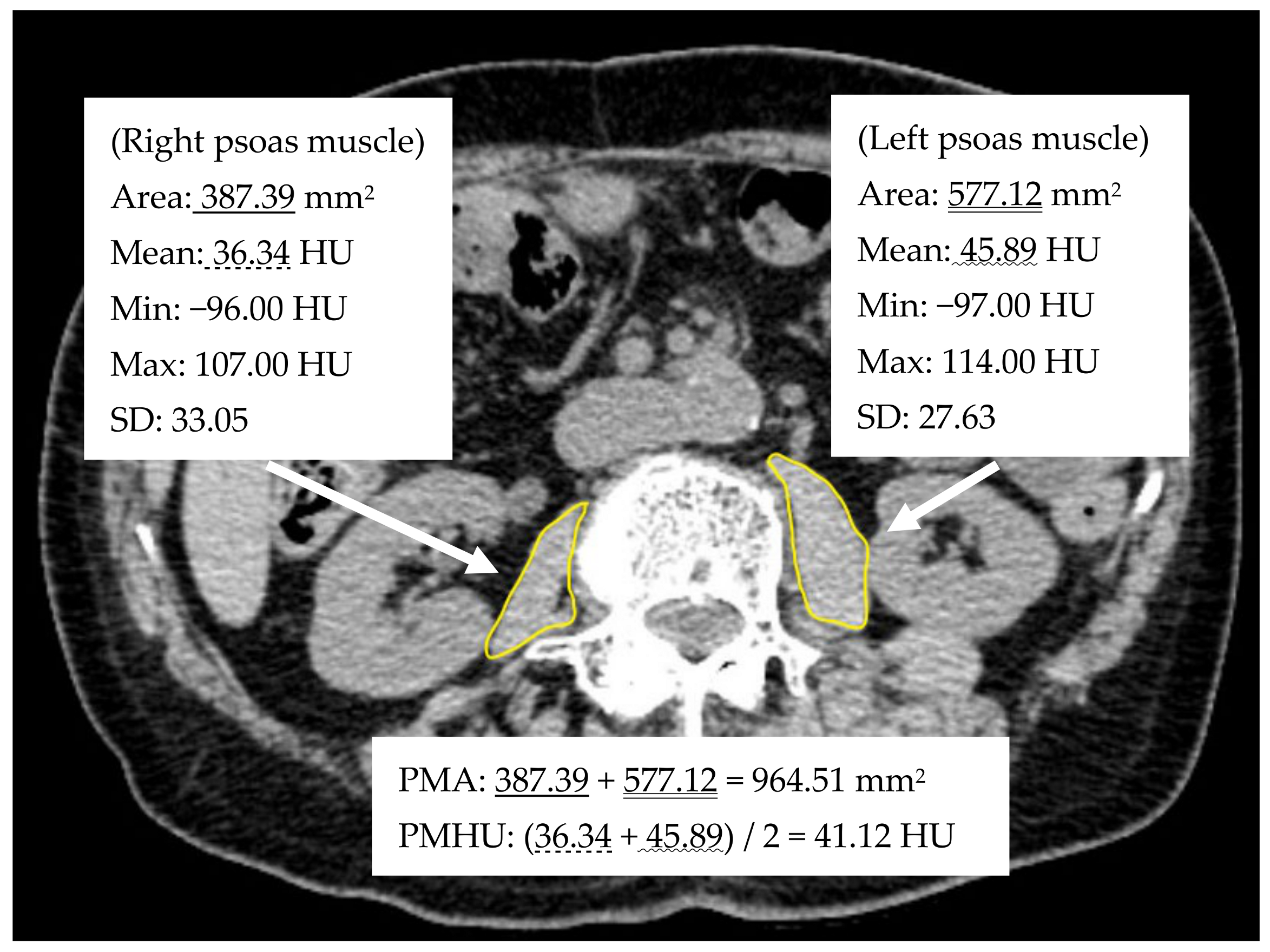
2.2. Image Analyses
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.-C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Fonteyne, V.; Ost, P.; Bellmunt, J.; Droz, J.P.; Mongiat-Artus, P.; Inman, B.; Paillaud, E.; Saad, F.; Ploussard, G. Curative Treatment for Muscle Invasive Bladder Cancer in Elderly Patients: A Systematic Review. Eur. Urol. 2018, 73, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chappidi, M.R.; Kates, M.; Patel, H.D.; Tosoian, J.J.; Kaye, D.R.; Sopko, N.A.; Lascano, D.; Liu, J.-J.; McKiernan, J.; Bivalacqua, T.J. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 256.e1–256.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Mayr, R.; Gierth, M.; Zeman, F.; Reiffen, M.; Seeger, P.; Wezel, F.; Pycha, A.; Comploj, E.; Bonatti, M.; Ritter, M.; et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 505–513. [Google Scholar] [CrossRef]
- Fukushima, H.; Takemura, K.; Suzuki, H.; Koga, F. Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer. Int. J. Mol. Sci. 2018, 19, 2999. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.-I.; Koga, F. Sarcopenia as a Prognostic Biomarker of Advanced Urothelial Carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef]
- Saitoh-Maeda, Y.; Kawahara, T.; Miyoshi, Y.; Tsutsumi, S.; Takamoto, D.; Shimokihara, K.; Hayashi, Y.; Mochizuki, T.; Ohtaka, M.; Nakamura, M.; et al. A low psoas muscle volume correlates with a longer hospitalization after radical cystectomy. BMC Urol. 2017, 17, 87. [Google Scholar] [CrossRef]
