Simple Summary
High-grade glioma (HGG) is a burdening oncological pathology for which maximum safe resection followed by combined chemoradiation therapy is still the gold standard. Despite these treatments the overall survival is less than 2 years. Alternative strategies are currently being investigated, including Focused Ultrasound (FUS). Given its non-invasiveness and promising pre-clinical results for tumor ablation, brain-blood barrier (BBB) opening and drug delivery, FUS is poised to achieve a therapeutic role in HGG treatment. This systematic review aims to identify the different modalities of how FUS can be used, how it impacts on survival in the clinic, based on the existing evidence. FUS-mediated tumor ablation still needs further investigation due to its controversial effects and complications. FUS-mediated BBB opening is showing positive results with low complication rate, as such potentially a gamechanger in future oncological treatments. Ongoing trials will clarify FUS impact on HGG patients.
Abstract
Background: Focused Ultrasound (FUS) is gaining a therapeutic role in neuro-oncology considering its novelty and non-invasiveness. Multiple pre-clinical studies show the efficacy of FUS mediated ablation and Blood-Brain Barrier (BBB) opening in high-grade glioma (HGG), but there is still poor evidence in humans, mainly aimed towards assessing FUS safety. Methods: With this systematic review our aim is, firstly, to summarize how FUS is proposed for human HGG treatment. Secondly, we focus on future perspectives and new therapeutic options. Using PRISMA 2020 guidelines, we reviewed case series and trials with description of patient characteristics, pre- and post-operative treatments and FUS outcomes. We considered nine case series (five about tumor ablation and four about BBB opening) with FUS-treated HGG patients between 1991 and 2021. Results: Sixty-eight patients were considered in total, mostly males (67.6%), with a mean age of 50.5 ± 15.3 years old. Major complication rates were found in the tumor ablation group (26.1%). FUS has been rarely applied for direct tumoral ablation in human HGG patients with controversial results, but at the best of current studies, FUS-mediated BBB opening is showing good results with very low complication rates, paving the way for a new reliable technique to improve local chemotherapy delivery and antitumoral immune response. Conclusions: FUS can become a complementary technique to surgical resection and standard radiochemotherapy in recurrent HGG. Ongoing trials could provide in the near future more data on FUS-mediated BBB opening impact on progression-free survival, overall survival and potential drug-delivery capacities.
1. Introduction
Ultrasonic waves are sound waves with a frequency > 20,000 Hz used for the first time during World War I for submarine detection [1,2]. During the 20th century progressively, ultrasound (US) started to be considered also as a medical tool able to diagnose and also to treat diseases such as autoimmune disorders [3,4] and started to be considered as a new tool to treat neurological pathologies: Lynn in 1942 and the Fry brothers in 1962 were the firsts to use this technology for tumor ablation [5,6,7]. At that time, the main problem impeding transcranial sonications for neuro-oncological purposes was the acoustic barrier represented by the skull, which lead to a loss of target accuracy and lack of temperature control; for these reasons these procedures were performed after bone flap removal [2,8,9].
During the past two decades, US has been widely used for intraoperative guidance and resection control in glioma surgery [10,11,12,13,14,15], in part due to its capacity to be complementary to other intraoperative techniques like 5-Aminolevulinic acid (5-ALA) fluorescence [16], intraoperative magnetic resonance imaging (MRI) [17], awake mapping [18,19,20] and asleep neuromonitoring [21,22,23].
Recently, the combination of stereotactic techniques, MRI and acoustic aberration phase correction permitted an increase in US accuracy [24,25]. High-intensity MRI-guided FUS has been used to induce a precise thermal ablation of the target tissue using a 650-Hz-frequency ultrasound energy, which can increase tissue temperature to 65 °C [26,27]. This technique led FUS to become an excellent alternative in functional neurosurgery (mostly for Parkinson’ disease and essential tremor), permitting non-invasive and rapid procedures [24,25]. New applications are being studied for neuromodulation and epilepsy with encouraging results [28]. The parallel introduction of transducers, water baths, degassed water and finally microbubbles considerably augmented the acoustic coupling, allowed a reduction of FUS-related side-effects such as thermal injury of the scalp and optic aberration of FUS waves [29,30]. Although promising, in neuro-oncology FUS direct ablation showed some limitations especially concerning a small treatment envelope and the time required to ablate largest tumors [31,32,33].
Different studies on animals and human models have been looking at FUS’ capabilities in high grade gliomas (HGG) ablation and in brain-blood barrier (BBB) opening in order to maximize the penetration of neo-adjuvant and adjuvant treatments to the central nervous system [34,35,36]. As we know BBB restricts and controls water-soluble substances movement between the blood and the parenchyma. This function can be altered in HGG, where endothelial cells lose their intercellular tight junctions and ability to prevent water-soluble molecules entry in the tumor, whilst permits a tumoral protection in chemotherapy delivery [37,38]. In fact, most chemotherapy drugs have large molecular weights, and considering BBB semi-permeability and selectiveness, the latter becomes a limiting obstacle in brain tumor treatment [39]. Multiple preclinical and clinical studies in animal glioma models have evaluated the safety and efficacy of BBB disruption with FUS [40,41,42]. Different large animal studies showed a possible drug delivery with FUS aid, especially concerning trastuzumab, doxorubicin, temozolomide (TMZ), methotrexate and carboplatin [39].
