Serum Total Bilirubin and Risk of Cancer: A Swedish Cohort Study and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. AMORIS Cohort
2.1.1. Study Population and Data Collection
2.1.2. Data Analysis
2.2. Systematic Review and Meta-Analysis
2.2.1. Literature Search Strategy
2.2.2. Meta-Analysis Statistical Techniques
3. Result
3.1. AMORIS Cohort
3.2. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyed Khoei, N.; Anton, G.; Peters, A.; Freisling, H.; Wagner, K.H. The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany. Antioxidants 2020, 9, 908. [Google Scholar] [CrossRef]
- Keshavan, P.; Schwemberger, S.J.; Smith, D.L.; Babcock, G.F.; Zucker, S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer 2004, 112, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.D.; Goessling, W.; Hoppin, A.G. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J. Biol. Chem. 1999, 274, 10852–10862. [Google Scholar] [CrossRef] [Green Version]
- Seyed Khoei, N.; Jenab, M.; Murphy, N.; Banbury, B.L.; Carreras-Torres, R.; Viallon, V.; Kühn, T.; Bueno-de-Mesquita, B.; Aleksandrova, K.; Cross, A.J.; et al. Circulating bilirubin levels and risk of colorectal cancer: Serological and Mendelian randomization analyses. BMC Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- King, D.; Armstrong, M.J. Overview of Gilbert’s syndrome. Drug Ther Bull. 2019, 57, 27–31. [Google Scholar] [CrossRef]
- Ohnaka, K.; Kono, S. Bilirubin, cardiovascular diseases and cancer: Epidemiological perspectives. Expert Rev. Endocrinol. Metab. 2010, 5, 891–904. [Google Scholar] [CrossRef]
