Simple Summary
Melanoma still represents a major challenge for health systems around the world. A classical treatment for patients with a high tumor burden or rapidly recurrent in-transit metastases is isolated limb perfusion (ILP) therapy instead of locoregional surgical resection, or when the latter is no longer feasible. In this era of modern systemic treatments for melanoma, it still remains interesting to analyze the role of management approaches for locoregionally-advanced disease, such as isolated limb perfusion (ILP). With this purpose, we conducted a systematic review updating the available literature on ILP for malignant melanomas. The main objectives of this review were to focus on the effectiveness and safety of ILP. In conclusion, ILP, with its low incidence of regional and systemic toxicity, is a valuable palliative treatment not only for patients with disease confined to a limb, but also for patients with a metastatic melanoma with a bulky or symptomatic disease, in order to improve their quality of life.
Abstract
Background. Isolated limb perfusion (ILP) is a locoregional procedure indicated by the unresectable melanoma of the limbs. Its complexity and highly demanding multidisciplinary approach means that it is a technique only implemented in a few referral centers around the globe. This report aims to examine its potential role in the era of targeted therapies and immunotherapy by conducting a systematic review of the literature on ILP. Methods. PubMed, Embase and Cochrane Library were searched. The eligibility criteria included publications from 2000–2020 providing valid data o effectiveness, survival or toxicity. Studies in which the perfusion methodology was not clearly described, letters to the editor, non-systematic reviews and studies that applied outdated clinical guidelines were excluded. To rule out studies of a low methodological quality and assess the risk of bias, the following aspects were also required: a detailed description of the applied ILP regimen, the clinical context, follow-up periods, analyzed clinical endpoints, and the number of analyzed ILPs. The disagreements were resolved by consensus. The results are presented in tables and figures. Results. Twenty-seven studies including 2637 ILPs were selected. The median overall response rate was 85%, with a median complete response rate of 58.5%. The median overall survival was 38 months, with a 5-year overall survival of 35%. The toxicity was generally mild according to Wieberdink toxicity criteria. Discussion. ILP still offer a high efficacy in selected patients. The main limitation of our review is the heterogeneity and age of most of the articles, as well as the absence of clinical trials comparing ILP with other procedures, making it difficult to transfer its results to the current era. Conclusions. ILP is still an effective and safe procedure for selected patients with unresectable melanoma of the limbs. In the era of targeted therapies and immunotherapy, ILP remains an acceptable and reasonable palliative treatment alternative, especially to avoid limb amputations. The ongoing clinical trials combining systemic therapies and ILP will provide more valuable information in the future to clarify the potential synergism of both strategies.
1. Introduction
Melanoma still represents a major challenge for health systems around the world, due to its rising incidence and the certainty that an early detection would mean a cure for most of the patients. Nevertheless, in the advanced stages and especially when some risk factors are present (node involvement, ulceration), melanoma cases imply a greater complexity which causes a high morbi-mortality. As stated, melanoma incidence continues to increase in the Western world: according to GLOBOCAN 2020 [1], the expected world number of new cases of CM was 324,635 in 2020, with an age-standardized incidence rate of 3.2 per 100,000/year and a mortality rate of 0.57 per 100,000/year.
Although most of the patients are diagnosed in early stages of the disease, approximately 5–8% of patients with melanoma recurrences will develop in-transit metastases, that is, multiple recurrent tumor deposits in the superficial lymphatic vessels, most often confined to the extremities [2]. Among these patients, the quality of life is greatly compromised, mainly due to tumor burden-related complications [3].
A classical option for patients with a high tumor burden or rapidly recurrent in-transit metastases is isolated limb perfusion (ILP) therapy instead of a locoregional surgical resection, or when the latter is no longer feasible. This technique was first unveiled in 1958 by Creech and Krementz [4]. The procedure consists of isolating the involved limb from the systemic circulation (using a properly placed pneumatic tourniquet or Esmarch bandage) and administering chemotherapy agents through a cannulated artery and vein using an extracorporeal bypass circuit, which allows the administration of a dose of cytostatics up to 20 times greater than the systemic dose [5]. In 1959, the first ILP intervention to treat a patient with an in-transit melanoma on one leg using melphalan took place (L-phenyl alanine mustard) [6]. In 1969, Stehlin combined moderate hyperthermia (40–41 °C) and ILP to enhance the effect of melphalan [7].