With this review, our aim is to summarize how FUS has been proposed for human HGG treatment as a useful technique to perform direct tumor ablation and BBB opening for chemotherapy. Secondarily, we point out which future perspectives and new therapeutic option are being achieved and studied with ongoing human trials.
2. Materials and Methods
Search Strategy, Inclusion Criteria, and Study Selection
The study protocol followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA-DTA) 2020 guidelines [43]. No PROSPERO registration was needed. We conducted a restricted search using the keywords “Focused Ultrasound” AND “Glioma” in May 2021 of the following databases: PubMed/Medline, Google Scholar and clinicaltrials.gov. This resulted in a total of 338 references (Figure 1).
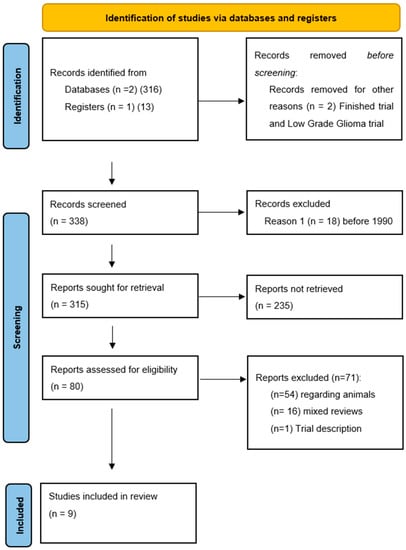
Figure 1.
PRISMA-P 2020 flow-chart and search strategy. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. doi:10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
The first two authors (LP and AM) independently screened all titles and abstracts, and full-text copies of all relevant articles were obtained with exclusion of no pertinent studies. In case of a discrepancy, the senior author (DM) arbitrated until a consensus among the authors was reached. The following inclusion criteria were used: (1) All studies reporting single or multiple cases of HGG treated with focused ultrasound; (2) studies published since 1990, as the standards of neurosurgery has significantly improved since then and results before this era are not comparable.
In total, 338 abstracts were screened, and 80 papers were retained for eligibility. During the review process, we searched for all reported cases where FUS was used on human patients harboring intracranial HGG. Research not involving HGG patients treated with focused ultrasound (5 articles) and before 1990 (18 articles) did not meet the inclusion criteria. Seventy-one articles were excluded, considering that 54 reported non-human research, 16 were mixed reviews on FUS in neurosurgery and one was a trial description. The references of the selected studies were checked to find all possible related articles (Figure 1). A large research was made on purpose at the beginning of the review process to prevent possible lack of eligible studies, which on a small pool of reported patients would have generated a major selection bias. No statistical analysis was performed.
3. Results
3.1. Patient Characteristics
We considered nine case series reporting patients treated for cerebral gliomas with FUS between 1991 and 2021. Sixty-eight patients were included (46 males and 22 females), of whom 63 benefitted from a previous treatment (surgery in 11 cases; radiotherapy in 52 cases) (Table 1). The mean age was of 50.5 ± 15.3 years (range, 17–80). Sixty-one patients harbored a glioblastoma, whilst seven had an anaplastic astrocytoma: all HGG were supratentorial. In our review, five papers included tumor ablation techniques whilst four analyzed BBB opening.

Table 1.
Demographic and pre-FUS session summary data of all-included studies.
3.2. FUS Devices for Direct Tumor Ablation
Guthkelch et al. [44,45,46] in 1991 used a radio-frequency signal that is amplified and converted to US by a curved piezoelectric transducer from a modified obstetrical ultrasound. Intracerebral temperatures were measured with flexible thermocouple probes introduced under CT-scan guidance through the craniotomized area.
In 2006, Park et al. [47] used transcranially the JC HIFU system (Chongqing HAIFU Medical Technology Co., LTD., Chongqing, China) on a 17 year-old female patient affected by an anaplastic astrocytoma. The patient had previously benefitted from a debulking through a craniotomy. The system is composed of a treatment table coupled to a high-frequency ultrasound and controlled by a central console. The US transducer can create a beam propagating through tissues with a temperature above 56 °C, provoking immediate thermal toxicity.
A similar FUS machine, used in further studies, is the ExAblate® (InSightec, Haifa, Israel). The latter consists in a 30 cm diameter hemispherical 512 up to 1024-element phased-array transducer coupled with a 512-channel driving system, a treatment planning workstation with MRI thermometry and dosimetry and a water cooling, circulation and degassing system, integrated with a clinical MRI Unit [33]. The US frequency is 620–720 KHz.
3.3. FUS Devices for BBB Opening
A French research consortium developed and used an implanted US device named SonoCloud® (Carthera®, Paris, France), consisting of a 10 mm diameter US transducer that had a resonance frequency of 1.05 MHz and was encased in an 11.5 mm diameter biocompatible housing [48]. The combination with ultrasound-specific microbubbles (Sonovue®, Bracco Imaging, Milan, Italy) permits a lower FUS treatment intensity. In Toronto Mainprize et al. used ExAblate® system to study chemotherapy BBB passage and concentration after its opening [49]. Finally Chen et al. [50] introduced a frameless neuronavigation US device called NaviFUS® (NaviFUS Inc., Taipei, Taiwan), that integrated with microbubbles is able to steer in real-time the transcranial ultrasound energy precisely and repeatedly at targeted central nervous system areas.