- Hu, X.; Dou, W.-C.; Shao, Y.-X.; Liu, J.-B.; Xiong, S.-C.; Yang, W.-X.; Li, X. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 747–754. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Mari, A.; Kimura, S.; Foerster, B.; Abufaraj, M.; D’Andrea, D.; Gust, K.M.; Shariat, S.F. A systematic review and meta-analysis of lymphovascular invasion in patients treated with radical cystectomy for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 293–305. [Google Scholar] [CrossRef]
- Robinson, S.M.; Denison, H.J.; Cooper, C.; Sayer, A.A. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, M.N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Smith, A.B.; Deal, A.M.; Yu, H.; Boyd, B.; Matthews, J.; Wallen, E.M.; Pruthi, R.S.; Woods, M.E.; Muss, H.; Nielsen, M.E. Sarcopenia as a Predictor of Complications and Survival Following Radical Cystectomy. J. Urol. 2014, 191, 1714–1720. [Google Scholar] [CrossRef]
- Miyake, M.; Morizawa, Y.; Hori, S.; Marugami, N.; Shimada, K.; Gotoh, D.; Tatsumi, Y.; Nakai, Y.; Inoue, T.; Anai, S.; et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer 2017, 17, 237. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, Y.; Nakamura, K.; Matsuoka, H.; Ogawa, C.; Masuyama, H. Pre-treatment psoas major volume is a predictor of poor prognosis for patients with epithelial ovarian cancer. Mol. Clin. Oncol. 2019, 11, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, C.-L.; Shen, X.; Huang, Y.-Y.; Zhang, F.-M.; Chen, X.-Y.; Ma, L.-L.; Chen, X.-L.; Yu, Z.; Wang, S.-L. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: A propensity score–matched analysis from a large-scale cohort. Surgery 2019, 166, 297–304. [Google Scholar] [CrossRef]