The tumor necrosis factor (TNF-α) is a cytokine with direct and indirect antitumor effects. Its effects may be mediated by a specific destructive effect against the tumor vasculature that is synergized with the cytotoxic effect of melphalan [8]. Since TNF-α is a key physiological mediator of the systemic inflammatory response, the systemic administration in doses with an antitumor effect has severe and potentially fatal side effects. Therefore, it can only be used clinically in ILP, and the continuous monitoring of the perfusion circuit leaks is an absolute requirement [9]. In 1994, Lienard et al. reported an additional positive effect with TNF-α and melphalan (TM-ILP) [10]. Subsequently, it is suggested on the basis of several case series that melphalan plus TNF-α ILP has higher overall and complete response rates than melphalan alone [11,12,13]. Despite this, phase III, randomized trials are lacking to fully elucidate the real value of ILP with or without TNF-α, and its eventual impact on survival over other strategies. Furthermore, the advent and development of immunotherapy and targeted therapies has dramatically changed the therapeutic landscape of patients with advanced melanoma, with a significant improvement in patient survival in the last decade. Therefore, in this era of modern systemic treatments for melanoma, it still remains interesting to analyze the role of management approaches for locoregionally advanced disease, such as isolated limb perfusion (ILP). With this purpose, we conducted a systematic review updating the available literature on ILP for malignant melanoma (MM). The main objectives of this review focused on the effectiveness and safety of ILP.
In this systematic review of the literature, we present 25 studies published between 2000 and 2019 that include a total of 2637 ILPs.
2. Materials and Methods
Between June and July 2021, searches in PUBMED, MEDLINE, and EMBASE were performed using the following list of keywords (intra-arterial chemotherapy, intra-arterial perfusion, isolated limb perfusion, cutaneous melanoma, malignant melanoma, transit metastases, satellite, locoregional metastases, melphalan, interferon alpha, tumor necrosis factor alpha, normothermia, hyperthermia, complete response, partial response, overall response, survival, overall survival, disease-free survival, toxicity, regional toxicity, systemic toxicity). We also searched the reference lists of previous systematic reviews, as well as the Cochrane database, where no studies were found.
To limit biases, the reviewers performed an exhaustive search of all relevant articles, explicit and reproducible selection criteria, assessment of the design and characteristics of the studies. The assessment of the methodological quality of the studies was carried out by five reviewers independently to avoid evaluation biases. We understand that one of the main biases of the review may be publication bias, since unpublished articles in the aforementioned databases were not taken into account.
Data analysis was performed using the SPSS version V28 program. The presentation of the tabulations was carried out using the Windows Excel and Word programs.
PRISMA statement has been followed to carry out this review and the Registration Number is reviewregistry1244.
2.1. Inclusion and Exclusion Criteria
Eligible studies had to meet the following inclusion criteria: (1) studies published between 2000 and 2020; (2) studies including subjects with unresectable MM of the extremities treated with any ILP regimen, regardless of temperature level (hyperthermia, normothermia) or the chemotherapy drug administered (melphalan, melphalan and TNF, others); (3) studies that analyze efficacy or effectiveness endpoints (clinical response, survival, recurrence rate, limb recovery rate); (4) studies that analyze safety parameters in terms of regional toxicity and/or systemic toxicity; (5) and Eligible study designs: randomized clinical trials (RCTs), cohort studies, case–control studies, and case series.