- Wagner, K.-H.; Wallner, M.; Moelzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin. Sci. (Lond.) 2015, 129, 1–25. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Wu, T.W.; Wu, J.; Li, R.K.; Mickle, D.; Carey, D. Albumin-bound bilirubins protect human ventricular myocytes against oxyradical damage. Biochem. Cell Biol. 1991, 69, 683–688. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Liou, I.W.; Weiss, N.S. Serum bilirubin and colorectal cancer risk: A population-based cohort study. Aliment. Pharmacol. Ther. 2006, 23, 1637–1642. [Google Scholar] [CrossRef]
- Kühn, T.; Sookthai, D.; Graf, M.E.; Schübel, R.; Freisling, H.; Johnson, T.; Katzke, V.; Kaaks, R. Albumin, bilirubin, uric acid and cancer risk: Results from a prospective population-based study. Br. J. Cancer 2017, 117, 1572–1579. [Google Scholar] [CrossRef] [Green Version]
- Zucker, S.D.; Horn, P.S.; Sherman, K.E. Serum bilirubin levels in the U.S. population: Gender effect and inverse correlation with colorectal cancer. Hepatology 2004, 40, 827–835. [Google Scholar] [CrossRef]
- Zucker, S.D.; Benedict, M.; Sherman, K.E. Serum bilirubin and risk of colorectal cancer. Aliment. Pharmacol Ther. 2006, 24, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Jirásková, A.; Novotný, J.; Novotný, L.; Vodicka, P.; Pardini, B.; Naccarati, A.; Schwertner, H.A.; Hubácek, J.A.; Puncochárová, L.; Šmerhovský, Z.; et al. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int. J. Cancer 2012, 131, 1549–1555. [Google Scholar] [CrossRef]
- Horsfall, L.J.; Burgess, S.; Hall, I.; Nazareth, I. Genetically raised serum bilirubin levels and lung cancer: A cohort study and Mendelian randomisation using UK Biobank. Thorax 2020, 75, 955–964. [Google Scholar] [CrossRef]
- Wen, C.-P.; Zhang, F.; Liang, D.; Wen, C.; Gu, J.; Skinner, H.D.; Chow, W.-H.; Ye, Y.; Pu, X.; Hildebrandt, M.A.; et al. The ability of bilirubin in identifying smokers with higher risk of lung cancer: A large cohort study in conjunction with global metabolomic profiling. Clin. Cancer Res. 2015, 21, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.E.; Kimm, H.; Jee, S.H. Combined effects of smoking and bilirubin levels on the risk of lung cancer in Korea: The severance cohort study. PLoS ONE 2014, 9, e103972. [Google Scholar] [CrossRef] [PubMed]