- Van Rijssen, L.B.; van Huijgevoort, N.C.; Coelen, R.J.; Tol, J.A.; Haverkort, E.B.; Nio, C.Y.; Busch, O.R.; Besselink, M.G. Skeletal Muscle Quality is Associated with Worse Survival After Pancreatoduodenectomy for Periampullary, Nonpancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 272–280. [Google Scholar] [CrossRef]
- Kitajima, Y.; Hyogo, H.; Sumida, Y.; Eguchi, Y.; Ono, N.; Kuwashiro, T.; Tanaka, K.; Takahashi, H.; Mizuta, T.; Ozaki, I.; et al. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J. Gastroenterol. Hepatol. 2013, 28, 1507–1514. [Google Scholar] [CrossRef]
- Cao, Q.; Xiong, Y.; Zhong, Z.; Ye, Q. Computed Tomography-Assessed Sarcopenia Indexes Predict Major Complications following Surgery for Hepatopancreatobiliary Malignancy: A Meta-Analysis. Ann. Nutr. Metab. 2019, 74, 24–34. [Google Scholar] [CrossRef]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, K.L.; Ke, J.-Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 2014, 16, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Rutten, I.J.; Ubachs, J.; Kruitwagen, R.F.; Beets-Tan, R.G.; Damink, S.O.; Van Gorp, T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, C.; Knipper, S.; Pecoraro, A.; Rosiello, G.; Luzzago, S.; Deuker, M.; Tian, Z.; Shariat, S.F.; Simeone, C.; Briganti, A.; et al. Patient frailty predicts worse perioperative outcomes and higher cost after radical cystectomy. Surg. Oncol. 2020, 32, 8–13. [Google Scholar] [CrossRef]
- Ploussard, G.; Daneshmand, S.; Efstathiou, J.A.; Herr, H.W.; James, N.D.; Rödel, C.M.; Shariat, S.F.; Shipley, W.U.; Sternberg, C.N.; Thalmann, G.N.; et al. Critical Analysis of Bladder Sparing with Trimodal Therapy in Muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2014, 66, 120–137. [Google Scholar] [CrossRef]