3.4. FUS for Tumor Ablation
In our review, we identified 23 patients who benefitted from a FUS tumor ablation procedure (Table 2). Five patients (8%, all in Guthkelch’s series [44]) did not had any other treatment purposed apart from FUS. This population was younger compared to BBB opening cohort. Eleven patients benefited from a pre-FUS tumor debulking and 18 from radiotherapy. Karnofsky performance score (KPS) prior to FUS was assessed only by Guthkelch et al. [44] (Table 3).

Table 2.
Demographic and pre- and post-FUS session summary data of FUS-tumor ablation studies.

Table 3.
Overall Tumor Ablation via FUS patient demographics, preoperative status and post-operative follow-up.
The authors used different power and length of ultrasound sonication with a mean value of 30.3 ± 6.8 sonications per patient. In 16 patients the number of sonications was not reported [44,47]. Depending on different authors, target position, beam temperature and low-power verification sessions before sonications were applied, with a lower temperature that was augmented once the target was confirmed by MRI or Computed Tomography (CT)-scan co-registration or overlap. Temperature was adapted considering patient compliance, discomfort and/or neurological symptoms. The mean follow-up for 19 patients was of 19.7 ± 14.6 months (four patients did not have a specified time range). In tumor ablation cohort, six patients (26.1%) presented a major FUS-related complication (four deep hematomas, one subcortical hematoma and one unwanted lesion in an eloquent area) (Table 3). No neurological outcome at long term follow-up was reported. No data was found concerning tumor volume stability.
3.5. FUS-Mediated BBB Opening
Forty-five patients were included in this subpopulation, presenting a mean age of 56.6 ± 5.3 years. In all cases, BBB opening was performed to enhance post-surgical chemotherapy. No data was found concerning pre-FUS tumor debulking procedure (Table 4 and Table 5), whereas 17 patients had pre-FUS radiotherapy. Four patients (8.8%, all in Mainprize’s series [49]) were enrolled in a safety and feasibility study so they had FUS as first treatment before any surgery or chemo-radiotherapy approach. In three studies, the KPS was considered before FUS delivery with a limit respectively at 70–100 (Mainprize et al. [49]), 90 (Idbaih et al. [51]), and 90 (Chen et al. [50]). Ultrasound sonications were augmented in number and intensity considering patient tolerance et lack of adverse events with a mean number of 53 ± 17. The BBB opening in three studies (Carpentier et al. [48], Idbaih et al. [51], and Chen et al. [52]) was mediated by intravenous (IV) bolus injection of SonoVue® microbubbles (Bracco Imaging, Milan, Italy) or by Definity microbubbles (Lantheus Medical Imaging, MA, USA). All the studies pointed to determine if BBB opening was safe, feasible, measurable, repeatable, and reversible without giving any major adverse event. French and Canadian collectives concurrently also administered chemotherapy (Carboplatin in Carpentier et al. [48] and Idbaih et al. [51], whilst Mainprize et al. [49] delivered Doxorubicin or TMZ). Chemotherapy delivery, penetrance and distribution still need further studies. Local inflammation and immunological induced response post-FUS were not explored. In those series no complications were found, and the temporary mean follow-up was of 13.7 ± 16.1 months. Progression-free survival (PFS) and overall survival (OS) were assessed only by Idbaih et al. [51] that demonstrated an increase of those parameters in FUS-BBB disruption (OS 12.94 vs. 8.64 months and PFS 4.11 vs. 2.73 months) compared to poor overture.

Table 4.
Demographic and pre- and post-FUS session summary data of FUS-BBB Opening studies.

Table 5.
Overall BBB Opening via FUS patient demographics, preoperative status and post-operative follow-up.
3.6. Ongoing Clinical Trials
Thirteen clinical trials are studying the impact of FUS on HGG treatment (11 about BBB opening and two about tumor ablation) (Table 6). Most of these studies are mainly focused on FUS-mediated BBB opening to increase chemotherapy delivery and distribution. The new ongoing clinical trials are performed with SonoCloud®, Exablate® and NaviFUS®. Zacharoulis et al. at Columbia University are evaluating FUS sonication with microbubbles aid to disrupt selectively and temporarily the BBB to augment oral Panobinostat concentration in children affected by diffuse midline gliomas (NCT04804709).

Table 6.
FUS clinical ongoing trials characteristics and promoters.
Sanai et al. at the Barrow Neurological Institute are using ascending doses of MR-guided FUS in combination with 5-ALA to assess safety and efficacity in recurrent HGG (NCT04559685). The measurement is made with Cleaved Caspase-3, MIB-1 level, GammaH2Ax in surgical specimen tissue. Another prospective study to be conducted at Besta Institute in Milan will evaluate the safety and feasibility of sonodynamic therapy with 5-ALA in patients with newly diagnosed GBM [53,54,55].