- Temme, E.H.; Zhang, J.; Schouten, E.G.; Kesteloot, H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001, 12, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.L.; Mägi, R.; et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef] [PubMed]
- Klonoff-Cohen, H.; Barrett-Connor, E.L.; Edelstein, S.L. Albumin levels as a predictor of mortality in the healthy elderly. J. Clin. Epidemiol. 1992, 45, 207–212. [Google Scholar] [CrossRef]
- Phillips, A.; Shaper, A.G.; Whincup, P.H. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 1989, 2, 1434–1436. [Google Scholar] [CrossRef]
- Sollie, S.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Santaolalla, A.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer 2019, 19, 858. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Zhang, P.; Xu, W.; Liu, Y.; Wang, B.; Jiang, T.; Hua, C.; Wang, X.; Xu, D.; Sun, B. Serum Uric Acid Increases Risk of Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2015, 2015, 764250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasak, A.M.; Rapp, K.; Hilbe, W.; Oberaigner, W.; Ruttmann, E.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H. The role of serum uric acid as an antioxidant protecting against cancer: Prospective study in more than 28 000 older Austrian women. Ann. Oncol. 2007, 18, 1893–1897. [Google Scholar] [CrossRef]
- Kolonel, L.N.; Yoshizawa, C.; Nomura, A.M.; Stemmermann, G.N. Relationship of serum uric acid to cancer occurrence in a prospective male cohort. Cancer Epidemiol. Biomark. Prev. 1994, 3, 225–228. [Google Scholar]