- PERIOP OG Working Group; Tully, R.; Loughney, L.; Bolger, J.; Sorensen, J.; McAnena, O.; Collins, C.G.; Carroll, P.A.; Arumugasamy, M.; Murphy, T.J.; et al. The effect of a pre- and post-operative exercise programme versus standard care on physical fitness of patients with oesophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (The PERIOP-OG Trial): Study protocol for a randomised controlled trial. Trials 2020, 21, 638. [Google Scholar] [CrossRef]
- Jones, C.; Eddleston, J.; McCairn, A.; Dowling, S.; McWilliams, D.; Coughlan, E.; Griffiths, R. Improving rehabilitation after critical illness through outpatient physiotherapy classes and essential amino acid supplement: A randomized controlled trial. J. Crit. Care 2015, 30, 901–907. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of Vitamin D Supplementation on Muscle Strength, Gait and Balance in Older Adults: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
| Variables | Total Cases (n = 177) | Men (n = 151) | Women (n = 26) | p-Value | |
|---|---|---|---|---|---|
| Median age at RC (IQR) | 70 (66–76) | 70 (66–76) | 72.5 (65–76) | 0.687 | |
| Median BMI (IQR) | 22.92 (20.94–24.95) | 23.11 (21.20–25.07) | 21.22 (19.03–22.68) | <0.01 | |
| ASA-PS | 1 | 21 (12%) | 17 (11%) | 4 (15%) | 0.755 |
| 2 | 120 (68%) | 103 (68%) | 17 (65%) | ||
| 3 | 36 (20%) | 31 (21%) | 5 (19%) | ||
| Clinical T stage, n (%) | NMIBC | 51 (29%) | 45 (30%) | 6 (23%) | 0.163 |
| 2 | 70 (40%) | 61 (40%) | 9 (35%) | ||
| 3 | 27 (15%) | 19 (13%) | 8 (31%) | ||
| 4 | 29 (16%) | 26 (17%) | 3 (12%) | ||
| Clinical N stage, n (%) | 0 | 154 (87%) | 132 (87%) | 22 (85%) | 0.752 |
| ≥1 | 23 (13%) | 19 (13%) | 4 (15%) | ||
| Neoadjuvant chemotherapy, n (%) | No | 102 (58%) | 90 (60%) | 12 (46%) | 0.207 |