Sunnybrook Health Sciences Centre in Toronto is leading three different trials on tumor ablation, BBB opening and Carboplatin delivery through BBB (NCT01473485; NCT03616860; NCT04440358). A multicenter prospective trial between Baltimore, Boston, Charlottesville and Morgantown is investigating the safety and feasibility of periphery tumor cavity BBB disruption with adjuvant planned temozolomide infusion (NCT03551249). In Taiwan, a new prospective pilot study is investigating the efficacy and safety of FUS in BBB opening with complementary administration of bevacizumab in patients with recurrent glioblastoma (NCT04446416). Another multicenter collective, composed by teams in Palo Alto, Baltimore, Boston and Cleveland, is studying BBB disruption combined with IV carboplatin for recurrent GBM treatment (NCT04417088).
In Korea, Chang et al. are studying BBB disruption along the periphery of tumor resection cavity with a subsequent planned adjuvant TMZ chemotherapy (NCT03712293). Three new trials conducted with Sonocloud® aid are studying drug delivery through BBB opening. A phase 1 and 2 trial driven by Carpentier et al. is evaluating Sonocloud® efficacity on the dose limiting toxicity (DLT) of escalating numbers of ultrasound beams at constant acoustic pressure and its safety and efficacy of BBB opening (NCT03744026).
At Northwestern University, Stupp et al. are evaluating with a phase 1 and 2 trial an intraoperative implantation of Sonocloud® in order to open safely and repeatedly the BBB for Albumin-bound Paclitaxel chemotherapy (NCT04528680). The aim is to establish a safe and effective dose of Albumin-bound Paclitaxel through BBB opening. Finally, the multicentric SonoFIRST trial is studying if Sonocloud® could improve the PFS of newly diagnosed GBM patients, treated by concurrent chemo-radiotherapy and adjuvant temozolomide compared with patients with standard of care alone (NCT04614493).
4. Discussion
In our systematic review, we have selected all the studies reporting the results of FUS application in treating human HGG. As a matter of fact, our study demonstrates how very few studies exist in the literature (n = 9), and they are all preliminary experiences/trials conducted on small numbers of patients (n = 68).
4.1. FUS Direct Tumor Ablation
Five different studies investigated the effects of FUS in direct tumor ablation for treating HGG patients (n = 23). In this subpopulation, complication rates were high, with more than one patient out of four developing a hematoma in the targeted FUS area. However, this could be due to the use of early devices in a series of patients that did not benefit from previous knowledge regarding selection criteria and risk factors for this specific treatment. Only 6.4% of the patients had to stop FUS delivery due to complications, which represents an overall good tolerance during treatment delivery. The lack of data on patients’ neurological outcome at follow-up could be explained by the fact that most of the studies were case series reports or preliminary reports of ongoing trials. Future studies should provide details on patient selection and outcome and could possibly allow us to better select patients who can benefit most and with the lower risk of complications.
Guthkelch et al. [44] pointed out that post-craniectomy FUS is effective in causing necrosis within the adequately-heated tumor volume with a mean temperature higher than 42 °C. On the other hand, one limitation was the non-uniform power distribution that does not permit a non-uniform tumoral sonication. Ram et al. [32] showed how MR-guided FUS gave immediate changes in contrast-enhanced T1-, T2- and diffusion-weighted MRI scans, in addition to signs of thermocoagulation of the tumor confirmed by histological examination. Only three patients with GBM underwent MR-guided FUS thermal ablation. First, they had a craniectomy 7–10 days before sonication to get a bone window for better ultrasound transmission [32]. One of these patients had a lesion caused by thermal ablation of the normal surrounding brain parenchyma outside the target in the pathway of transmission of the ultrasound waves, leading to neurological deficits [32]. Park et al. [47] used FUS in one patient for whom any other therapeutic option was judged inadequate, showing a tumor volume and peripherical oedema diminution in the 6 months follow-up.
In 2010, McDannold et al. [33] managed, for the first time, to focus the ultrasound beams transcranially into the brain and visualizing heating with MR temperature imaging ExAblate 3000 system in three GBM patients. They were unable to reach a complete tumor ablation due to low power of FUS device (650–800 W), and the trial was stopped when a fourth patient suffered a cavitation induced fatal intracranial hemorrhage [33]. In 2014, Coluccia et al. [31] reported a case where a 63-year-old patient was treated for a centrally located recurrent GBM: intraoperative MR thermometry identified 17 of the 25 sonications as capable of thermocoagulation, with temperature between 55 °C and 65 °C. Immediate post-procedural diffusion-weighted MRI identified multiple bright lesions representing the thermally coagulated tissue in the targeted tumor volume, and during the follow-up the patient showed a neurological improvement of his pre-procedural deficits [31]. In tumor ablation papers, complete follow-up data are missing and complication rates seem high, a possible explanation could be that those were pioneering studies with small patient samples. For that reason, ongoing trials should validate the real potential of FUS ablation power.
The authors used different power and length of ultrasound sonication with a mean value of 30.3 ± 6.8 sonications per patient. In 16 patients the number of sonications was not reported [44,47]. Depending on different authors, target position, beam temperature and low-power verification sessions before sonications were applied, with a lower temperature that was augmented once the target was confirmed by MRI or CT-scan co-registration or overlap. Temperature was adapted considering patient compliance, discomfort and/or neurological symptoms. The mean follow-up for 19 patients was of 19.7 ± 14.6 months (four patients did not have a specified time range). In tumor ablation cohort, six patients (26.1%) presented a major FUS-related complication (four deep hematomas, one subcortical hematoma and one unwanted lesion in an eloquent area) (Table 3). No neurological outcome at long term follow-up was reported. No data was found concerning tumor volume stability.