- Van Hemelrijck, M.; Garmo, H.; Binda, E.; Hayday, A.; Karagiannis, S.N.; Hammar, N.; Walldius, G.; Lambe, M.; Jungner, I.; Holmberg, L. Immunoglobulin E and cancer: A meta-analysis and a large Swedish cohort study. Cancer Causes Control. 2010, 21, 1657–1667. [Google Scholar] [CrossRef]
- Holme, I.; Aastveit, A.H.; Jungner, I.; Walldius, G. Relationships between lipoprotein components and risk of myocardial infarction: Age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS). J. Intern. Med. 2008, 264, 30–38. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- Horsfall, L.J.; Rait, G.; Walters, K.; Swallow, D.M.; Pereira, S.P.; Nazareth, I.; Petersen, I. Serum bilirubin and risk of respiratory disease and death. JAMA 2011, 305, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Inoguchi, T.; Nohara, Y.; Nojiri, C.; Nakashima, N. Association of serum bilirubin levels with risk of cancer development and total death. Sci. Rep. 2021, 11, 13224. [Google Scholar] [CrossRef]
- Troche, J.R.; Mayne, S.T.; Freedman, N.D.; Shebl, F.M.; Guertin, K.A.; Cross, A.J.; Abnet, C.C. Alcohol Consumption-Related Metabolites in Relation to Colorectal Cancer and Adenoma: Two Case-Control Studies Using Serum Biomarkers. PLoS ONE 2016, 11, e0150962. [Google Scholar] [CrossRef] [Green Version]
- Walia, T.; Quevedo, J.F.; Hobday, T.J.; Croghan, G.; Jatoi, A. Colorectal cancer patients with liver metastases and severe hyperbilirubinemia: A consecutive series that explores the benefits and risks of chemotherapy. Ther. Clin. Risk Manag. 2008, 4, 1363–1366. [Google Scholar]
- Ching, S.; Ingram, D.; Hahnel, R.; Beilby, J.; Rossi, E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J. Nutr. 2002, 132, 303–306. [Google Scholar] [CrossRef]
- Saffari-Chaleshtori, J.; Heidarian, E.; Shafiee, S.M. Apoptotic Effects of Bilirubin on Skin Cancer Cell Lines SK-MEL-3 (Melanoma) and A431 (Non-Melanoma). Anticancer Agents Med. Chem. 2021, 21, 1871–1882. [Google Scholar] [CrossRef]