| Yes | 75 (42%) | 61 (40%) | 14 (54%) | ||
| Median PMA (mm2) (IQR) | 1129 (894–1455) | 1242 (983–1510) | 679 (521–887) | <0.01 | |
| Median PMI (cm2/m2) (IQR) | 4.31 (3.41–5.41) | 4.66 (3.66–5.60) | 2.88 (2.34–3.89) | <0.01 | |
| Median PMHU (HU) (IQR) | 43.14 (39.26–47.54) | 43.40 (39.56–47.75) | 40.93 (34.56–45.16) | 0.043 |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Category | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age | <70 | Reference | Reference | ||||
| ≥70 | 2.093 | 1.239–3.533 | 0.006 | 1.734 | 1.010–2.977 | 0.046 | |
| Sex | Men | Reference | Reference | ||||
| Women | 2.210 | 1.189–4.109 | 0.012 | 2.116 | 1.132–3.954 | 0.019 | |
| ASA-PS | 1,2 | Reference | |||||
| 3 | 1.624 | 0.926–2.849 | 0.091 | ||||
| Clinical T stage | <3 | Reference | Reference | ||||
| ≥3 | 1.782 | 1.705–2.956 | 0.025 | 1.665 | 1.001–2.769 | 0.049 | |
| Clinical N stage | 0 | Reference | |||||
| ≥1 | 0.659 | 0.283–1.530 | 0.33 | ||||
| Neoadjuvant chemotherapy | No | Reference | |||||
| Yes | 0.877 | 0.512–1.502 | 0.633 | ||||
| BMI | Not low | Reference | |||||
| Low | 0.909 | 0.507–1.631 | 0.749 | ||||
| PMA | Not low | Reference | |||||
| Low | 1.332 | 0.692–2.564 | 0.391 | ||||
| PMI | Not low | Reference | |||||
| Low | 1.019 | 0.530–1.959 | 0.954 | ||||
| PMHU | Not low | Reference | Reference | ||||
| Low | 1.924 | 1.132–3.270 | 0.016 | 1.758 | 1.014–3.048 | 0.044 | |
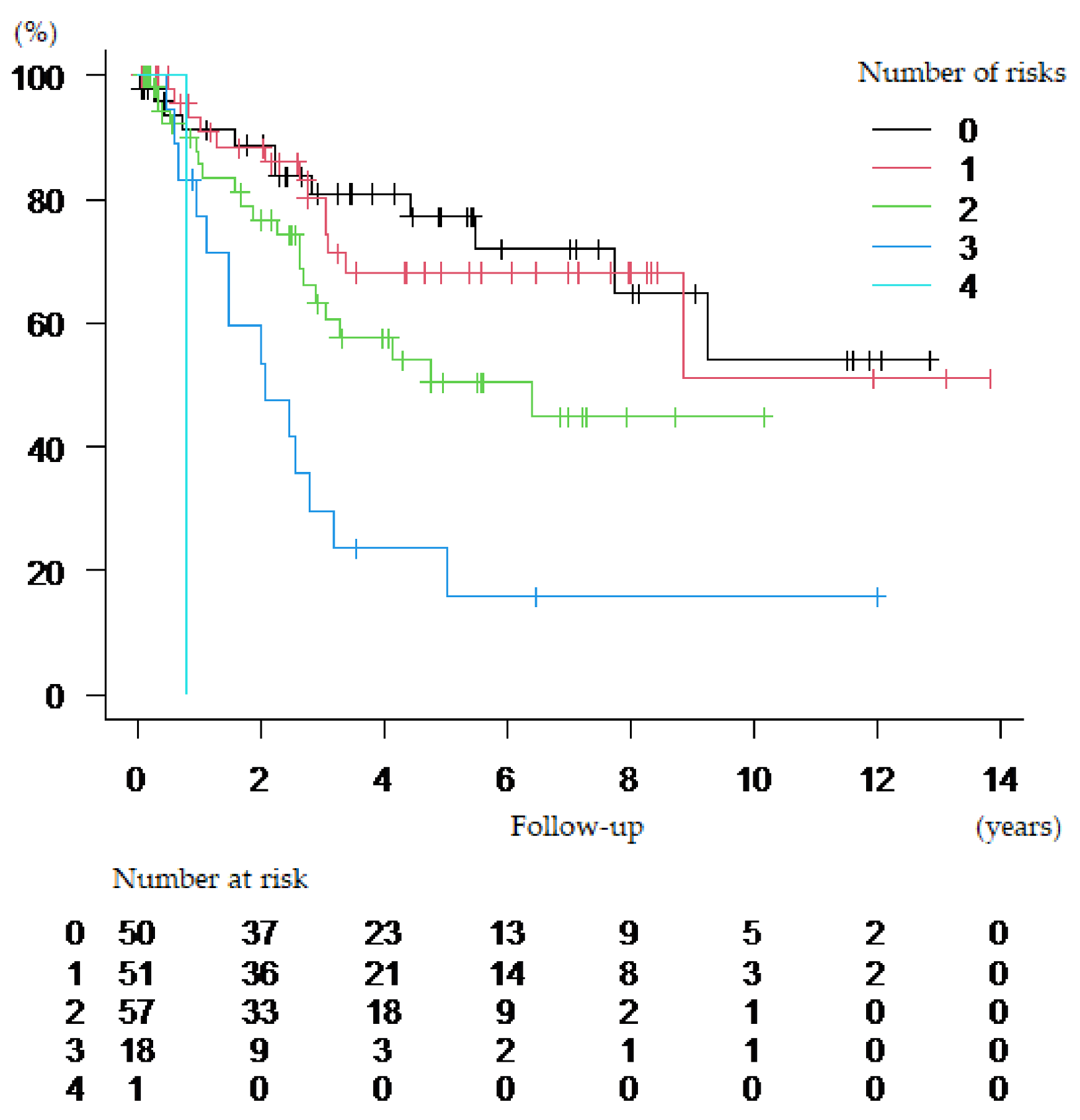
| Risk Factors | 0 | 1 |
|---|---|---|
| 1. Age | <70 | ≥70 |
| 2. Sex | Men | Women |
| 3. Clinical T stage | <3 | ≥3 |
| 4. PMHU | Men: ≥39.56 HU Women: ≥34.56 HU | Men: <39.56 HU Women: <34.56 HU |
| Risk Category | ||
| Low-risk | If <2 risk factors present | |