4.2. FUS-Mediated BBB Opening
Despite abundant preclinical evidence indicating the efficacy of BBB disruption using low-intensity FUS for enhanced delivery of various chemotherapeutic agents and viral vectors to the CNS, evidence regarding safety and efficacy of this treatment method in humans with brain tumors remains limited. In our review, among the patients (n = 45) who received FUS-mediated BBB opening for treatment of cerebral HGG, no complications were found, and patients’ mean follow-up was 13.7 ± 16.1 months. Mainprize et al. [49] firstly demonstrated a possible safe and effective BBB transient opening with systemically administered chemotherapy with FUS and microbubbles before surgical debulking in a phase I single arm open label study. In fact, in this study five patients benefitted of TMZ (n = 4) or Doxorubicin (n = 1) administration with FUS, microbubbles injection (Definity®; Lantheus Medical Imaging, North Billerica, MA, USA) and MRI control. The authors demonstrated a radiographic evidence of an immediate 15–50% increased contrast enhancement on T1-weighted MRI at BBB level, without any significant ultrasound related clinical or radiological adverse event, validating the BBB opening. One hour prior to the procedure, the patient received the subtherapeutic dose of chemotherapy and in the following day, the patient had a tumor resection. The procedure was well-tolerated, with a successful opening of the BBB based on increased gadolinium enhancement at T1-weighted MRI [49]. Tissue liquid-chromatography mass spectrometry analysis demonstrated greater concentration of liposomal doxorubicin and oral TMZ in brain regions where BBB disruption occurred compared to areas without BBB disruption [49]. Ultrasound contrast agents, such as microbubbles, amplify focal heating during sonication and are used to reduce the time-averaged power needed during transcranial FUS ablation; for this purpose real-time passive acoustic mapping is really helpful to avoid unwanted cavitation [56,57,58,59].
Quantitative analysis of microbubbles is also helpful to understand their circulation and plan microbubble-mediated treatments [60]. The French Carthera group developed an implantable, low-intensity pulsed ultrasound device system that permits BBB disruption using pulsed ultrasound in combination with systemically injected microbubbles [48]. In 2016, Carpentier et al. [48] showed the preliminary results on 15 patients that benefitted from repeated sonication that permitted safe delivery of systemic chemotherapy with carboplatin. The BBB was disrupted at acoustic pressure levels up to 1.1 MPa without detectable adverse effects [48]. Subsequently, Ibdaih et al. [51] showed that patients who had a good post-FUS BBB disruption had an augmentation of PFS and OS. At least one sonication was achieved in 19 patients. BBB disruption was evaluated with contrast-enhanced T1-weighted brain MRI and was visible after 52 out of 65 ultrasound sessions [51]. The treatment was safe without serious adverse events or carboplatin related neurotoxicity. Finally, Chen et al. [50,52] reported promising results with NaviFUS®. In animal models, preliminary evidence of FUS-induced immune modulation is being provided as an additional therapeutic benefit by converting the immunosuppressive tumor microenvironment into an immunostimulatory tumor microenvironment [50,52]. On human patients, a safe, dose-dependent and auto-reversible BBB opening is achieved without major adverse events and with a simple and easy-to-use machine [50,52]. NaviFUS® seems to be extremely reliable for neurosurgeons as it does not need for a head-holder and a rapid trajectory planning can be performed with the aid of standard neuronavigation [50,52].
4.3. Ongoing Trials and Future Perspectives
To the best of our knowledge, most of the trials are involving adult patients and especially recurrent cases of GBM. BBB opening seems the most investigated subject. The promising results of French and Taiwanese collectives could pave the way for new treatment definition in HGG. Furthermore, US-guided therapy, as for general radiology, might be an option for brain therapy in the future: US transparent cranial prosthesis could be implanted after tumor resection in order to allow both diagnostic US-direct imaging alone, or as a guidance for FUS mediated therapies [55]. FUS implementation could, in fact, change in a cost-effective way new protocolled second-line treatment.
Ongoing trials should give concrete data on clinical status at long-term follow-up, validating the true ratio of adverse events. Even if excellent results were shown on animal models in post-FUS immunological acquired and innate awakening, major investigations should analyze this matter. A thoughtful consideration should be made regarding the therapeutic agents that are most likely to benefit from BBB opening to potentially make an oncological difference in future clinical studies: in fact, administration should be fast, easy, reproducible and effective.
The necessary repeated administration of the molecule should in particular be taken into account, permitting an effective and precise drug-delivery through the BBB.
Finally, two emerging preclinical applications of FUS could represent a future application in human brain tumor treatment. The first one takes advantage of BBB opening (using FUS in combination with microbubbles) to enhance the release of biomarkers from the brain tumor to the blood circulation, thereby allowing FUS-enabled brain tumor liquid biopsies [61]. Another important application will be the control of anti-tumor effect and functions of engineered immune cells such as chimeric antigen receptor T cells (CAR-T cells) within tumors. This “acoustogenetic” control, mediated by the heat generated by short pulses of FUS on a promoter for the heat-shock protein. can reversibly activate engineered T cells [62]. Besides efficacy, new future studies should evaluate carefully and exhaustively the safety profile of FUS ablation in order to compare and judge it relative to that of the more invasive but possibly proving ultimately safer Laser-Induced Interstitial Thermotherapy ablation [63,64].
