Proteomics as a Complementary Technique to Characterize Bladder Cancer
Abstract
Simple Summary
Abstract
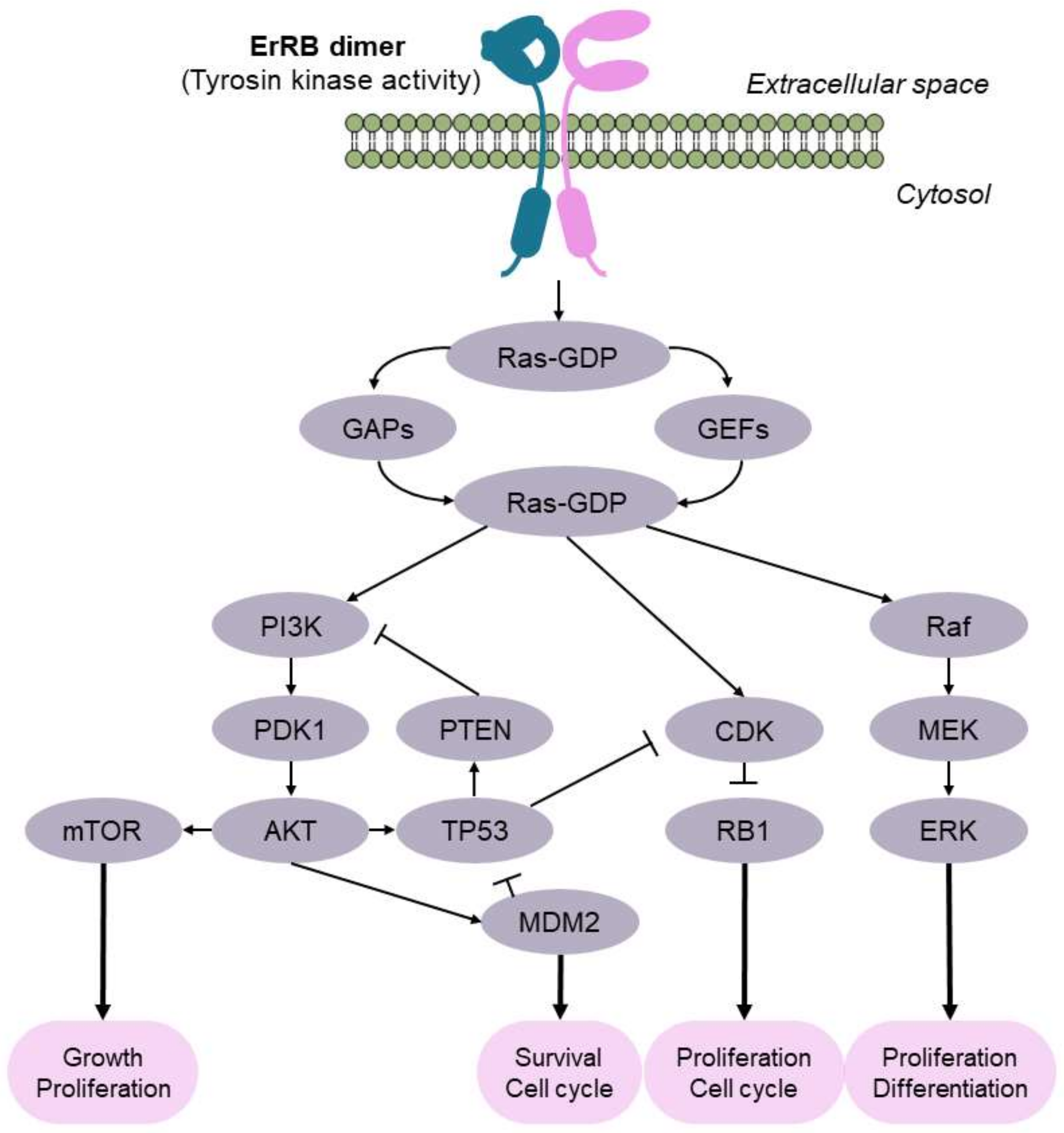
1. Introduction
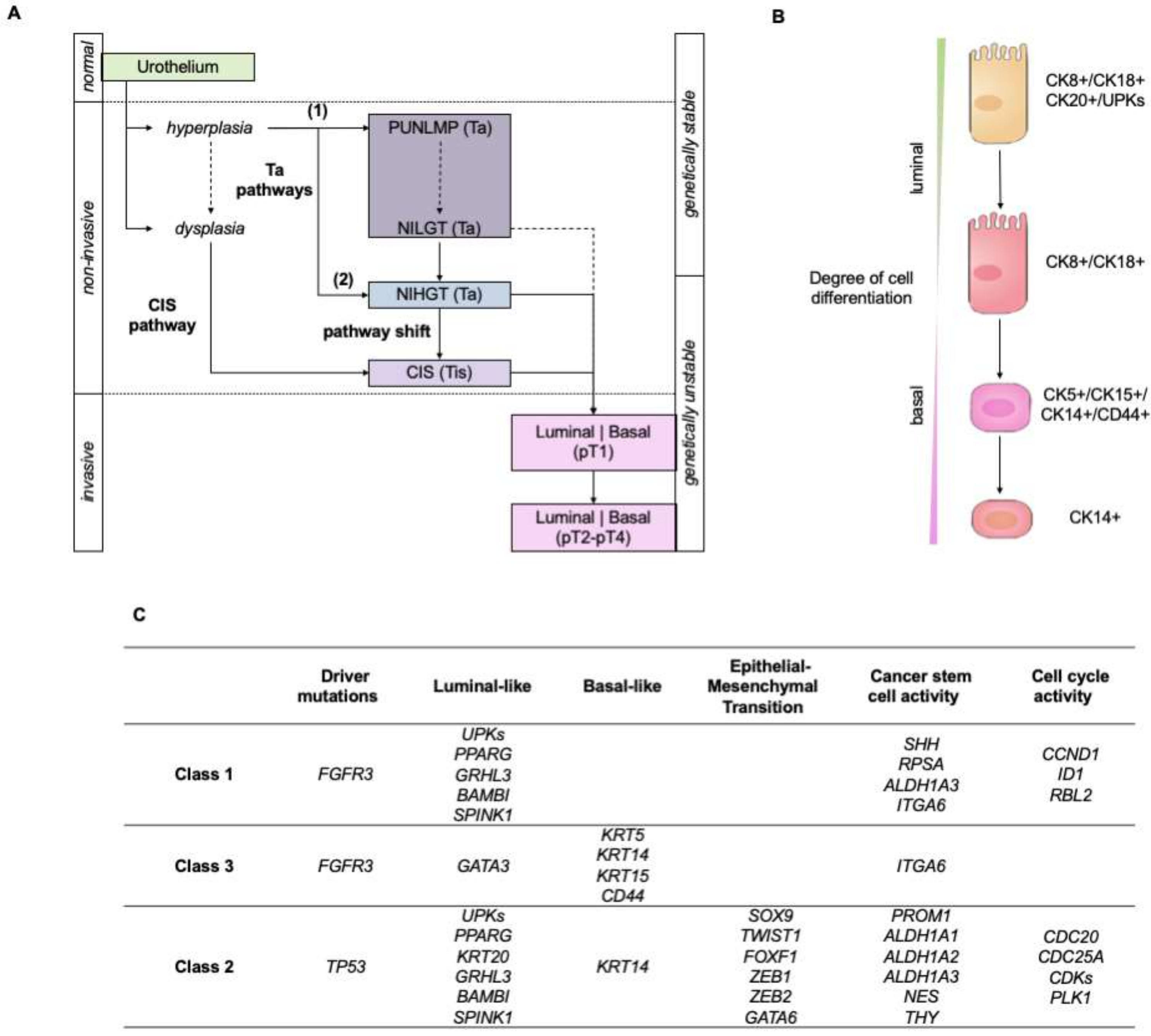
The Pathways of Bladder Tumorigenesis
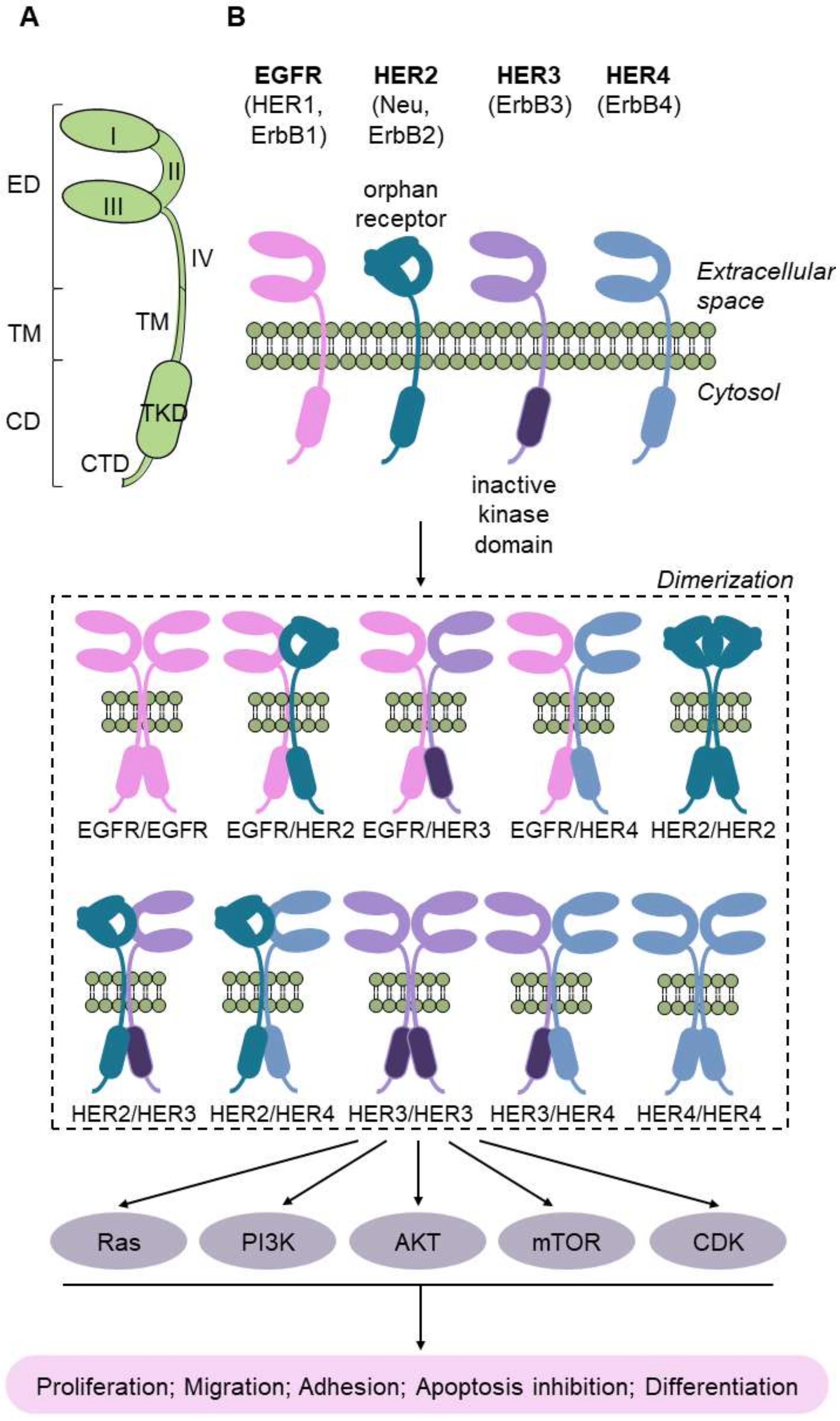
2. Omics Sciences in the Molecular Classification of Bladder Cancer
2.1. Molecular Classifications of Bladder Cancer Based on the Transcriptome
2.2. Molecular Classifications of Bladder Cancer Based on Proteomics
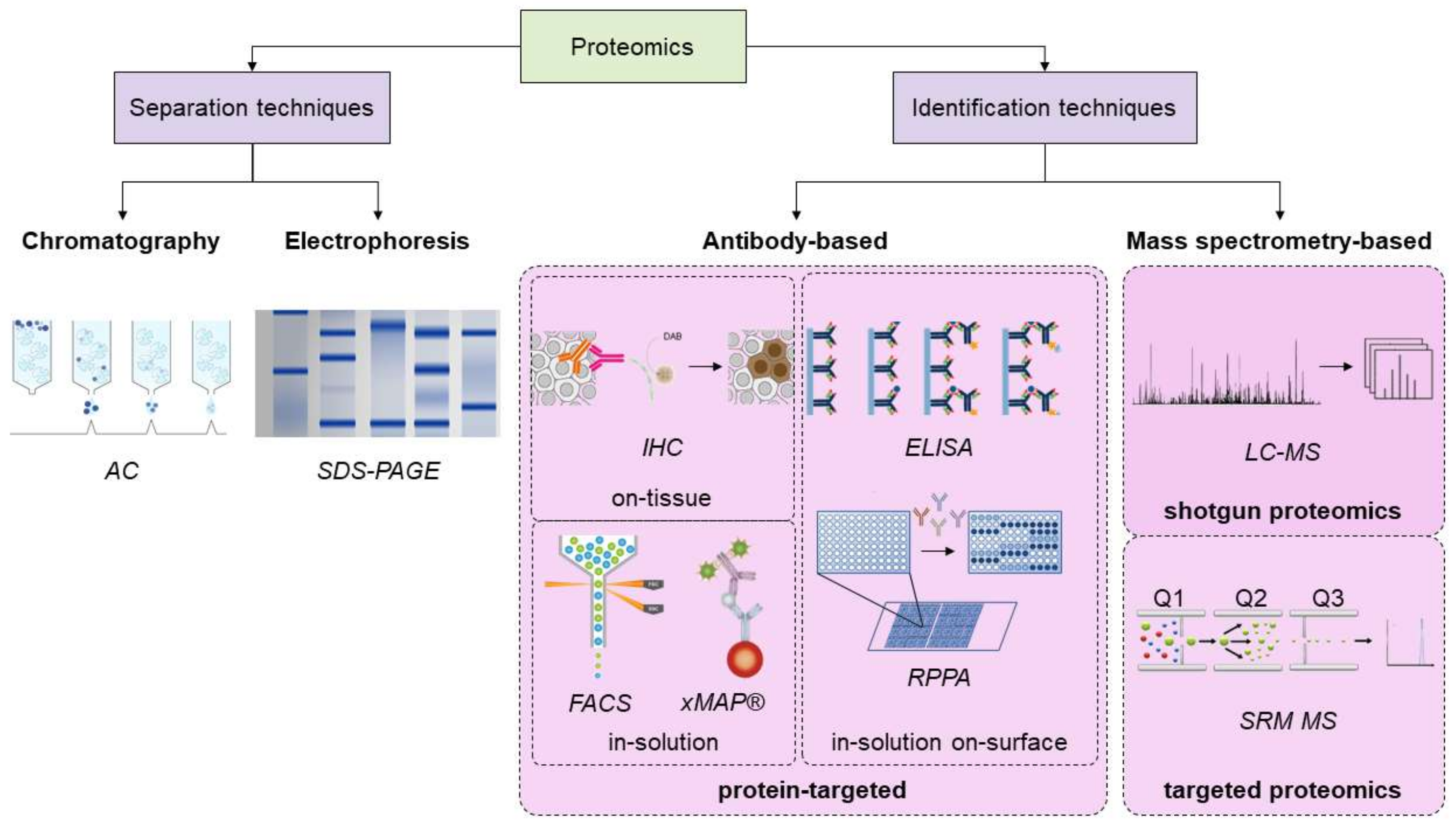
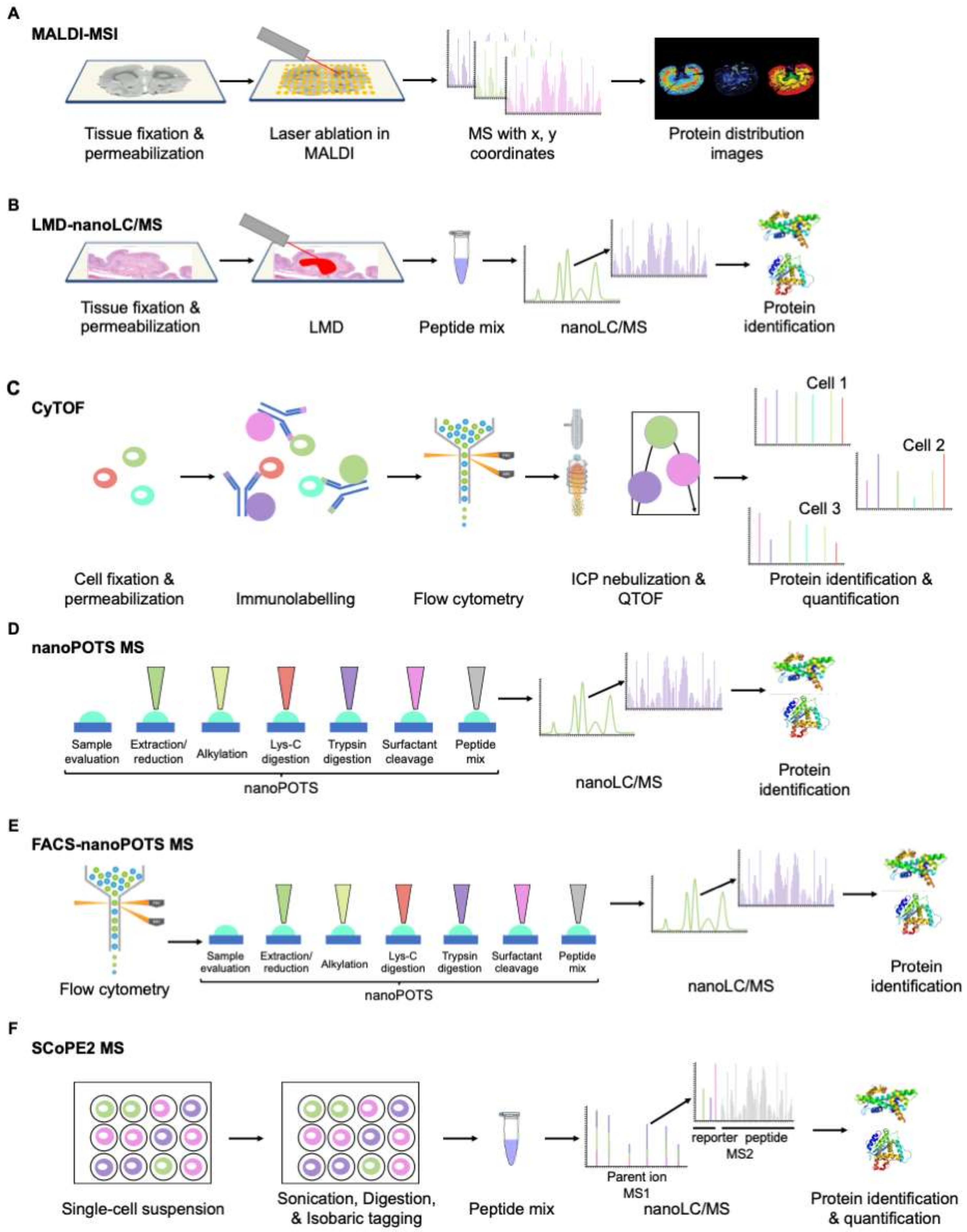
3. Overview of the Proteomics Techniques, and Novel Advances in the Field
4. Insights into the Histopathology of Bladder Cancer by Proteomics
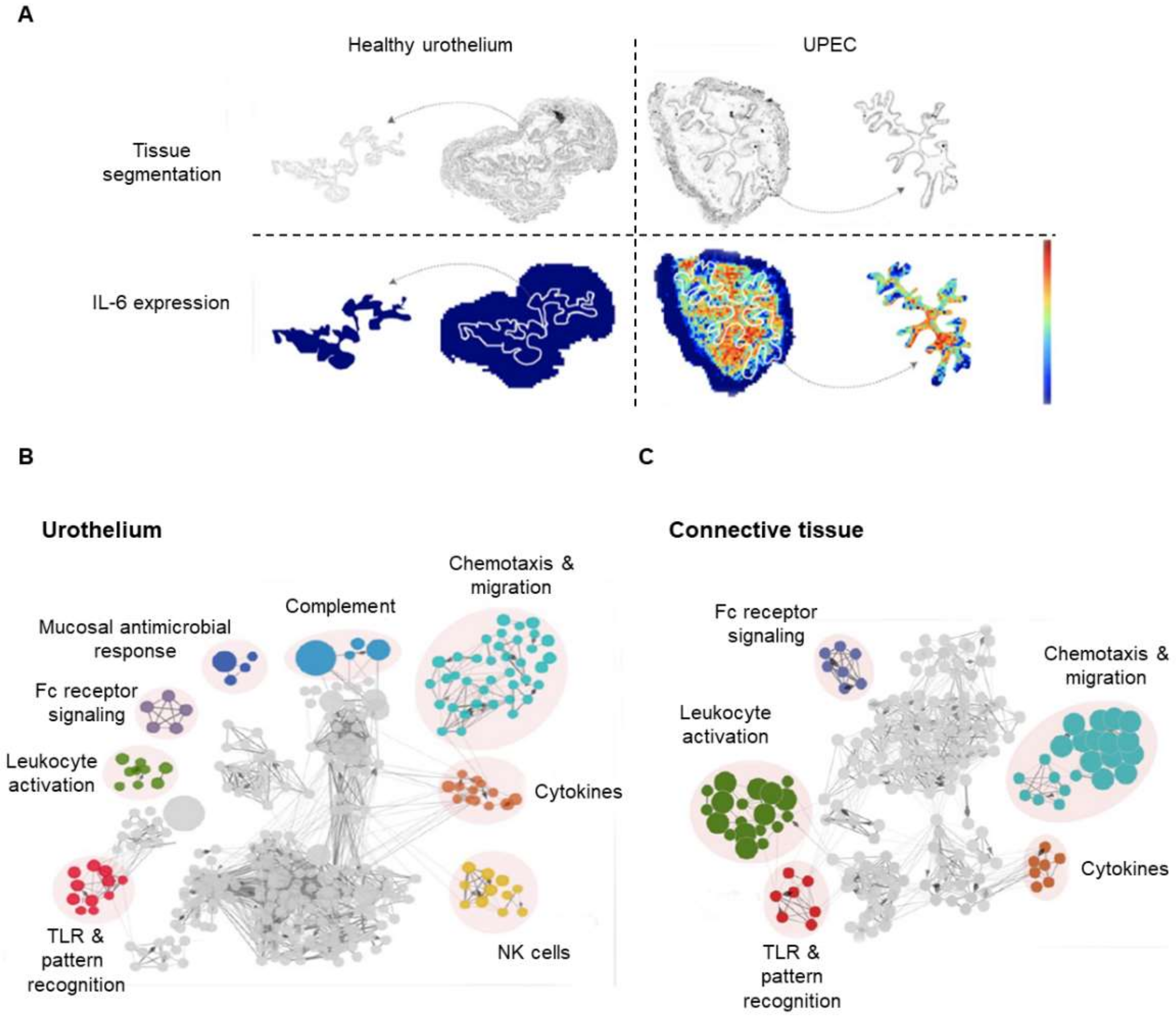
4.1. Recent Contributions to the Proteomics of the Urothelium
4.2. Recent Contributions to the Proteomics of the Lamina Propria
4.3. Recent Contributions to the Proteomics of the Muscularis Propria
4.4. Recent Contributions to the Proteomics of the Serosa Adventitia
4.5. The Dawn of the Histoproteomics of Bladder Cancer
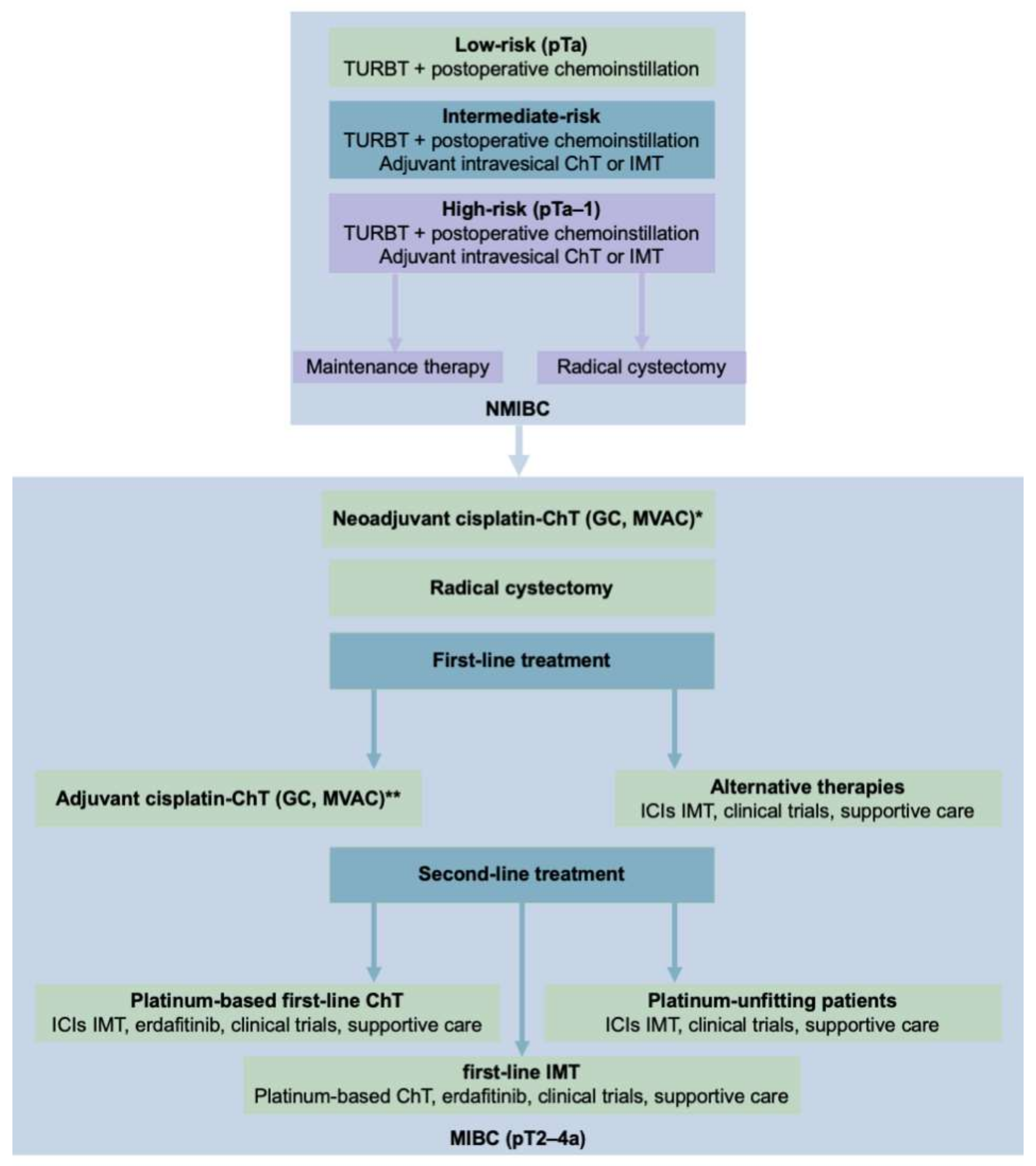
5. Proteomics in the Therapy of Bladder Cancer
5.1. Response to Platinum-Based Therapies in Bladder Cancer
5.2. Response to Second-Line Therapies in Bladder Cancer
5.2.1. Immunotherapy
5.2.2. Targeted Chemotherapy
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, I.; Rais-Bahrami, S.; Daugherty, M.; Agarwal, P.K.; Vourganti, S.; Bratslavsky, G. Primary urethral carcinoma: A Surveillance, Epidemiology, and End Results data analysis identifying predictors of cancer-specific survival. Urol Ann. 2018, 10, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 2009, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.; Dalbagni, G. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Sylvester, R.J.; Collette, L. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Schrier, B.P.; Hollander, M.P.; van Rhijn, B.W.; Kiemeney, L.A.; Witjes, J.A. Prognosis of muscle-invasive bladder cancer: Difference between primary and progressive tumours and implications for therapy. Eur. Urol. 2004, 45, 292–296. [Google Scholar] [CrossRef]
- Kayama, E.; Kikuchi, E.; Fukumoto, K. History of Non-Muscle-Invasive Bladder Cancer May Have a Worse Prognostic Impact in cT2-4aN0M0 Bladder Cancer Patients Treated With Radical Cystectomy. Clin. Genitourin. Cancer 2018, 16, e969–e976. [Google Scholar] [CrossRef] [PubMed]
- Pietzak, E.J.; Zabor, E.C.; Bagrodia, A. Genomic Differences Between “Primary” and “Secondary” Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy. Eur. Urol. 2019, 75, 231–239. [Google Scholar] [CrossRef]
- Reid, M.D.; Osunkoya, A.O.; Siddiqui, M.T.; Looney, S.W. Accuracy of grading of urothelial carcinoma on urine cytology: An analysis of interobserver and intraobserver agreement. Int. J. Clin. Exp. Pathol. 2012, 5, 882–891. [Google Scholar]
- Tosoni, I.; Wagner, U.; Sauter, G. Clinical significance of interobserver differences in the staging and grading of superficial bladder cancer. BJU Int. 2000, 85, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.N.; Sauter, G.; Epstein, J.I. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; IARC Press: Lyon, France, 2004. [Google Scholar]
- Klaile, Y.; Schlack, K.; Boegemann, M.; Steinestel, J.; Schrader, A.J.; Krabbe, L.M. Variant histology in bladder cancer: How it should change the management in non-muscle invasive and muscle invasive disease? Transl. Androl. Urol. 2016, 5, 692–701. [Google Scholar] [CrossRef]
- Xylinas, E.; Rink, M.; Robinson, B.D. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy. Eur. J. Cancer 2013, 49, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, L.; Chen, Y.; Hou, J. The prognostic value of histological subtype in patients with metastatic bladder cancer. Oncotarget 2017, 8, 28408–28417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, L.; Wang, G.; Hu, J.; Sun, T.; Fu, B. Do histological variants in urothelial carcinoma of the bladder portend poor prognosis? A systematic review and meta-analysis. Oncotarget 2017, 8, 48263–48271. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; D’Andrea, D.; Korn, S. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, C.; Tang, Y. Glandular differentiation in pT1 urothelial carcinoma of bladder predicts poor prognosis. Sci. Rep. 2019, 9, 5323. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Dasari, S.; Long, W.; Mohan, C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am. J. Cancer Res. 2019, 9, 1104–1117. [Google Scholar]
- Bratu, O.; Marcu, D.; Anghel, R.; Spinu, D.; Iorga, L.; Balescu, I.; Bacalbasa, N.; Diaconu, C.; Savu, C.; Savu, C.; et al. Tumoral markers in bladder cancer (Review). Exp. Ther Med. 2021, 22, 773. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.W.; Vis, A.N.; van der Kwast, T.H. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J. Clin. Oncol. 2003, 21, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, G.; Eriksson, P.; Patschan, O. Molecular changes during progression from nonmuscle invasive to advanced urothelial carcinoma. Int. J. Cancer 2020, 146, 2636–2647. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Kruhøffer, M.; Thykjaer, T. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004, 64, 4040–4048. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, J.P.; Sahoo, D.; Chin, R.K. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl. Acad. Sci. USA 2012, 109, 2078–2083. [Google Scholar] [CrossRef]
- Jung, S.; Wu, C.; Eslami, Z. The role of immunohistochemistry in the diagnosis of flat urothelial lesions: A study using CK20, CK5/6, P53, Cd138, and Her2/Neu. Ann. Diagn. Pathol. 2014, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Ranjzad, P.; Marriage, F. Cytokeratin 15 marks basal epithelia in developing ureters and is upregulated in a subset of urothelial cell carcinomas. PLoS ONE 2013, 8, e81167. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Ochoa, A.; McConkey, D.J. Genetic Alterations in the Molecular Subtypes of Bladder Cancer: Illustration in the Cancer Genome Atlas Dataset. Eur. Urol. 2017, 72, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R. Urothelial tumorigenesis: A tale of divergent pathways. Nat. Rev. Cancer 2005, 5, 713–725. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, J.; Majewski, T. Evidence for alternative candidate genes near RB1 involved in clonal expansion of in situ urothelial neoplasia. Lab. Investig. 2006, 86, 175–190. [Google Scholar] [CrossRef]
- Hurst, C.D.; Alder, O.; Platt, F.M. Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell 2017, 32, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Fajkovic, H.; Halpern, J.A.; Cha, E.K. Impact of gender on bladder cancer incidence, staging, and prognosis. World J. Urol. 2011, 29, 457–463. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2018, 174, 1033. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Kompier, L.C.; Lurkin, I.; van der Aa, M.N. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 2010, 5, e13821. [Google Scholar] [CrossRef]