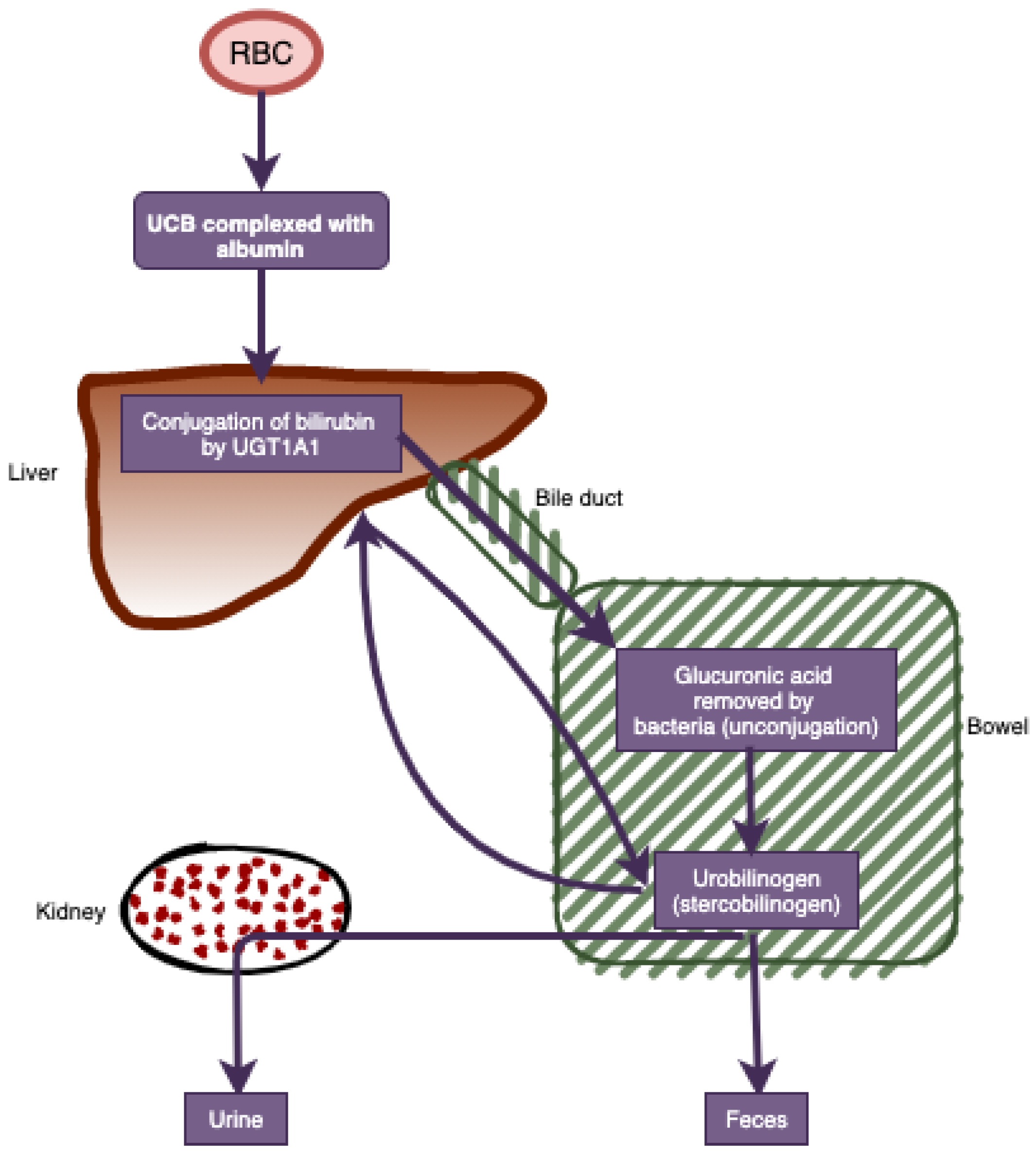
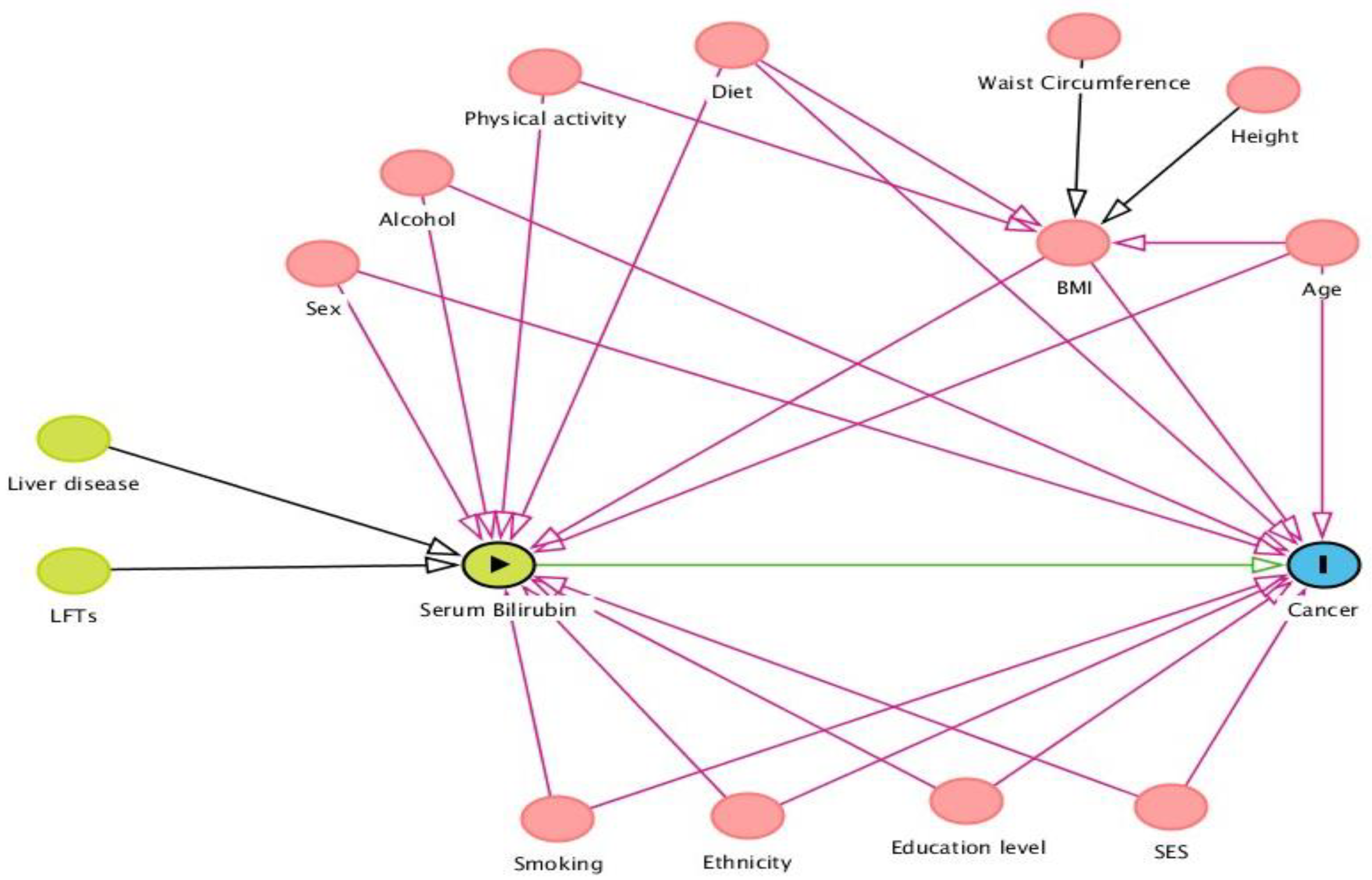

| Non-Cancer (n = 109,350) | Cancer (n = 27,695) | Total (n = 137,045) | |
|---|---|---|---|
| Sex | |||
| Male | 58,800 (53.8) | 15,498 (56) | 74,298 (54.2) |
| Female | 50,550 (46.2) | 12,197 (44) | 62,747 (45.8) |
| Age (Median, SD) | 46.3 (14.9) | 53.3 (12) | 11.3 (7) |
| SES | |||
| Low | 48,145 (44) | 11,450 (41.3) | 59,595 (43.5) |
| Medium | 48,643 (44.5) | 13,826 (49.9) | 62,469 (45.6) |
| High | 12,562 (11.5) | 2419 (8.7) | 14,981 (10.9) |
| Bilirubin | |||
| Continuous | 11.3 (7.2) | 11.4 (5.9) | |
| Q1 | 35,214 (32.2) | 7982 (28.8) | 43,196 (31.5) |
| Q3 | 22,645 (20.7) | 6089 (22) | 28,734 (21) |
| Q3 | 25,612 (23.4) | 6983 (25.2) | 32,595 (23.8) |
| Q4 | 25,879 (23.7) | 6641 (24) | 32,520 (23.7) |
| Number of comorbidities | |||
| 0 | 101,058 (92.4) | 25,533 (92.2) | 126,591 (92.4) |
| 1 | 5582 (5.1) | 1562 (5.6) | 7144 (5.2) |
| 2 | 1537 (1.4) | 356 (1.3) | 1893 (1.4) |
| ≥3 | 1173 (1.1) | 244 (0.9) | 1417 (1.0) |
| Fasting status | |||
| No | 34,336 (31.4) | 8554 (30.9) | 42,890 (31.3) |
| Yes | 52,628 (48.1) | 15,394 (55.6) | 68,022 (49.6) |
| NK | 22,386 (20.5) | 3747 (13.5) | 26,133 (19.1) |
| Education category | |||
| 1 | 30,861 (28.2) | 8842 (31.9) | 39,704 (29) |
| 2 | 44,618 (40.8) | 10,978 (39.6) | 55,596 (40.6) |
| 3 | 26,762 (24.5) | 6346 (22.9) | 33,108 (24.2) |
| NK | 7108 (6.5) | 1529 (5.5) | 8637 (6.3) |
| BMI (mean, SD) | |||
| 24.6 (5.7) | 24.9 (3.9) | 24.6 (5.4) | |
| ≤24.9 | 5810 (5.3) | 1326 (4.8) | 7136 (5.2) |
| 25–29.9 | 2963 (2.7) | 865 (3.1) | 3828 (2.8) |
| ≥30 | 767 (0.7) | 212 (0.8) | 979 (0.7) |
| Missing | 99,810 (91.3) | 25,292 (91.3) | 125,102 (91.3) |
| Overall N = 27,695 | Men N = 15,498 | Women N = 12,197 | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | HR | 95%CI | |