5. Conclusions
FUS has been rarely applied for direct tumoral ablation in human HGG patients, but in current studies, it has shown some technical pitfalls and complications probably due to pioneer studies and lack of well-established inclusion criteria. Ongoing and future studies should provide data to improve patient selection and improve the risk-benefit ratio.
On the counterpart, FUS-mediated BBB opening is showing good results with very low complication rates. It seems to be a reliable technique to improve in local chemotherapy delivery and antitumoral immune response. Furthermore, this application could be complementary to surgical resection and standard radiochemotherapy in recurrent HGG. Ongoing trials should, in the near future, provide more data on FUS-mediated BBB opening impact on PFS and OS.
Author Contributions
Conceptualization L.P., A.M., F.P., D.M.; Data curation L.P., A.M., G.J., A.N.; Formal analysis L.P., A.M., T.R.M., F.P., D.M.; Data Interpretation L.P., A.M., G.J., A.N., F.D., J.P., T.R.M., S.M., K.S., F.P., D.M.; Writing L.P., A.M., T.R.M., F.P., D.M.; Critical Manuscript Revision L.P., A.M., G.J., A.N., F.D., J.P., T.R.M., S.M., K.S., F.P., D.M. All authors have read and agreed to the published version of the manuscript.
Funding
D.M. is supported by the ISREC Foundation, Swiss Bridge Foundation, Swiss Innovation Agency-Innosuisse, Ligue Genevoise contre le cancer, Ernst and Lucie Schmidheiny Foundation, PHRT-ETH Zurich, Association Frederic Fellay and the Henri Dubois-Ferrière Dinu Lipatti Foundation.
Data Availability Statement
All data are available in the text.
Conflicts of Interest
The authors have no conflict of interest to disclose.
Abbreviations
| HGG | High-grade glioma |
| FUS | Focused Ultrasound |
| BBB | Blood-Brain Barrier |
| US | Ultrasound |
| 5-ALA | 5-Aminolevulinic Acid |
| MRI | Magnetic Resonance Imaging |
| TMZ | Temozolomide |
| KPS | Karnofsky Performance Status |
| CT | Computed Tomography |
| RT | Radiotherapy |
| H | Hematoma |
| TND | Transient Neurological Deficit |
| NL | New Lesion |
| NA | Not Assessed |
| IV | Intravenous |
| GBM | Glioblastoma |
| AA | Anaplastic Astrocytoma |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| DLT | Dose Limiting Toxicity |
| RT | Radiotherapy |
References
- Briquard, P. Paul langevin. Ultrasonics 1972, 10, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Van Tiggelen, R.; Pouders, E. Ultrasound and computed tomography: Spin-offs of the world wars. JBR-BTR 2003, 86, 235–241. [Google Scholar] [PubMed]
- Gersten, J.W.; Kawashima, E. Recent advances in fundamental aspects of ultrasound and muscle. Br. J. Phys. Med. 1955, 18, 106–109. [Google Scholar] [PubMed]
- Gersten, J.W. Relation of ultrasound effects to the orientation of tendon in the ultrasound field. Arch. Phys. Med. Rehabil. 1956, 37, 201–209. [Google Scholar]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller, A.E. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, W.J.; Meyers, R. Ultrasonic method of modifying brain structures. Stereotact. Funct. Neurosurg. 1962, 22, 315–327. [Google Scholar] [CrossRef]
- Fry, W.J.; Mosberg, W.H.; Barnard, J.W.; Fry, F.J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 1954, 11, 471–478. [Google Scholar] [CrossRef]
- Vallancien, G.; Harouni, M.; Veillon, B.; Mombet, A.; Prapotnich, D.; Brisset, J.M.; Bougaran, J. Focused extracorporeal pyrotherapy: Feasibility study in man. J. Endourol. 1992, 6, 173–181. [Google Scholar] [CrossRef]
- Visioli, A.; Rivens, I.; ter Haar, G.; Horwich, A.; Huddart, R.; Moskovic, E.; Padhani, A.; Glees, J. Preliminary results of a phase I dose escalation clinical trial using focused ultrasound in the treatment of localised tumours. Eur. J. Ultrasound 1999, 9, 11–18. [Google Scholar] [CrossRef]
- Moiraghi, A.; Pallud, J. Intraoperative ultrasound techniques for cerebral gliomas resection: Usefulness and pitfalls. Ann. Transl. Med. 2020, 8, 523. [Google Scholar] [CrossRef]
- Moiraghi, A.; Prada, F.; Delaidelli, A.; Guatta, R.; May, A.; Bartoli, A.; Saini, M.; Perin, A.; Walchli, T.; Momjian, S.; et al. Navigated intraoperative 2-dimensional ultrasound in high-grade glioma surgery: Impact on extent of resection and patient outcome. Oper. Neurosurg. 2020, 18, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Prada, F.; Del Bene, M.; Moiraghi, A.; DiMeco, F. Echographic brain semeiology and topographic anatomy according to surgical approaches. In Intraoperative Ultrasound (IOUS) in Neurosurgery; Springer: Berlin, Germany, 2016; ISBN 9783319252681. [Google Scholar]