- Cetintas, V.B.; Batada, N.N. Is there a causal link between PTEN deficient tumors and immunosuppressive tumor microenvironment? J. Transl. Med. 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Kulac, I.; Arslankoz, S.; Netto, G.J.; Ertoy Baydar, D. Reduced immunohistochemical PTEN staining is associated with higher progression rate and recurrence episodes in non-invasive low-grade papillary urothelial carcinoma of the bladder. Virchows Arch. 2018, 472, 969–974. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, R.; Blanco Gómez, B.; Vázquez-Estévez, S.; Pérez-Fentes, D.; Núñez, C. Blood-based protein biomarkers in bladder urothelial tumors. J. Proteom. 2021, 247, 104329. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, G.; Lauss, M.; Lövgren, K. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 2012, 18, 3377–3386. [Google Scholar] [CrossRef]
- Sjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M. Molecular classification of urothelial carcinoma: Global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 2017, 242, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Marzouka, N.A.; Eriksson, P.; Rovira, C.; Liedberg, F.; Sjödahl, G.; Höglund, M. A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci. Rep. 2018, 8, 3737. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Eriksson, P.; Marzouka, N.A.; Liedberg, F.; Sjödahl, G.; Höglund, M. Molecular pathology of the luminal class of urothelial tumors. J. Pathol. 2019, 249, 308–318. [Google Scholar] [CrossRef]
- Rebouissou, S.; Bernard-Pierrot, I.; de Reyniès, A. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl. Med. 2014, 6, 244ra91. [Google Scholar] [CrossRef] [PubMed]
- Lebret, T.; Neuzillet, Y.; Houede, N. Identification of targeted therapy for an aggressive subgroup of muscle-invasive bladder cancers. Mol. Cell Oncol. 2015, 2, e999507. [Google Scholar] [CrossRef][Green Version]
- Choi, W.; Porten, S.; Kim, S. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.; Zhang, M.; Zhang, L. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W.; Dinney, C.P. Genetic subtypes of invasive bladder cancer. Curr. Opin. Urol. 2015, 25, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Chai, S.; Mose, L.E. Claudin-low bladder tumors are immune infiltrated and actively immune suppressed. JCI Insight 2016, 1, e85902. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Nikolos, F.; Chen, F. Prognostic Power of a Tumor Differentiation Gene Signature for Bladder Urothelial Carcinomas. J. Natl. Cancer Inst. 2018, 110, 448–459. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Aine, M.; Eriksson, P.; Liedberg, F.; Höglund, M.; Sjödahl, G. On Molecular Classification of Bladder Cancer: Out of One, Many. Eur. Urol. 2015, 68, 921–923. [Google Scholar] [CrossRef]
- Seiler, R.; Ashab, H.A.D.; Erho, N. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Cheng, W.; Fu, D.; Xu, F.; Zhang, Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. 2020, 69, 3–14. [Google Scholar] [CrossRef]
- Wehrenberg, B.; Ochoa, A.; Choi, W.; McConkey, D. SRC drives invasion of luminal, but not basal, bladder cancer. In Proceedings of the AACR Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019. [Google Scholar] [CrossRef]
- Nomura, S.; Suzuki, Y.; Akatsuka, J. Expression of epithelial-Mesenchymal transition related markers in Plasmacytoid Urothelial carcinoma of the urinary bladder. BMC Urol. 2020, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, G.; Wang, K. Collagen Type VI Alpha 3 Chain Promotes Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Transforming Growth Factor β (TGF-β)/Smad Pathway. Med. Sci. Monit. 2018, 24, 5346–5354. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Hao, Z.; Han, J.L.; Zhu, D.J.; Jin, Z.F.; Xie, W.L. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol. Oncol. 2014, 32, 855–863. [Google Scholar] [CrossRef] [PubMed]
- de Velasco, G.; Trilla-Fuertes, L.; Gamez-Pozo, A. Urothelial cancer proteomics provides both prognostic and functional information. Sci. Rep. 2017, 7, 15819. [Google Scholar] [CrossRef]
- Stroggilos, R.; Mokou, M.; Latosinska, A.; Makridakis, M.; Lygirou, V.; Mavrogeorgis, E.; Drekolias, D.; Frantzi, M.; Mullen, W.; Fragkoulis, C.; et al. Proteome-based classification of Nonmuscle Invasive Bladder Cancer. Int. J. Cancer 2020, 146, 281–294. [Google Scholar] [CrossRef]
- Aebersold, R.; Burlingame, A.L.; Bradshaw, R.A. Western blots versus selected reaction monitoring assays: Time to turn the tables? Mol. Cell Proteom. 2013, 12, 2381–2382. [Google Scholar] [CrossRef]
- Graham, H.; Chandler, D.J.; Dunbar, S.A. The genesis and evolution of bead-based multiplexing. Methods 2019, 158, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Shah, J.B.; Xing, J. A multiplexed, particle-based flow cytometric assay identified plasma matrix metalloproteinase-7 to be associated with cancer-related death among patients with bladder cancer. Cancer 2010, 116, 4513–4519. [Google Scholar] [CrossRef]
- Pan, L.; Aguilar, H.A.; Wang, L.; Iliuk, A.; Tao, W.A. Three-Dimensionally Functionalized Reverse Phase Glycoprotein Array for Cancer Biomarker Discovery and Validation. J. Am. Chem. Soc. 2016, 138, 15311–15314. [Google Scholar] [CrossRef] [PubMed]
- Stack, E.C.; Wang, C.; Roman, K.A.; Hoyt, C.C. Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014, 70, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rizk, E.M.; Gartrell, R.D.; Barker, L.W. Prognostic and Predictive Immunohistochemistry-Based Biomarkers in Cancer and Immunotherapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 291–299. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Q.; Svec, F.; Fréchet, J.M. Porous polymer monolithic column with surface-bound gold nanoparticles for the capture and separation of cysteine-containing peptides. Anal. Chem. 2010, 82, 3352–3358. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N. Corrigendum: Global quantification of mammalian gene expression control. Nature 2013, 495, 126–127. [Google Scholar] [CrossRef]
- Hortin, G.L.; Sviridov, D. The dynamic range problem in the analysis of the plasma proteome. J. Proteom. 2010, 73, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Seeley, E.H.; Schwamborn, K.; Caprioli, R.M. Imaging of intact tissue sections: Moving beyond the microscope. J. Biol. Chem. 2011, 286, 25459–25466. [Google Scholar] [CrossRef] [PubMed]
- Sprung, R.W.; Martinez, M.A.; Carpenter, K.L. Precision of multiple reaction monitoring mass spectrometry analysis of formalin-fixed, paraffin-embedded tissue. J. Proteome Res. 2012, 11, 3498–3505. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Clair, G.; Chrisler, W.B. Proteomic Analysis of Single Mammalian Cells Enabled by Microfluidic Nanodroplet Sample Preparation and Ultrasensitive NanoLC-MS. Angew. Chem. Int. Ed. Engl. 2018, 57, 12370–12374. [Google Scholar] [CrossRef] [PubMed]
- Longuespée, R.; Alberts, D.; Pottier, C. A laser microdissection-based workflow for FFPE tissue microproteomics: Important considerations for small sample processing. Methods 2016, 104, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Simonds, E.F.; Qiu, P. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, Y.; Liu, J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst 2019, 144, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.T. Single-cell Proteomics: Progress and Prospects. Mol. Cell Proteom. 2020, 19, 1739–1748. [Google Scholar] [CrossRef]
- Longuespée, R.; Casadonte, R.; Schwamborn, K.; Reuss, D.; Kazdal, D.; Kriegsmann, K.; von Deimling, A.; Weichert, W.; Schirmacher, P.; Kriegsmann, J.; et al. Proteomics in Pathology. Proteomics 2018, 18, 1700361. [Google Scholar] [CrossRef]