| Continuous | 0.99 | 0.99–1.00 | 0.97 | 0.94–1.01 | 0.99 | 0.98–1.00 |
| Q1 ≤8 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| Q2 9–10 | 0.97 | 0.94–1.00 | 1.00 | 0.82–1.22 | 0.99 | 0.94–1.04 |
| Q3 11–13 | 0.96 | 0.92–0.99 | 0.85 | 0.70–1.04 | 1.00 | 0.95–1.05 |
| Q4 ≥14 | 0.96 | 0.93–1.00 | 1.07 | 0.90–1.29 | 0.98 | 0.93–1.04 |
| Continuous | Q1 ≤8 | Q2 9–10 | Q3 11–13 | Q4 ≥13 | |
|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Any Cancer | 0.99 (0.99–1.00) | 1.00 (Ref) | 0.97 (0.94–1.00) | 0.96 (0.92–0.99) | 0.96 (0.93–1.00) |
| Colorectal | 0.99 (0.98–1.00) | 1.00 (Ref) | 0.94 (0.85–1.03) | 0.91 (0.82–1.00) | 0.92 (0.84–1.02) |
| Lung | 0.94 (0.93–0.95) | 1.00 (Ref) | 0.74 (0.64–0.84) | 0.66 (0.58–0.75) | 0.50 (0.44–0.59) |
| Breast | 1.00 (0.99–1.01) | 1.00 (Ref) | 1.09 (1.00–1.20) | 1.06 (0.97–1.17) | 1.13 (1.01–1.25) |
| Gynaecological | 0.99 (0.98–1.00) | 1.00 (Ref) | 1.02 (0.92–1.14) | 0.97 (0.86–1.09) | 0.86 (0.76–0.99) |
| Prostate | 1.00 (0.99–1.00) | 1.00 (Ref) | 0.94 (0.86–1.03) | 0.97 (0.89–1.05) | 1.00 (0.93–1.09) |
| Bladder | 0.99 (0.97–1.99) | 1.00 (Ref) | 0.89 (0.75–1.07) | 0.88 (0.75–1.05) | 0.91 (0.77–1.08) |
| Melanoma | 1.01 (1.00–1.01) | 1.00 (Ref) | 1.15 (0.97–1.35) | 1.08 (0.92–1.28) | 1.25 (1.06–1.47) |
| Haematological | 1.00 (0.99–1.01) | 1.00(Ref) | 1.06 (0.92–1.23) | 0.94 (0.81–1.08) | 1.09 (0.95–1.26) |
| Overall N = 1875 | Smokers N = 82 | Non-Smokers N = 102 | ||||
|---|---|---|---|---|---|---|
| Bilirubin levels | ||||||
| Mean (SD) | 10.3 (4.6) | 10.1 (8.4) | 10.3 (3.6) | |||
| HR | 95%CI | HR (N) | 95%CI | HR (N) | 95%CI | |
| Continuous | 0.94 | 0.93–0.95 | 1.00 | 0.96–1.04 | 0.92 | 0.87–0.97 |
| Q1 ≤8 | 1.00 | Ref | 1.00 (29) | Ref | 1.00 (40) | Ref |
| Q2 9–10 | 0.73 | 0.64–0.84 | 0.59 25 | 0.31–1.10 | 0.93 (14) | 0.54–1.61 |