- Prada, F.; Vitale, V.; Del Bene, M.; Boffano, C.; Sconfienza, L.M.; Pinzi, V.; Mauri, G.; Solbiati, L.; Sakas, G.; Kolev, V.; et al. Contrast-enhanced MR Imaging versus Contrast-enhanced US: A comparison in glioblastoma surgery by using intraoperative fusion imaging. Radiology 2017, 285, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Unsgaard, G.; Gronningsaeter, A.; Ommedal, S.; Hernes, T.A.N. Brain operations guided by real-time two-result of improved image quality. Neurosurgery 2002, 51, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Incekara, F.; Smits, M.; Dirven, L.; Bos, E.M.; Balvers, R.K.; Haitsma, I.K.; Schouten, J.W.; Vincent, A.J.P.E. Intraoperative B-mode ultrasound guided surgery and the extent of glioblastoma resection: A randomized controlled trial. Front. Oncol. 2021, 11, 649797. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Moiraghi, A.; Roux, A.; Peeters, S.; Pelletier, J.B.; Baroud, M.; Trancart, B.; Oppenheim, C.; Lechapt, E.; Benevello, C.; Parraga, E.; et al. Feasibility, safety and impact on overall survival of awake resection for newly diagnosed supratentorial IDH-wildtype glioblastomas in adults. Cancers 2021, 13, 2911. [Google Scholar] [CrossRef]
- Pallud, J.; Zanello, M.; Moiraghi, A.; Peeters, S.; Trancart, B.; Edjlali, M.; Oppenheim, C.; Varlet, P.; Chrétien, F.; Dhermain, F.; et al. Surgery of insular diffuse gliomas-part 1: Transcortical awake resection is safe and independently improves overall survival. Neurosurgery 2021, 89, 565–578. [Google Scholar] [CrossRef]
- Pallud, J.; Roux, A.; Trancart, B.; Peeters, S.; Moiraghi, A.; Edjlali, M.; Oppenheim, C.; Varlet, P.; Chrétien, F.; Dhermain, F.; et al. Surgery of insular diffuse gliomas-part 2: Probabilistic cortico-subcortical atlas of critical eloquent brain structures and probabilistic resection map during transcortical awake resection. Neurosurgery 2021, 89, 579–590. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method: Clinical article. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef]
- Boex, C.; Haemmerli, J.; Momjian, S.; Schaller, K. Prognostic values of motor evoked potentials in insular, precental, or postcentral resections. J. Clin. Neurophysiol. 2016, 33, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Boëx, C.; Goga, C.; Bérard, N.; Al Awadhi, A.; Bartoli, A.; Meling, T.; Bijlenga, P.; Schaller, K. Intraoperative subcortico-cortical evoked potentials of the visual pathway under general anesthesia. Clin. Neurophysiol. 2021, 132, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.E.; Dallapiazza, R.; Huss, D.; Warren, A.L.; Sperling, S.; Gwinn, R.; Shah, B.B.; Elias, W.J. A randomized, sham-controlled trial of transcranial magnetic resonance-guided focused ultrasound thalamotomy trial for the treatment of tremor-dominant, idiopathic Parkinson disease. Neurosurgery 2016, 63, 154. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Meng, Y.; Suppiah, S.; Mithani, K.; Solomon, B.; Schwartz, M.L.; Lipsman, N. Current and emerging brain applications of MR-guided focused ultrasound. J. Ther. Ultrasound 2017, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.R.; Dallapiazza, R.; Jeff Elias, W. Neurological applications of transcranial high intensity focused ultrasound. Int. J. Hyperth. 2015, 31, 285–291. [Google Scholar] [CrossRef]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.H.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.S.; Jung, H.H.; Zadicario, E.; Rachmilevitch, I.; Tlusty, T.; Vitek, S.; Chang, J.W. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: Efficiency of acoustic energy delivery through the skull. J. Neurosurg. 2016, 124, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Hynynen, K.; McDannold, N.; Clement, G.; Jolesz, F.A.; Zadicario, E.; Killiany, R.; Moore, T.; Rosen, D. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain--a primate study. Eur. J. Radiol. 2006, 59, 149–156. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O’Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Ram, Z.; Cohen, Z.R.; Harnof, S.; Tal, S.; Faibel, M.; Nass, D.; Maier, S.E.; Hadani, M.; Mardor, Y. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006, 59, 949–955. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging—Guided focused ultrasound surgery of brain tumors. Neurosurgery 2010, 66, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, C.D.; Livingstone, M.S.; McDannold, N. Combined ultrasound and MR imaging to guide focused ultrasound therapies in the brain. Phys. Med. Biol. 2013, 58, 4749–4761. [Google Scholar] [CrossRef] [Green Version]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bijgaart, R.J.E.; Eikelenboom, D.C.; Hoogenboom, M.; Fütterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and mechanical high-intensity focused ultrasound: Perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol. Immunother. 2017, 66, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Zünkeler, B.; Carson, R.E.; Olson, J.; Blasberg, R.G.; DeVroom, H.; Lutz, R.J.; Saris, S.C.; Wright, D.C.; Kammerer, W.; Patronas, N.J.; et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J. Neurosurg. 1996, 85, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L.; Hochberg, F.H.; Rabinov, J.D.; Sorensen, A.G.; Lev, M.; Kim, L.; Weisskoff, R.M.; Gonzalez, R.G.; Gyldensted, C.; Rosen, B.R. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J. Neurosurg. 1999, 90, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused ultrasound strategies for brain tumor therapy. Oper. Neurosurg. 2020, 19, 9–18. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef] [Green Version]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- McDannold, N.J.; Vykhodtseva, N.I.; Hynynen, K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006, 241, 95–106. [Google Scholar] [CrossRef]