- Longuespée, R.; Casadonte, R.; Schwamborn, K.; Kriegsmann, M. Proteomics in Pathology: The Special Issue. Proteom. Clin. Appl. 2019, 13, e1800167. [Google Scholar] [CrossRef]
- Cunha, G.R.; Fujii, H.; Neubauer, B.L.; Shannon, J.M.; Sawyer, L.; Reese, B.A. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J. Cell Biol. 1983, 96, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Sekkingstad, M.; Meloy, B.A. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differ. Res. Biol. Divers. 1983, 24, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tasian, G.; Cunha, G.; Baskin, L. Smooth muscle differentiation and patterning in the urinary bladder. Differ. Res. Biol. Divers. 2010, 80, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Robboy, S.J.; Kurita, T.; Baskin, L.; Cunha, G.R. New insights into human female reproductive tract development. Differ. Res. Biol. Divers. 2017, 97, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Gevaert, T.; Ganzer, R.; de Ridder, D.; Neuhaus, J. Comparative immunohistochemical characterization of interstitial cells in the urinary bladder of human, guinea pig and pig. Histochem. Cell Biol. 2018, 149, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Xu, A.S.; Ren, K.Y.M. Tertiary Lymphoid Structures Associate with Tumour Stage in Urothelial Bladder Cancer. Bladder Cancer 2017, 3, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Czerniak, B.; Ochoa, A. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat. Rev. Urol. 2014, 11, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Varley, C.L.; Garthwaite, M.A.; Cross, W.; Hinley, J.; Trejdosiewicz, L.K.; Southgate, J. PPAR gamma-regulated tight junction development during human urothelial cytodifferentiation. J. Cell Physiol. 2006, 208, 407–417. [Google Scholar] [CrossRef]
- Walz, T.; Häner, M.; Wu, X.R. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: A closed “twisted ribbon” structure. J. Mol. Biol. 1995, 248, 887–900. [Google Scholar] [CrossRef]
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, L.A.; Chlapowski, F.J.; Bonneville, M.A. Lumenal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images. J. Cell Biol. 1972, 53, 73–91. [Google Scholar] [CrossRef]
- Tratnjek, L.; Romih, R.; Kreft, M.E. Differentiation-dependent rearrangements of actin filaments and microtubules hinder apical endocytosis in urothelial cells. Histochem. Cell Biol. 2017, 148, 143–156. [Google Scholar] [CrossRef]
- Kageyama, S.; Yoshiki, T.; Isono, T. High expression of human uroplakin Ia in urinary bladder transitional cell carcinoma. Jpn. J. Cancer Res. 2002, 93, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Mohanty, S.K.; Kunju, L.P. Uroplakin II outperforms uroplakin III in diagnostically challenging settings. Histopathology 2014, 65, 132–138. [Google Scholar] [CrossRef]
- Tian, W.; Guner, G.; Miyamoto, H. Utility of uroplakin II expression as a marker of urothelial carcinoma. Hum. Pathol. 2015, 46, 58–64. [Google Scholar] [CrossRef] [PubMed]
- N’Dow, J.; Pearson, J.P.; Bennett, M.K.; Neal, D.E.; Robson, C.N. Mucin gene expression in human urothelium and in intestinal segments transposed into the urinary tract. J. Urol. 2000, 164, 1398–1404. [Google Scholar] [CrossRef]
- Walsh, M.D.; Hohn, B.G.; Thong, W. Mucin expression by transitional cell carcinomas of the bladder. Br. J. Urol. 1994, 73, 256–262. [Google Scholar] [CrossRef]
- Kaur, S.; Momi, N.; Chakraborty, S. Altered expression of transmembrane mucins, MUC1 and MUC4, in bladder cancer: Pathological implications in diagnosis. PLoS ONE 2014, 9, e92742. [Google Scholar] [CrossRef]
- Stojnev, S.; Ristic-Petrovic, A.; Velickovic, L.J. Prognostic significance of mucin expression in urothelial bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4945–4958. [Google Scholar] [PubMed]
- Zhang, X.G.; Zhang, T.; Li, C.Y.; Zhang, M.H.; Chen, F.M. CD164 promotes tumor progression and predicts the poor prognosis of bladder cancer. Cancer Med. 2018, 7, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; van Seuningen, I. Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20 signature is associated with poor survival in human carcinomas. J. Transl. Med. 2018, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Jin, L. Biological and Clinical Significance of GATA3 Detected from TCGA Database and FFPE Sample in Bladder Cancer Patients. Onco Targets Ther. 2020, 13, 945–958. [Google Scholar] [CrossRef]
- Kong, D.B.; Chen, F.; Sima, N. Focal adhesion kinases crucially regulate TGFβ-induced migration and invasion of bladder cancer cells via Src kinase and E-cadherin. Onco Targets Ther. 2017, 10, 1783–1792. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Wu, X. Simultaneous deactivation of FAK and Src improves the pathology of hypertrophic scar. Sci. Rep. 2016, 6, 26023. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Tiede, S.; Meyer-Schaller, N.; Kalathur, R.K.R. The FAK inhibitor BI 853520 exerts anti-tumor effects in breast cancer. Oncogenesis 2018, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Moradi Binabaj, M.; Bahrami, A.; Khazaei, M. The prognostic value of cyclin D1 expression in the survival of cancer patients: A meta-analysis. Gene 2020, 728, 144283. [Google Scholar] [CrossRef]
- Xu, S.; Gu, G.; Ni, Q. The expression of AEG-1 and Cyclin D1 in human bladder urothelial carcinoma and their clinicopathological significance. Int. J. Clin. Exp. Med. 2015, 8, 21222–21228. [Google Scholar]
- Jäger, T.; Becker, M.; Eisenhardt, A. The prognostic value of cadherin switch in bladder cancer. Oncol. Rep. 2010, 23, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Bindels, E.M.; Vermey, M.; van den Beemd, R.; Dinjens, W.N.; van der Kwast, T.H. E-cadherin promotes intraepithelial expansion of bladder carcinoma cells in an in vitro model of carcinoma in situ. Cancer Res. 2000, 60, 177–183. [Google Scholar] [PubMed]
- Guo, C.C.; Bondaruk, J.; Yao, H. Assessment of Luminal and Basal Phenotypes in Bladder Cancer. Sci. Rep. 2020, 10, 9743. [Google Scholar] [CrossRef]
- Samaratunga, H.; Delahunt, B.; Egevad, L. Pleomorphic giant cell carcinoma of the urinary bladder: An extreme form of tumour de-differentiation. Histopathology 2016, 68, 533–540. [Google Scholar] [CrossRef]
- Mai, K.T.; Bateman, J.; Djordjevic, B.; Flood, T.A.; Belanger, E.C. Clear Cell Urothelial Carcinoma. Int. J. Surg. Pathol. 2017, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Trpkov, K.; Lopez-Beltran, A.; Grignon, D.; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: Report from the International Society of Urologic Pathology consensus conference. Am. J. Surg. Pathol. 2014, 38, e20–e34. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Algaba, F.; Berney, D.M. Handling and reporting of transurethral resection specimens of the bladder in Europe: A web-based survey by the European Network of Uropathology (ENUP). Histopathology 2011, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Wirtz, R.M.; Gross-Weege, M. mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants. Int. J. Mol. Sci. 2018, 19, 3396. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, A.; Mohamedali, Z.; Jones, E.; Masterson, J.; Gilks, C.B. Comparison of the WHO/ISUP classification and cytokeratin 20 expression in predicting the behavior of low-grade papillary urothelial tumors. World/Health Organization/Internattional Society of Urologic Pathology. Mod. Pathol. 2001, 14, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.; Denzinger, S.; Fritsche, H.M. Introduction and first clinical application of a simplified immunohistochemical validation system confirms prognostic impact of KI-67 and CK20 for stage T1 urothelial bladder carcinoma: Single-center analysis of eight biomarkers in a series of three hundred six patients. Clin. Genitourin. Cancer 2013, 11, 537–544. [Google Scholar] [CrossRef]