| Q3 11–13 | 0.65 | 0.58–0.75 | 0.53 33 | 0.27–1.03 | 1.02 (15) | 0.61–1.70 |
| Q4 ≥14 | 0.50 | 0.44–0.59 | 0.84 15 | 0.44–1.60 | 0.45 (13) | 0.24–0.86 |
| p-trend | 0.000 | 0.241 | 0.040 | |||
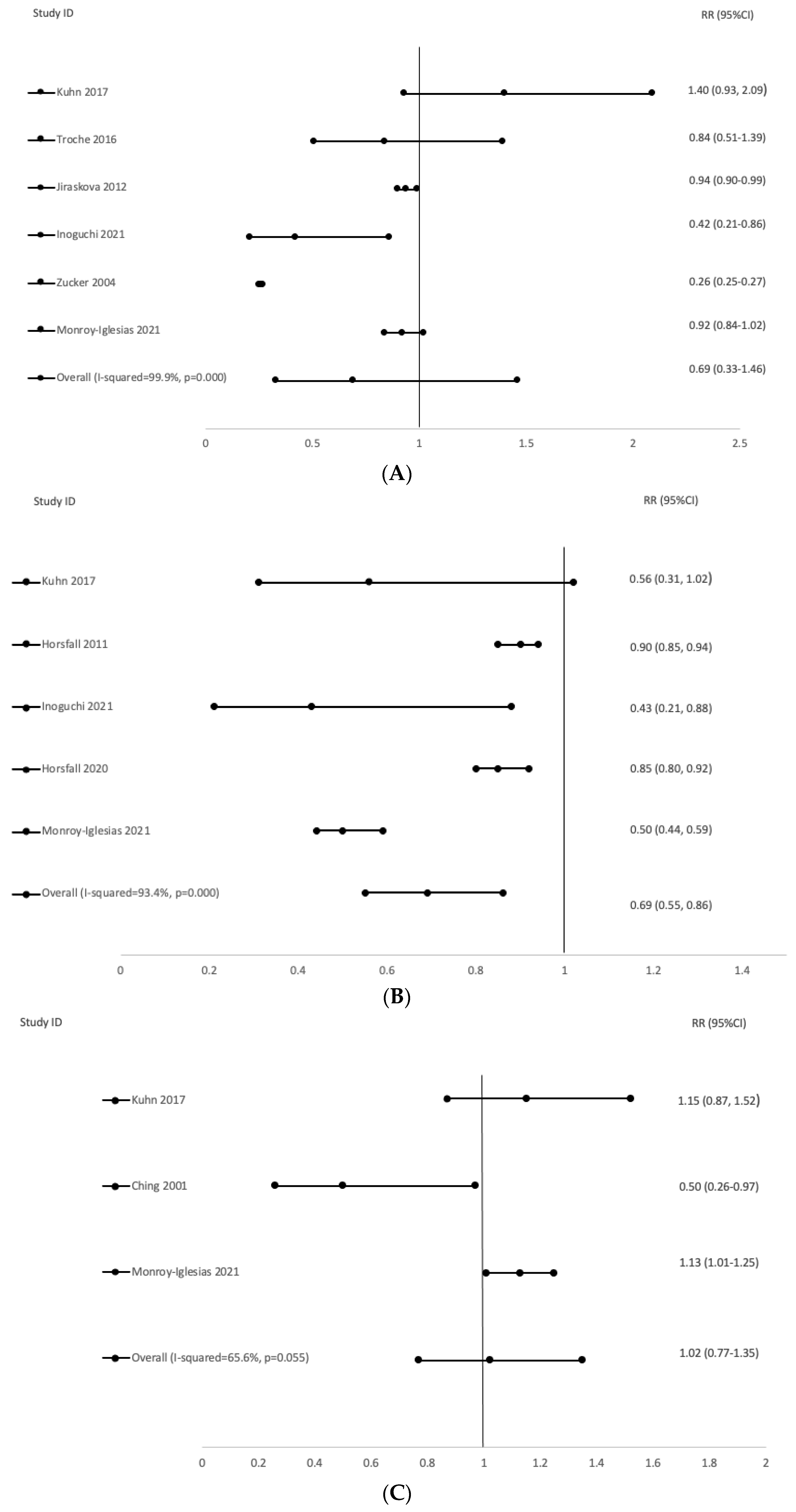
| Author, Year | Country | Study Type | Population | Follow-Up | Exposure | Outcome | Findings | Confounders/Adjustments | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Kuhn, 2017 | EPIC | Cohort | 627 BC, 554 PC, 195 LC, 256 CRC cases | 14.8 | Serum bilirubin | BC, PC, CRC, LC risk | No significant associations | Age, smoking, alcohol, aspirin use, PA, WC, BMI, height, education level. BC +hormonal factors CRC + diet | Also looking at albumin and uric acid |
| Inoguchi, 2021 | Japan | Cohort | 403 LC; 315 CRC | 4.7 | Serum bilirubin | LC, CRC, BC, PC, cervical risk | Decreased LC risk in men (HR 0.47; 95%CI 0.27–0.82); no significant association for women. Decreased CRC risk (HR 0.42; 95%CI 0.21–0.86). | ||
| Troche, 2016 | USA (PLCO, NCAS) | Case-Control (2) | PLCO- 252 cases, 250 controls; NCAS- 120 cases, 77 controls | 15 | Serum bilirubin stratified by alcohol consumption | CRC risk | No significant associations in either study | Age, sex and smoking | |
| Jirásková, 2011 | Czech Republic | Case-Control | 777 cases and 986 controls | NK | Serum bilirubin | CRC risk | Decreased CRC risk (OR 0.94; 95%CI 0.90–0.99) Decreased CRC risk men (0.93; 95%CI 0.87–0.99) Decreased CRC risk women (0.92; 95%CI 0.85–1.00, p = 0.05) | Sex and age | Per mmol increase of serum bilirubin |
| Zucker, 2004 | USA | Cohort | 83 cases | NK | Serum bilirubin | CRC risk | Decreased CRC risk (OR 0.257; 95%CI 0.254–0.260) | Per 1 mg/dL increase in bilirubin levels. | |