| Intermediate-risk | If 2 risk factors present | |
| High-risk | If ≥3 risk factors present | |
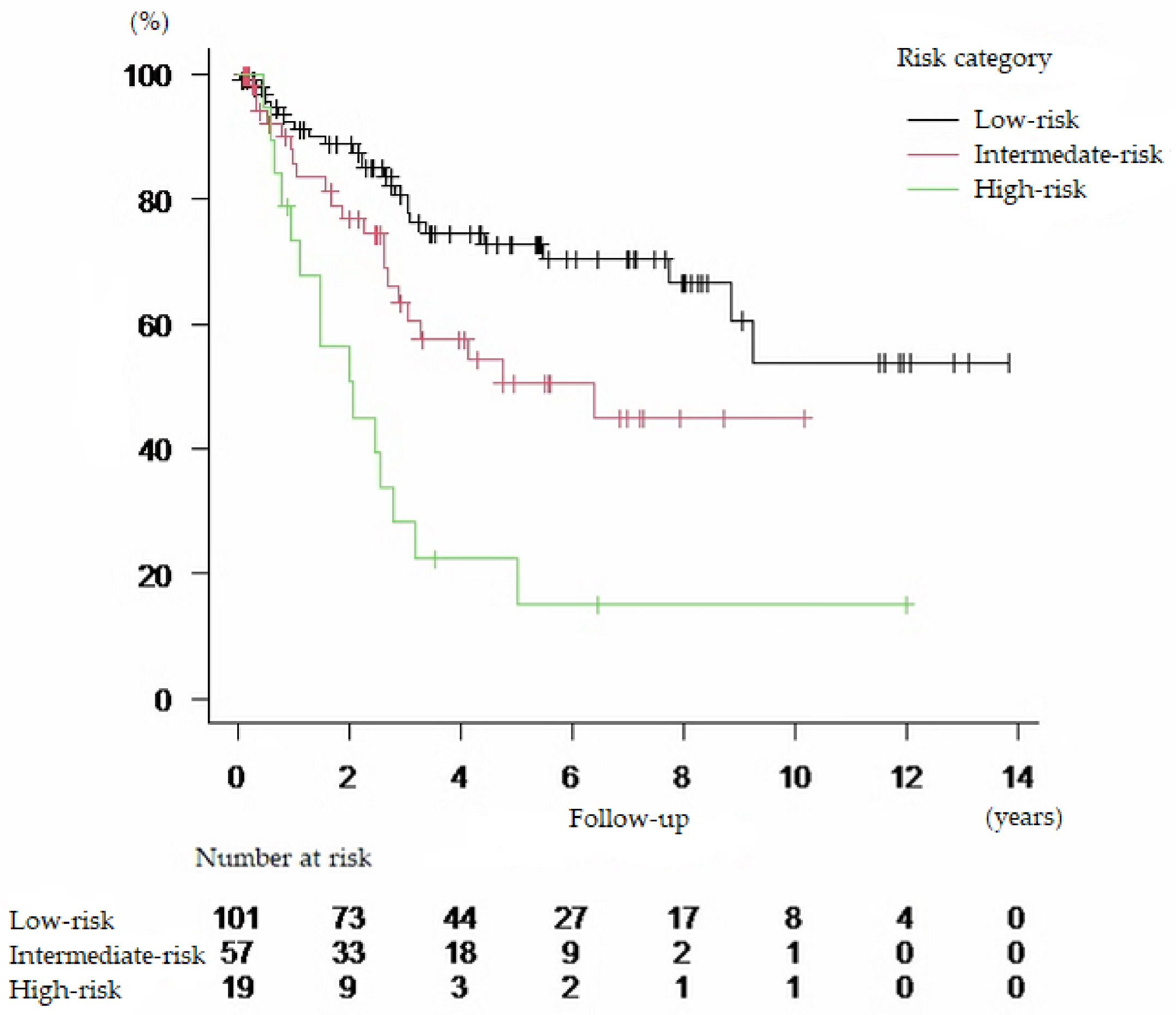
| Risk Category | n | HR | 95% CI | p Value |
|---|---|---|---|---|
| Low-risk | 101 | Reference | ||
| Intermediate-risk | 57 | 1.902 | 1.061–3.411 | 0.031 |
| High-risk | 19 | 4.597 | 2.408–8.775 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugino, Y.; Sasaki, T.; Kato, M.; Masui, S.; Nishikawa, K.; Okamoto, T.; Kajiwara, S.; Shibahara, T.; Onishi, T.; Tanaka, S.; et al. Prognostic Effect of Preoperative Psoas Muscle Hounsfield Unit at Radical Cystectomy for Bladder Cancer. Cancers 2021, 13, 5629. https://doi.org/10.3390/cancers13225629
Sugino Y, Sasaki T, Kato M, Masui S, Nishikawa K, Okamoto T, Kajiwara S, Shibahara T, Onishi T, Tanaka S, et al. Prognostic Effect of Preoperative Psoas Muscle Hounsfield Unit at Radical Cystectomy for Bladder Cancer. Cancers. 2021; 13(22):5629. https://doi.org/10.3390/cancers13225629
Chicago/Turabian StyleSugino, Yusuke, Takeshi Sasaki, Manabu Kato, Satoru Masui, Kouhei Nishikawa, Takashi Okamoto, Shinya Kajiwara, Takuji Shibahara, Takehisa Onishi, Shiori Tanaka, and et al. 2021. "Prognostic Effect of Preoperative Psoas Muscle Hounsfield Unit at Radical Cystectomy for Bladder Cancer" Cancers 13, no. 22: 5629. https://doi.org/10.3390/cancers13225629
APA StyleSugino, Y., Sasaki, T., Kato, M., Masui, S., Nishikawa, K., Okamoto, T., Kajiwara, S., Shibahara, T., Onishi, T., Tanaka, S., Kanda, H., Matsuura, H., & Inoue, T. (2021). Prognostic Effect of Preoperative Psoas Muscle Hounsfield Unit at Radical Cystectomy for Bladder Cancer. Cancers, 13(22), 5629. https://doi.org/10.3390/cancers13225629