- Bertz, S.; Otto, W.; Denzinger, S. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur. Urol. 2014, 65, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Southgate, J.; Lobban, D.; Harnden, P. Expression of cytokeratin 20 in urinary cytology of patients with bladder carcinoma. Cancer 1998, 83, 1052–1054. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, P.; Liu, J.; Wang, F.; Xi, S. HER2 and Src co-regulate proliferation, migration and transformation by downstream signaling pathways in arsenite-treated human uroepithelial cells. Metallomics 2018, 10, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Liu, J.; Zhou, Q.; Li, S.; Liu, W.; Xi, S. Long-term treatment with arsenite activates HER1 and HER2 through upregulating EGF, TGFα, and HSP90 in a human uroepithelial cell line. Cell Biol. Toxicol. 2020, 36, 279–284. [Google Scholar] [CrossRef]
- Zinnall, U.; Weyerer, V.; Compérat, E. Micropapillary urothelial carcinoma: Evaluation of HER2 status and immunohistochemical characterization of the molecular subtype. Hum. Pathol. 2018, 80, 55–64. [Google Scholar] [CrossRef]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur. Urol. 2011, 60, 350–357. [Google Scholar] [CrossRef]
- Hammam, O.; Nour, H.H.; Mosaad, M. The clinical significance of HER2 protein amplification/expression in urinary bladder lesion. Arab. J. Urol. 2015, 13, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Campanile, A.; Antic, T. Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients with ERBB Alterations. J. Clin. Oncol. 2016, 34, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Colopy, S.A.; Bjorling, D.E.; Mulligan, W.A.; Bushman, W. A Population of Progenitor Cells in the Basal and Intermediate Layers of the Murine Bladder Urothelium Contributes to Urothelial Development and Regeneration. Dev. Dyn. 2014, 243, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.J.; Kershners, A.M.; Beachys, P.A. The Stromal Niche for Epithelial Stem Cells: A Template for Regeneration and a Brake on Malignancy. Cancer Cell 2017, 32, 404–410. [Google Scholar] [CrossRef]
- Papafotiou, G.; Paraskevopoulou, V.; Vasilaki, E.; Kanaki, Z.; Paschalidis, N.; Klinakis, A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun. 2016, 7, 11914. [Google Scholar] [CrossRef]
- Narla, S.T.; Bushnell, D.S.; Schaefer, C.M.; Nouraie, M.; Bates, C.M. Keratinocyte Growth Factor reduces injury and leads to early recovery from cyclophosphamide bladder injury. Am. J. Pathol. 2020, 190, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ross, W.T.; Mysorekar, I.U. Urothelial generation and regeneration in development, injury, and cancer. Dev. Dyn. 2017, 246, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Xiao, J.; Mo, Q. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef]
- van Marck, V.; Stove, C.; Jacobs, K.; van den Eynden, G.; Bracke, M. P-cadherin in adhesion and invasion: Opposite roles in colon and bladder carcinoma. Int. J. Cancer 2011, 128, 1031–1044. [Google Scholar] [CrossRef]
- Wang, P.; Lin, S.L.; Zhang, L.H. The prognostic value of P-cadherin in non-muscle-invasive bladder cancer. Eur. J. Surg. Oncol. 2014, 40, 255–259. [Google Scholar] [CrossRef]
- Mandeville, J.A.; Silva Neto, B.; Vanni, A.J. P-cadherin as a prognostic indicator and a modulator of migratory behaviour in bladder carcinoma cells. BJU Int. 2008, 102, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Genitsch, V.; Kollár, A.; Vandekerkhove, G. Morphologic and genomic characterization of urothelial to sarcomatoid transition in muscle-invasive bladder cancer. Urol. Oncol. 2019, 37, 573.e19–573.e29. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.H.; Vahabi, B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic Clin. Pharmacol. Toxicol. 2016, 119, 57–62. [Google Scholar] [CrossRef]
- Gabella, G. Lamina propria: The connective tissue of rat urinary bladder mucosa. Neurourol. Urodyn. 2019, 38, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, T.; Vanstreels, E.; Daelemans, D. Identification of different phenotypes of interstitial cells in the upper and deep lamina propria of the human bladder dome. J. Urol. 2014, 192, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Vakar-Lopez, F.; Shen, S.S.; Zhang, S.; Tamboli, P.; Ayala, A.G.; Ro, J.Y. Muscularis mucosae of the urinary bladder revisited with emphasis on its hyperplastic patterns: A study of a large series of cystectomy specimens. Ann. Diagn. Pathol. 2007, 11, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; McCloskey, K.D. Lamina propria: The functional center of the bladder? Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef]
- Drumm, B.T.; Koh, S.D.; Andersson, K.E.; Ward, S.M. Calcium signalling in Cajal-like interstitial cells of the lower urinary tract. Nat. Rev. Urol. 2014, 11, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, L.; Maddur, S.; Puri, P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J. Pediatr. Surg. 2000, 35, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Weiqun, Y.; Mark, Z.L.; Warren, H.G. Cellular expression profile for interstitial cells of Cajal in bladder—A cell often misidentified as myocyte or myofibroblast. PLoS ONE 2012, 7, e48897. [Google Scholar] [CrossRef]
- Gevaert, T.; Ridder, D.; Vanstreels, E. The stem cell growth factor receptor KIT is not expressed on interstitial cells in bladder. J. Cell Mol. Med. 2017, 21, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Pezzone, M.A.; Watkins, S.C.; Alber, S.M.; King, W.E.; de Groat, W.C. Identification of c-kit-positive cells in the mouse ureter: The interstitial cells of Cajal of the urinary tract. Am. J. Physiol. Renal Physiol. 2003, 284, F925–F929. [Google Scholar] [CrossRef]
- Lagou, M.; de Vente, J.; Kirkwood, T.B.; Hedlund, P.; Andersson, K.E. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int. 2006, 97, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.H.; Roy, R.; Hollywood, M.A.; Thornbury, K.D.; McHale, N.G. Platelet-derived growth factor receptor-alpha cells in mouse urinary bladder: A new class of interstitial cells. J. Cell Mol. Med. 2012, 16, 691–700. [Google Scholar] [CrossRef]
- Rasmussen, H.; Rumessen, J.J.; Hansen, A.; Smedts, F.; Horn, T. Ultrastructure of Cajal-like interstitial cells in the human detrusor. Cell Tissue Res. 2009, 335, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C.; Guasti, D.; del Popolo, G.; Faussone-Pellegrini, M.S. Telocytes subtypes in human urinary bladder. J. Cell Mol. Med. 2014, 18, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Triguero, D.; Lafuente-Sanchis, A.; Garcia-Pascual, A. Proliferation of interstitial cells in the cyclophosphamide-induced cystitis and the preventive effect of imatinib. BioMed Res. Int. 2017, 2017, 3457093. [Google Scholar] [CrossRef] [PubMed]