- Salameh, J.-P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef] [PubMed]
- Guthkelch, A.N.; Carter, L.P.; Cassady, J.R.; Hynynen, K.H.; Iacono, R.P.; Johnson, P.C.; Obbens, E.A.M.T.; Roemer, R.B.; Seeger, J.F.; Shimm, D.S.; et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J. Neurooncol. 1991, 10, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Hynynen, K.; Roemer, R.; Guthkelch, A.N.; Fleischer, A.S.; Shively, J. An ultrasound window to perform scanned, focused ultrasound hyperthermia treatments of brain tumors. Med. Phys. 1987, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; Roemer, R.; Anhalt, D.; Johnson, C.; Xu, Z.X.; Swindell, W.; Cetas, T. A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. Int. J. Hyperth. 1987, 3, 21–35. [Google Scholar] [CrossRef]
- Park, J.W.; Jung, S.; Jung, T.Y.; Lee, M.C. Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: A preliminary report. AIP Conf. Proc. 2006, 829, 238. [Google Scholar]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: A clinical safety and feasibility study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-T.; Chai, W.-Y.; Lin, Y.-J.; Lin, C.-J.; Chen, P.-Y.; Tsai, H.-C.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-T.; Lin, Y.-J.; Chai, W.-Y.; Lin, C.-J.; Chen, P.-Y.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: Clinical trial protocol. Ann. Transl. Med. 2020, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, L.; D’Ammando, A.; Gionso, M.; Sheybani, N.D.; Lopes, M.-B.; Moore, D.; Allen, S.; Gatesman, J.; Porto, E.; Timbie, K.; et al. Intracranial sonodynamic therapy with 5-aminolevulinic acid and sodium fluorescein: Safety study in a porcine model. Front. Oncol. 2021, 11, 679989. [Google Scholar] [CrossRef]
- D’Ammando, A.; Raspagliesi, L.; Gionso, M.; Franzini, A.; Porto, E.; Di Meco, F.; Durando, G.; Pellegatta, S.; Prada, F. Sonodynamic therapy for the treatment of intracranial gliomas. J. Clin. Med. 2021, 10, 1101. [Google Scholar] [CrossRef]
- Prada, F.; Franzini, A.; Moosa, S.; Padilla, F.; Moore, D.; Solbiati, L.; DiMeco, F.; Legon, W. In vitro and in vivo characterization of a cranial window prosthesis for diagnostic and therapeutic cerebral ultrasound. J. Neurosurg. 2020, 134, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.T.; Apostolakis, I.; Konofagou, E.E. Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles. Phys. Med. Biol. 2018, 63, 65009. [Google Scholar] [CrossRef] [PubMed]
- Pouliopoulos, A.N.; Jimenez, D.A.; Frank, A.; Robertson, A.; Zhang, L.; Kline-Schoder, A.R.; Bhaskar, V.; Harpale, M.; Caso, E.; Papapanou, N.; et al. Temporal stability of lipid-shelled microbubbles during acoustically-mediated blood-brain barrier opening. Front. Phys. 2020, 8, 137. [Google Scholar] [CrossRef]
- Konofagou, E.E.; Tunga, Y.-S.; Choia, J.; Deffieuxa, T.; Baseria, B.; Vlachosa, F. Ultrasound-induced blood-brain barrier opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef]
- Konofagou, E.E. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics 2012, 2, 1223–1237. [Google Scholar] [CrossRef]
- Prada, F.; Gennari, A.G.; Linville, I.M.; Mutersbaugh, M.E.; Chen, Z.; Sheybani, N.; DiMeco, F.; Padilla, F.; Hossack, J.A. Quantitative analysis of in-vivo microbubble distribution in the human brain. Sci. Rep. 2021, 11, 11797. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, G.; Ye, D.; Nazeri, A.; Yue, Y.; Liu, W.; Wang, X.; Dunn, G.P.; Petti, A.A.; Leuthardt, E.C.; et al. Focused ultrasound-enabled brain tumor liquid biopsy. Sci. Rep. 2018, 8, 6553. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y.; et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jermakowicz, W.J.; Mahavadi, A.K.; Cajigas, I.; Dan, L.; Guerra, S.; Farooq, G.; Shah, A.H.; D’Haese, P.F.; Ivan, M.E.; Jagid, J.R.; et al. Predictive modeling of brain tumor laser ablation dynamics. J. Neurooncol. 2019, 144, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, O.; Arzumanov, G.; Luther, E.; McMahon, J.T.; Malcolm, J.G.; Mansour, S.; Lee, I.Y.; Willie, J.T.; Komotar, R.J.; Danish, S.F. Magnetic resonance-guided laser interstitial thermal therapy for posterior fossa neoplasms. J. Neurooncol. 2020, 149, 533–542. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).