| Horsfall, 2011 | USA | Cohort | 1341 cases | 8.3 | Serum bilirubin | LC risk | Decreased LC risk in men (IRR 0.92; 95%CI 0.89–0.95) Decreased LC risk in women (IRR 0.89; 95%CI 0.86–0.93) | Age, BMI, systolic blood pressure, smoking status, alcohol intake and SES | Per 0.1 mg/dL increase in bilirubin |
| Horsfall, 2020 | UK | Cohort | 2002 cases | NK | Serum bilirubin | LC risk | Decreased LC risk (IRR 0.85; 95%CI 0.80–0.92) | Age, gender, calendar year, ethnicity, height, weight, recruitment centre and smoking status | Per 5 nmol/L increase in bilirubin levels. |
| Ching, 2001 | Australia | Case-Control | 153 cases, 151 controls | NK | Serum bilirubin | BC risk | Decreased BC risk (OR 0.50; 95%CI 0.26–0.97) | Age at menarche, parity, dietary fat and alcohol intake |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Iglesias, M.J.; Moss, C.; Beckmann, K.; Hammar, N.; Walldius, G.; Bosco, C.; Van Hemelrijck, M.; Santaolalla, A. Serum Total Bilirubin and Risk of Cancer: A Swedish Cohort Study and Meta-Analysis. Cancers 2021, 13, 5540. https://doi.org/10.3390/cancers13215540
Monroy-Iglesias MJ, Moss C, Beckmann K, Hammar N, Walldius G, Bosco C, Van Hemelrijck M, Santaolalla A. Serum Total Bilirubin and Risk of Cancer: A Swedish Cohort Study and Meta-Analysis. Cancers. 2021; 13(21):5540. https://doi.org/10.3390/cancers13215540
Chicago/Turabian StyleMonroy-Iglesias, Maria J., Charlotte Moss, Kerri Beckmann, Niklas Hammar, Goran Walldius, Cecilia Bosco, Mieke Van Hemelrijck, and Aida Santaolalla. 2021. "Serum Total Bilirubin and Risk of Cancer: A Swedish Cohort Study and Meta-Analysis" Cancers 13, no. 21: 5540. https://doi.org/10.3390/cancers13215540
APA StyleMonroy-Iglesias, M. J., Moss, C., Beckmann, K., Hammar, N., Walldius, G., Bosco, C., Van Hemelrijck, M., & Santaolalla, A. (2021). Serum Total Bilirubin and Risk of Cancer: A Swedish Cohort Study and Meta-Analysis. Cancers, 13(21), 5540. https://doi.org/10.3390/cancers13215540