- Volmar, K.E.; Chan, T.Y.; de Marzo, A.M.; Epstein, J.I. Florid von Brunn nests mimicking urothelial carcinoma: A morphologic and immunohistochemical comparison to the nested variant of urothelial carcinoma. Am. J. Surg. Pathol. 2003, 27, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Wiener, D.P.; Koss, L.G.; Sablay, B.; Freed, S.Z. The prevalence and significance of Brunn’s nests, cystitis cystica and squamous metaplasia in normal bladders. J. Urol. 1979, 122, 317–321. [Google Scholar] [CrossRef]
- Eastman, R., Jr.; Leaf, E.M.; Zhang, D. Fibroblast growth factor-10 signals development of von Brunn’s nests in the exstrophic bladder. Am. J. Physiol. Renal Physiol. 2010, 299, F1094–F1110. [Google Scholar] [CrossRef]
- Lawson, A.R.J.; Abascal, F.; Coorens, T.H. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 2020, 370, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Garcia, J.A.; Reynolds, J. Transcriptomic and Protein Analysis of Small-cell Bladder Cancer (SCBC) Identifies Prognostic Biomarkers and DLL3 as a Relevant Therapeutic Target. Clin. Cancer Res. 2019, 25, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef]
- Bottek, J.; Soun, C.; Lill, J.K. Spatial proteomics revealed a CX3CL1-dependent crosstalk between the urothelium and relocated macrophages through IL-6 during an acute bacterial infection in the urinary bladder. Mucosal Immunol. 2020, 13, 702–714. [Google Scholar] [CrossRef]
- Conroy, M.J.; Lysaght, J. CX3CL1 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, Y.; Chan, Q.; Jiang, L.; Dong, J. CX3CL1 promotes lung cancer cell migration and invasion via the Src/focal adhesion kinase signaling pathway. Oncol. Rep. 2019, 41, 1911–1917. [Google Scholar] [CrossRef]
- Krantz, D.; Mints, M.; Winerdal, M. IL-16 processing in sentinel node regulatory T cells is a factor in bladder cancer immunity. Scand. J. Immunol. 2020, 92, e12926. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.H.; Meng, E.; Young, J.S. The physiological function of lower urinary tract smooth muscle. Auton. Neurosci. 2010, 154, 3–13. [Google Scholar] [CrossRef]
- Sánchez Freire, V.; Burkhard, F.C.; Schmitz, A.; Kessler, T.M.; Monastyrskaya, K. Structural differences between the bladder dome and trigone revealed by mRNA expression analysis of cold-cut biopsies. BJU Int. 2011, 108, E126–E135. [Google Scholar] [CrossRef] [PubMed]
- Roosen, A.; Wu, C.; Sui, G.; Chowdhury, R.A.; Patel, P.M.; Fry, C.H. Characteristics of spontaneous activity in the bladder trigone. Eur. Urol. 2009, 56, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Shen, S.S.; Lapetino, S. Diagnostic utility of antibody to smoothelin in the distinction of muscularis propria from muscularis mucosae of the urinary bladder: A potential ancillary tool in the pathologic staging of invasive urothelial carcinoma. Am. J. Surg. Pathol. 2009, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bowens, N.; Parmacek, M.S. Chapter 82—Development of the smooth muscle cell lineage. Fundam. Biol. Mech. Dis. 2012, 2, 1109–1116. [Google Scholar] [CrossRef]
- Lindh, C.; Nilsson, R.; Lindstrom, M.L.; Lundi, L.; Elmberger, G. Detection of smoothelin expression in the urinary bladder is strongly dependent on pretreatment conditions: A critical analysis with possible consequences for cancer staging. Virchows Arch. 2011, 458, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Beukers, W.; Meijer, T.; Vissers, C.J. Down-staging (<pT2) of urothelial cancer at cystectomy after the diagnosis of detrusor muscle invasion (pT2) at diagnostic transurethral resection (TUR): Is prediction possible? Virchows Arch. 2012, 461, 149–156. [Google Scholar] [CrossRef]
- Khayyata, S.; Dudas, M.; Rohan, S.M. Distribution of smoothelin expression in the musculature of the genitourinary tract. Mod. Pathol. 2009, 22, 154–204. [Google Scholar] [CrossRef]
- Chakravarthy, R.; Ahmed, K.; Abbasi, S.; Cotterill, A.; Parveen, N. In response-a modified staining protocol for Smoothelin immunostaining. Virchows Arch. 2011, 459, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Soh, S.; Satoh, T. Distribution of lymphatic vessel network in normal urinary bladder. Urology 2008, 72, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, Y.; Lebret, T.; Molinie, V. Perivesical fat invasion in bladder cancer: Implications for prognosis comparing pT2b, pT3a and pT3b stages and consequences for adjuvant chemotherapy indications. BJU Int. 2012, 110, 1736–1741. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Finley, D.S.; Calvert, V.S.; Inokuchi, J. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J. Urol. 2009, 182, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Monteiro, C.; Cunha, V. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J. Exp. Clin. Cancer Res. 2012, 31, 32. [Google Scholar] [CrossRef]
- Hariharan, N.; Ashcraft, K.A.; Svatek, R.S. Adipose Tissue-Secreted Factors Alter Bladder Cancer Cell Migration. J. Obes. 2018, 2018, 9247864. [Google Scholar] [CrossRef]
- Michishita, M.; Tomita, K.I.; Yano, K.; Kasahara, K.I. Mast cell accumulation and degranulation in rat bladder with partial outlet obstruction. Adv. Ther. 2015, 32, 16–28. [Google Scholar] [CrossRef]
- Sullivan, M.P.; Cristofaro, V.; Radisavljevic, Z.M.; Yalla, S.V. Regional distribution and molecular interaction of caveolins in bladder smooth muscle. BJU Int. 2012, 110, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, K.; Uchiyama, M.; Yamochi, T. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J. Biol. Chem. 2007, 282, 10117–10131. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, C.; Tae, N.; Lee, S. Syntenin regulates TGF-beta1-induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-beta type I receptor internalization. Oncogene 2016, 35, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, M.; Nohtomi, K.; Mori, Y. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem. Biophys. Res. Commun. 2018, 495, 223–229. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Roth, D.M. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J. Biol. Chem. 2005, 180, 31036–31044. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Hanzal-Bayer, M.; Hancock, J.F. Biogenesis of caveolae: A structural model for caveolin-induced domain formation. J. Cell Sci. 2006, 119, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Yu, L.; Guili, V. Nerve Growth Factor signaling from membrane microdomains to the nucleus: Differential regulation by caveolins. Int. J. Mol. Sci. 2017, 18, 693. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Toufaily, C.; Annabi, B. Caveolin and cavin family members: Dual roles in cancer. Biochimie 2014, 107, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Chen, F.; Pan, Q. The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther. 2017, 10, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.; Jameel, Z.; McCain, D.A.; Hassoun, P.; Bamboat, Z.M. Massive malignant solitary fibrous tumor arising from the bladder serosa: A case report. J. Med. Case Rep. 2015, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Kratiras, Z.; Spapis, V.; Koniaris, E.; Kozyrakis, D.; Skriapas, K. Malignant solitary fibrous tumor of urinary bladder: A rare clinical entity. Arch. Ital. Urol. Androl. 2019, 91, 135–136. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ochiai, A. Special cancer microenvironment in human colonic cancer: Concept of cancer microenvironment formed by peritoneal invasion (CMPI) and implication of subperitoneal fibroblast in cancer progression. Pathol. Int. 2016, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Kodera, Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J. Gastroenterol. 2016, 22, 6829–6840. [Google Scholar] [CrossRef] [PubMed]
- Habuka, M.; Fagerberg, L.; Hallström, B.M.; Pontén, F.; Yamamoto, T.; Uhlen, M. The Urinary Bladder Transcriptome and Proteome Defined by Transcriptomics and Antibody-Based Profiling. PLoS ONE 2015, 10, e0145301. [Google Scholar] [CrossRef] [PubMed]
- Witzke, K.E.; Großerueschkamp, F.; Jütte, H. Integrated Fourier Transform Infrared Imaging and Proteomics for Identification of a Candidate Histochemical Biomarker in Bladder Cancer. Am. J. Pathol. 2019, 189, 619–631. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Zhang, J. AHNAK2 is a Novel Prognostic Marker and Oncogenic Protein for Clear Cell Renal Cell Carcinoma. Theranostics 2017, 7, 1100–1113. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Shi, X.; Yue, B.; Hao, J. AHNAK2 is a potential prognostic biomarker in patients with PDAC. Oncotarget 2017, 8, 31775–31784. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, K.; Woo, J.; Park, J.; Kim, H.; Lee, K.E.; Kim, H.; Kim, Y.; Moon, K.C.; Kim, J.Y.; et al. Quantitative Proteomic Analysis Identifies AHNAK (Neuroblast Differentiation-associated Protein AHNAK) as a Novel Candidate Biomarker for Bladder Urothelial Carcinoma Diagnosis by Liquid-based Cytology. Mol. Cell Proteom. 2018, 17, 1788–1802. [Google Scholar] [CrossRef]
- Sheppard, H.M.; Feisst, V.; Chen, J.; Print, C.; Dunbar, P.R. AHNAK is downregulated in melanoma, predicts poor outcome, and may be required for the expression of functional cadherin-1. Melanoma Res. 2016, 26, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Sohn, M.; Lim, H.J. Ahnak functions as a tumor suppressor via modulation of TGFβ/Smad signaling pathway. Oncogene 2014, 33, 4675–4684. [Google Scholar] [CrossRef]
- Ho, M.E.; Quek, S.I.; True, L.D. Bladder cancer cells secrete while normal bladder cells express but do not secrete AGR2. Oncotarget 2016, 7, 15747–15756. [Google Scholar] [CrossRef] [PubMed]
- Steurer, S.; Singer, J.M.; Rink, M.; Chun, F.; Dahlem, R.; Simon, R.; Burandt, E.; Stahl, P.; Terracciano, L.; Schlomm, T.; et al. MALDI imaging-based identification of prognostically relevant signals in bladder cancer using large-scale tissue microarrays. Urol Oncol. 2014, 32, 1225–1233. [Google Scholar] [CrossRef]
- Brooks, M.; Mo, Q.; Krasnow, R.; Ho, P.L.; Lee, Y.C.; Xiao, J.; Kurtova, A.; Lerner, S.; Godoy, G.; Jian, W.; et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget. 2016, 7, 82609–82619. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Vos, D.R.N.; Jansen, I.; Lucas, M.; Paine, M.R.L.; de Boer, O.J.; Meijer, S.L.; Savci-Heijink, C.D.; Marquering, H.A.; de Bruin, D.M.; Heeren, R.M.A.; et al. Strategies for managing multi-patient 3D mass spectrometry imaging data. J. Proteom. 2019, 193, 184–191. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef] [PubMed]
- van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Dunbrack, R.L.; Brennan, T.A.; Andrake, M.D.; Zhou, Y.; Serebriiskii, I.G.; Slifker, M.; Alpaugh, K.; Dulaimi, E.; Palma, N.; et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder. Cancer Eur. Urol. 2015, 68, 959–967. [Google Scholar] [CrossRef]
- Liu, D.; Plimack, E.R.; Hoffman-Censits, J.; Garraway, L.A.; Bellmunt, J.; van Allen, E.; Rosenberg, J.E. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA Oncol. 2016, 2, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Le Goux, C.; Vacher, S.; Schnitzler, A.; Barry Delongchamps, N.; Zerbib, M.; Peyromaure, M.; Sibony, M.; Allory, Y.; Bieche, I.; Damotte, D.; et al. Assessment of prognostic implication of a panel of oncogenes in bladder cancer and identification of a 3-gene signature associated with recurrence and progression risk in non-muscle-invasive bladder cancer. Sci. Rep. 2020, 10, 16641. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Inamoto, T.; Tsujino, T.; Matsui, Y.; Konuma, T.; Nishimura, K.; Uchimoto, T.; Tsutsumi, T.; Matsunaga, T.; Maenosono, R.; et al. Increased BUB1B/BUBR1 expression contributes to aberrant DNA repair activity leading to resistance to DNA-damaging agents. Oncogene 2021. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, F.H.; de Jong, J.; Fransen van de Putte, E.E.; Michaut, M.; Schlicker, A.; Peters, D.; Velds, A.; Nieuwland, M.; van den Heuvel, M.M.; Kerkhoven, R.M.; et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol. 2016, 69, 384–388. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, W.; Zhang, Z.; Song, R.; Zeng, S.; Sun, Y.; Xu, C. Prognostic role of HER2 expression in bladder cancer: A systematic review and meta-analysis. Int. Urol. Nephrol. 2015, 47, 87–94. [Google Scholar] [CrossRef]
- Bahrami, S.; Kazemi, B.; Zali, H.; Black, P.C.; Basiri, A.; Bandehpour, M.; Hedayati, M.; Sahebkar, A. Discovering Therapeutic Protein Targets for Bladder Cancer Using Proteomic Data Analysis. Curr. Mol. Pharmacol. 2020, 13, 150–172. [Google Scholar] [CrossRef]
- Balluff, B.; Elsner, M.; Kowarsch, A.; Rauser, S.; Meding, S.; Schuhmacher, C.; Feith, M.; Herrmann, K.; Röcken, C.; Schmid, R.M.; et al. Classification of HER2/neu status in gastric cancer using a breast-cancer derived proteome classifier. J. Proteome Res. 2010, 9, 6317–6322. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Feng, W.; Luo, Y.; Lai, H.; Li, Q.; Xiu, B.; Li, Y.; Li, Y.; Huang, S.; et al. A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine 2020, 62, 103074. [Google Scholar] [CrossRef]
- Mo, Q.; Li, R.; Adeegbe, D.O.; Peng, G.; Chan, K.S. Integrative multi-omics analysis of muscle-invasive bladder cancer identifies prognostic biomarkers for frontline chemotherapy and immunotherapy. Commun. Biol. 2020, 3, 784. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Vander Velde, N.; Guerin, A.; Ionescu-Ittu, R.; Shi, S.; Wu, E.Q.; Lin, S.W.; Hsu, L.I.; Saum, K.U.; de Ducla, S.; Wang, J.; et al. Comparative Effectiveness of Non-cisplatin First-line Therapies for Metastatic Urothelial Carcinoma: Phase 2 IMvigor210 Study Versus US Patients Treated in the Veterans Health Administration. Eur. Urol. Oncol. 2019, 2, 12–20. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Loriot, Y.; Durán, I.; Ravaud, A.; Retz, M.; Vogelzang, N.J.; Nelson, B.; Wang, J.; Shen, X.; Powles, T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021, 80, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.L.; Harrison, M.R.; Deal, A.M.; Ramalingam, S.; Whang, Y.E.; Brower, B.; Dunn, M.; Osterman, C.K.; Heiling, H.M.; Bjurlin, M.A.; et al. Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Bladder Cancer. J. Clin. Oncol. 2021, 39, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J. Clin. Oncol. 2017, 35, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Pfizer. Study of Sasanlimab (PF-06801591) in Combination with Bacillus Calmette-Guerin (BCG) in Participants with High-Risk Non-Muscle Invasive Bladder Cancer (CREST). Identifier: NCT04165317. Available online: https://clinicaltrials.gov/ct2/show/NCT04165317 (accessed on 6 October 2021).
- Ruijin Hospital. Chemotherapy Combined with Tislelizumab as Bladder Sparing Option for Patients with Muscle Invasive Bladder Cancer. Identifier: NCT04909775. Available online: https://clinicaltrials.gov/ct2/show/NCT04909775 (accessed on 6 October 2021).
- AstraZeneca. Study of Tremelimumab in Patients with Advanced Solid Tumors. Identifier: NCT02527434. Available online: https://clinicaltrials.gov/ct2/show/NCT02527434 (accessed on 6 October 2021).
- Grivas, P.; Puligandla, M.; Cole, S.; Courtney, K.D.; Dreicer, R.; Gartrell, B.A.; Cetnar, J.P.; Dall’era, M.; Galsky, M.D.; Jain, R.K.; et al. PrE0807 phase Ib feasibility trial of neoadjuvant nivolumab (N)/lirilumab (L) in cisplatin-ineligible muscle-invasive bladder cancer (BC). J. Clin. Oncol. 2019, 37, TPS4594. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Currie, G.; Abella, E.; Vaena, D.A.; Kalebasty, A.R.; Curigliano, G.; Tupikowski, K.; Andric, Z.G.; Lugowska, I.; Kelly, W.K. FIERCE-22: Clinical activity of vofatamab (V) a FGFR3 selective inhibitor in combination with pembrolizumab (P) in WT metastatic urothelial carcinoma, preliminary analysis. J. Clin. Oncol 2019, 37, 4511. [Google Scholar] [CrossRef]
- Lindis Biotech GmbH. Investigation of Safety and Tolerability of Catumaxomab in Patients with NMIBC. Identifier: NCT04819399. Available online: https://clinicaltrials.gov/ct2/show/NCT04819399 (accessed on 7 October 2021).
- Memorial Sloan Kettering Cancer Center. Study of Gemcitabine, Carboplatin, and Panitumumab (GCaP) as Neoadjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. Identifier: NCT01916109. Available online: https://clinicaltrials.gov/ct2/show/NCT01916109 (accessed on 7 October 2021).
- Wong, Y.N.; Litwin, S.; Vaughn, D.; Cohen, S.; Plimack, E.R.; Lee, J.; Song, W.; Dabrow, M.; Brody, M.; Tuttle, H.; et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J. Clin. Oncol. 2012, 30, 3545–3551. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Daignault, S.; Agarwal, N.; Grivas, P.D.; Siefker-Radtke, A.O.; Puzanov, I.; MacVicar, G.R.; Levine, E.G.; Srinivas, S.; Twardowski, P.; et al. A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 2014, 120, 2684–2693. [Google Scholar] [CrossRef]
- Hoffmann-La Roche. A Study to Determine Best Tumor Response with Trastuzumab Emtansine in Human Epidermal Growth Factor Receptor 2 (HER2) Overexpressing Solid Tumors (KAMELEON). Identifier: NCT02999672. Available online: https://clinicaltrials.gov/ct2/show/NCT02999672 (accessed on 7 October 2021).
- The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School. A Clinical Study of RC48-ADC Combined with Triplizumab For Treatment of Myometrial Invasive Bladder Cancer. Identifier: NCT05016973. Available online: https://clinicaltrials.gov/ct2/show/NCT05016973 (accessed on 7 October 2021).
- Lattanzi, M.; Rosenberg, J.E. The emerging role of antibody-drug conjugates in urothelial carcinoma. Expert Rev. Anticancer Ther. 2020, 20, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef]
- Podojil, J.R.; Glaser, A.P.; Baker, D.; Courtois, E.T.; Fantini, D.; Yu, Y.; Eaton, V.; Sivajothi, S.; Chiang, M.; Das, A.; et al. Antibody targeting of B7-H4 enhances the immune response in urothelial carcinoma. Oncoimmunology 2020, 9, 1744897. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, L.; Lu, Q.; Huang, H.; Feng, C.; Tao, Y.; Li, Z.; Hu, J.; Lai, Z.; Wang, Q.; et al. A combination of ssGSEA and mass cytometry identifies immune microenvironment in muscle-invasive bladder cancer. J. Clin. Lab. Anal. 2021, 35, e23754. [Google Scholar] [CrossRef]
- Read, S.; Greenwald, R.; Izcue, A.; Robinson, N.; Mandelbrot, D.; Francisco, L.; Sharpe, A.H.; Powrie, F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006, 177, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Lombard-Banek, C.; Schiel, J.E. Mass Spectrometry Advances and Perspectives for the Characterization of Emerging Adoptive Cell Therapies. Molecules 2020, 25, 1396. [Google Scholar] [CrossRef]
- Perna, F.; Berman, S.H.; Soni, R.K.; Mansilla-Soto, J.; Eyquem, J.; Hamieh, M.; Hendrickson, R.C.; Brennan, C.W.; Sadelain, M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017, 32, 506–519.e5. [Google Scholar] [CrossRef]
- Enblad, G.; Karlsson, H.; Gammelgård, G.; Wenthe, J.; Lövgren, T.; Amini, R.M.; Wikstrom, K.I.; Essand, M.; Savoldo, B.; Hallböök, H.; et al. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin. Cancer Res. 2018, 24, 6185–6194. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, J.; Liu, Z.; Zhang, J.; Chang, J. Label-Free Quantitative Proteomics Combined with Biological Validation Reveals Activation of Wnt/β-Catenin Pathway Contributing to Trastuzumab Resistance in Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 1981. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chang, J.; Liu, M.; Yuan, J.; Zhang, J.; Qin, J.; Xia, X.; Wang, Y. Quantitative proteomics profiling reveals activation of mTOR pathway in trastuzumab resistance. Oncotarget 2017, 8, 45793–45806. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Liao, W.L.; Zhao, L.; Whitcomb, E.; Henderson, L.; O’Day, E.; Xu, P.; Thyparambil, S.; Krizman, D.; Bengali, K.; et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: Comparison to IHC and FISH. Gastric Cancer 2016, 19, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- An, E.; Ock, C.Y.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Liao, W.L.; Cecchi, F.; Blackler, A.; et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann. Oncol. 2017, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, K.; Bellmunt, J. Erdafitinib for the treatment of metastatic bladder cancer. Expert Rev. Clin. Pharmacol. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Bayer. Phase 1b/2 Study of Rogaratinib (BAY1163877) in Combination with Atezolizumab in Urothelial Carcinoma (FORT-2). Identifier: NCT03473756. Available online: https://clinicaltrials.gov/ct2/show/NCT03473756 (accessed on 7 October 2021).
- National Cancer Institute (NCI). Olaparib in Treating Patients with Metastatic or Advanced Urothelial Cancer with DNA-Repair Defects. Identifier: NCT03375307. Available online: https://clinicaltrials.gov/ct2/show/NCT03375307 (accessed on 8 October 2021).
- AstraZeneca. Open-Label, Randomised, Multi-Drug, Biomarker-Directed, Phase 1b Study in Pts w/Muscle Invasive Bladder Cancer (BISCAY). Identifier: NCT02546661. Available online: https://clinicaltrials.gov/ct2/show/NCT02546661 (accessed on 8 October 2021).
- National Cancer Institute (NCI). Testing the Effectiveness of Two Immunotherapy Drugs (Nivolumab and Ipilimumab) with One Anti-cancer Targeted Drug (Cabozantinib) for Rare Genitourinary Tumors. Identifier: NCT03866382. Available online: https://clinicaltrials.gov/ct2/show/NCT03866382 (accessed on 8 October 2021).
- Powles, T.; Huddart, R.A.; Elliott, T.; Sarker, S.J.; Ackerman, C.; Jones, R.; Hussain, S.; Crabb, S.; Jagdev, S.; Chester, J.; et al. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J. Clin. Oncol. 2017, 35, 48–55. [Google Scholar] [CrossRef]
- Miller, K.; Morant, R.; Stenzl, A.; Zuna, I.; Wirth, M. A Phase II Study of the Central European Society of Anticancer-Drug Research (CESAR) Group: Results of an Open-Label Study of Gemcitabine plus Cisplatin with or without Concomitant or Sequential Gefitinib in Patients with Advanced or Metastatic Transitional Cell Carcinoma of the Urothelium. Urol. Int. 2016, 96, 5–13. [Google Scholar] [CrossRef]
- Krook, M.A.; Lenyo, A.; Wilberding, M.; Barker, H.; Dantuono, M.; Bailey, K.M.; Chen, H.Z.; Reeser, J.W.; Wing, M.R.; Miya, J.; et al. Efficacy of FGFR Inhibitors and Combination Therapies for Acquired Resistance in FGFR2-Fusion Cholangiocarcinoma. Mol. Cancer Ther. 2020, 19, 847–857. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Keenan, T.E.; Li, T.; Tayob, N.; Trippa, L.; Pastorello, R.G.; Richardson Iii, E.T.; Dillon, D.; Amoozgar, Z.; Overmoyer, B.; et al. Nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer: A phase II and biomarker study. NPJ Breast Cancer 2021, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Sologova, S.S.; Mokhosoev, I.M.; Terentiev, A.A. Predictive biomarkers for systemic therapy of hepatocellular carcinoma. Expert Rev. Mol. Diagn. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Kim, H.P.; Kang, M.J.; Cho, B.K.; Han, S.W.; Kim, T.Y.; Yi, E.C. Phosphoproteomic analysis identifies activated MET-axis PI3K/AKT and MAPK/ERK in lapatinib-resistant cancer cell line. Exp. Mol. Med. 2013, 45, e64. [Google Scholar] [CrossRef] [PubMed][Green Version]
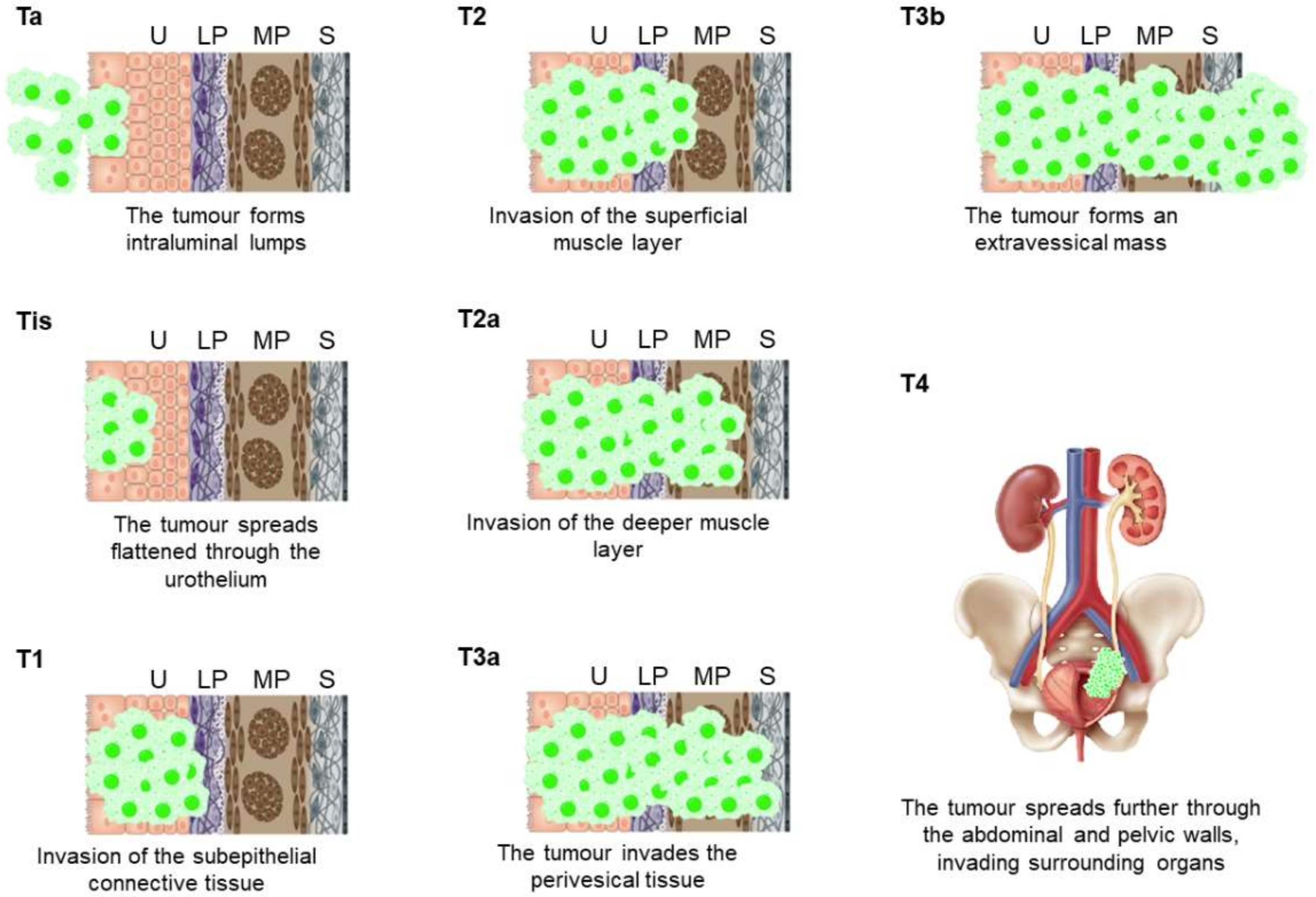


| T—Primary tumor | ||
| Tx | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| Ta | Non-invasive papillary carcinoma | |
| Tis | Carcinoma in situ: “flat tumor” | |
| T1 | Tumor invades subepithelial connective tissue | |
| T2 | Tumor invades muscle: | |
| T2a | Tumor invades superficial muscle (inner half) | |
| T2b | Tumor invades deep muscle (outer half) | |
| T3 | Tumor invades perivesical tissue: | |
| T3a | Microscopically | |
| T3b | Macroscopically (extravesical mass) | |
| T4 | Tumor invades surrounding organs: | |
| T4a | Tumor invades prostate stroma, seminal vesicles, uterus, or vagina | |
| T4b | Tumor invades pelvic wall or abdominal wall | |
| N—Regional lymph nodes | ||
| Nx | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis in a single lymph node in the pelvis | |
| N2 | Metastasis in multiple regional lymph nodes in the pelvis | |
| N3 | Metastasis in a common iliac lymph node(s) | |
| M—Distant metastasis | ||
| M0 | No distant metastasis | |
| M1 | Distant metastasis: | |
| M1a | Non-regional lymph nodes | |
| M1b | Other distant metastasis | |
| Urothelial-Type Tumors | Non-Urothelial-Type Tumors | ||||
|---|---|---|---|---|---|
| Non-Invasive | Invasive | ||||
| WHO 2004 | WHO 2016 | WHO 2004 | WHO 2016 | WHO 2004 | WHO 2016 |
| Urothelial carcinoma in situ | With squamous differentiation | * | Squamous cell neoplasm | ||
| Papillary urothelial carcinoma, low grade | With glandular differentiation | * | Glandular neoplasm | ||
| Papillary urothelial carcinoma, high grade | With small cell differentiation | * | - | Urachal carcinoma | |
| Papillary urothelial neoplasm of low malignant potential | With trophoblastic differentiation | * | - | Tumors of Müllerian type | |
| Urothelial papilloma | Nested | Neuroendocrine tumors | |||
| Inverted urothelial papilloma | Microcystic | Melanocytic tumors | |||
| - | Urothelial proliferation of uncertain malignant potential (hyperplasia) | Micropapillary | Mesenchymal tumors | ||
| - | Urothelial dysplasia | Lymphoepithelioma-like | Urothelial tract hematopoietic and lymphoid tumors | ||
| Lymphoma-like | - | Miscellaneous | |||
| Plasmacytoid | Plasmacytoid/signet ring cell/diffuse | ||||
| Sarcomatoid | |||||
| Giant cell | |||||
| Undifferentiated | Poorly differentiated | ||||
| - | Lipid rich | ||||
| - | Clear cell | ||||
| Patients/Phase | Schema | Setting | Study Conclusions | Year, [Ref.] |
|---|---|---|---|---|
| 386/II | Nivolumab 3 mg/kg IV | mMBIC | OS was 7.0 months, and effectiveness was irrespective of PD-L1 expression levels | 2017, [221] |
| 119/II | Carboplatin vs. Atezolizumab 1200 mg IV | mMIBC | OS was 15.0 months with atezolizumab, and 12.1 months with any platinum-based chemotherapy, and 8.7 months with carboplatin/gemcitabine. First-line atezolizumab for cisplatin-unfit mMIBC provided an OS benefit over platinum-based treatments | 2019, [222] |
| 310/II | Atezolizumab 1200 mg IV | mMIBC | Increased levels of PD-L1 were associated with better responses. All TCGA 2014 subtypes responded to the therapy, although it was significantly higher in the luminal cluster II | 2016, [223] |
| 931/III | Vinflunine, paclitaxel, or docetaxel vs. Atezolizumab 1200 mg IV | mMIBC | Updated OS demonstrated long-term stable remission. After 24 months, the OS was 23% to atezolizumab, and 13% to the alternative chemotherapy | 2021, [224] |
| 39/II | GC + Pembrolizumab 200 mg IV | MIBC (T2-4a, N0-1, M0) | With neoadjuvant GC + pembrolizumab, 56% of patients (95% CI, 40 to 72) achieved <pT2N0, and 36% (95% CI, 21 to 53) achieved pT0N0 | 2021, [225] |
| 542/III | Vinflunine, paclitaxel, or docetaxel vs. pembrolizumab 200 mg IV | mMIBC | After 24 months of follow-up, long-term outcomes were better with pembrolizumab over chemotherapy | 2019, [226] |
| 374/II | Pembrolizumab 200 mg IV | mMIBC | 8.9%, and 19.7% of patients achieved complete and partial response, respectively, with a median of 30.1 months (95% CI). Patients with unaffected lymph nodes had better outcome rates | 2020, [227] |
| 44/Ib | Avelumab 10 mg/kg IV | mMIBC | Avelumab was safe, and it was associated with a large median duration of response, and a prolonged survival rate | 2017, [228] |
| 249/I | Avelumab IV 10 mg/kg IV | mMIBC | Avelumab as neoadjuvant in platinum-treated patients was safe, and demonstrated the best overall complete or partial response | 2018, [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cortés, R.; Vázquez-Estévez, S.; Fernández, J.Á.; Núñez, C. Proteomics as a Complementary Technique to Characterize Bladder Cancer. Cancers 2021, 13, 5537. https://doi.org/10.3390/cancers13215537
López-Cortés R, Vázquez-Estévez S, Fernández JÁ, Núñez C. Proteomics as a Complementary Technique to Characterize Bladder Cancer. Cancers. 2021; 13(21):5537. https://doi.org/10.3390/cancers13215537
Chicago/Turabian StyleLópez-Cortés, Rubén, Sergio Vázquez-Estévez, Javier Álvarez Fernández, and Cristina Núñez. 2021. "Proteomics as a Complementary Technique to Characterize Bladder Cancer" Cancers 13, no. 21: 5537. https://doi.org/10.3390/cancers13215537
APA StyleLópez-Cortés, R., Vázquez-Estévez, S., Fernández, J. Á., & Núñez, C. (2021). Proteomics as a Complementary Technique to Characterize Bladder Cancer. Cancers, 13(21), 5537. https://doi.org/10.3390/cancers13215537