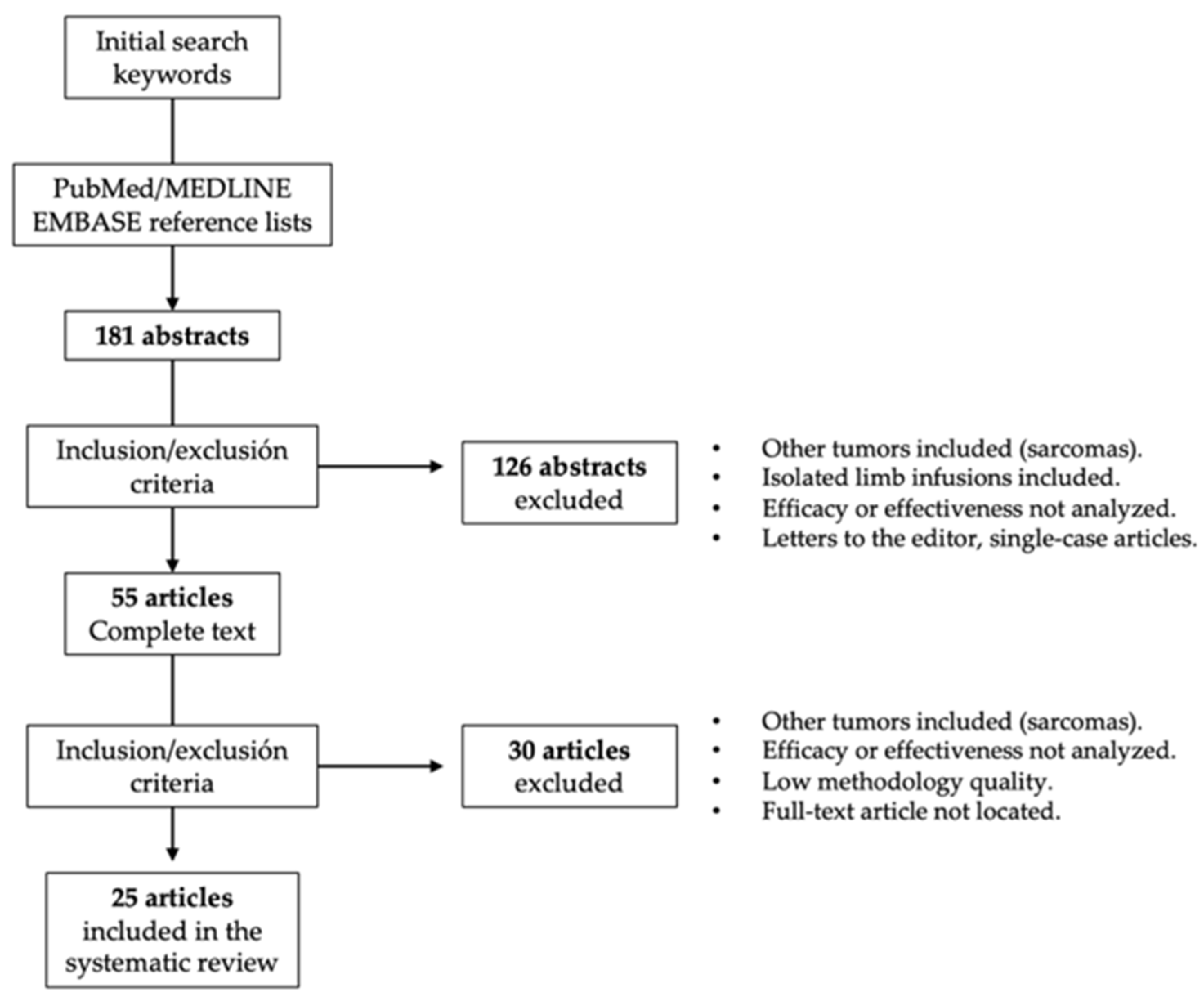
Studies in which the perfusion methodology (chemotherapeutic drug, temperature regimen, etc.) was not clearly described, studies that did not report valid results on clinical effectiveness or toxicity, letters to the editor, non-systematic reviews, and studies that applied outdated clinical guidelines were excluded from this systematic review (Figure 1). To rule out studies of low methodological quality, the following aspects were also required: detailed description of the applied ILP regimen, clinical context, follow-up periods, analyzed clinical endpoints, and number of analyzed ILPs.
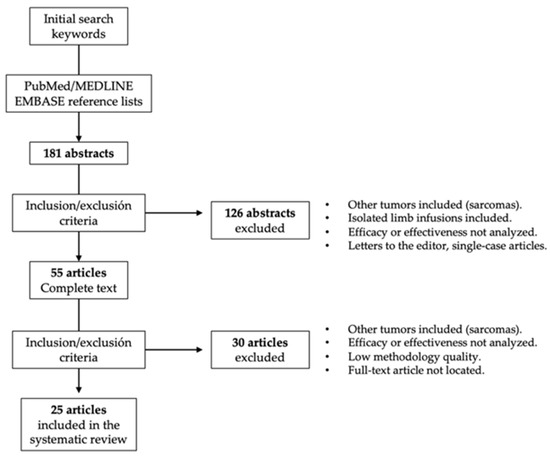
Figure 1.
Procedure for the selection of studies included in this systematic review.
Abstracts for which we could not located a full-text article were excluded, as were publications in a language other than English. Five reviewers collected the data independently by tabulating the study intervention characteristics and comparing them against the planned groups for each synthesis. Disagreements were resolved by consensus.
2.2. Outcome Measures
The RECIST and WHO criteria to assess tumor response to nonsurgical treatments were applied to extract data on objective clinical responses to ILP [14,15]. Therefore, the percentage of patients who achieved complete response (CR), partial response (PR), and overall response (OR) were the efficacy endpoints analyzed. Studies that did not provide direct information on these measures were also included if they could be calculated from the available data. In that regard, OR was calculated as the sum of CR and PR. The measure used to synthesize these results was the median, providing the interquartile range in all instances.
Survival after ILP was also analyzed. At this point, data on overall survival were extracted in terms of percentage (3-year and 5-year overall survival) and median overall survival. Median progression-free survival was also registered. Other secondary endpoints drawn from the analyzed studies were time to local progression (TTLP), time to systemic progression (TTSP), melanoma specific survival and the rate of limb recovery.
For the assessment of regional toxicity, studies describing the results according to the Wieberdink classification system for regional toxicity were included in the review [16]. For the analysis of systemic toxicity, the Common Terminology Criteria for Adverse Events version 3.0 [17], version 4.0 [18], version 5.0 [19] and the WHO classification of chemotherapy toxicity [20] were contemplated.
3. Results
Twenty-five studies were included in this review, representing a total of 2637 ILPs (Table 1), with a median of 91 ILPs included (range 17–380). Most were observational (88%, n = 22), while there were only three clinical trials [21,22,23] (12%), and only one of them was a randomized clinical trial comparing two chemotherapy regimens [21]. Four studies (16%) reported results on repeated ILPs [24,25,26,27]. All studies provided efficacy data in terms of the clinical response and toxicity except the study by Alexander et al. [28], which only reported efficacy data. The mean age of the patients was 64.07 years.

Table 1.
Studies of ILP for unresectable locally advanced melanoma of the limbs included in the systematic review.
3.1. Clinical Response
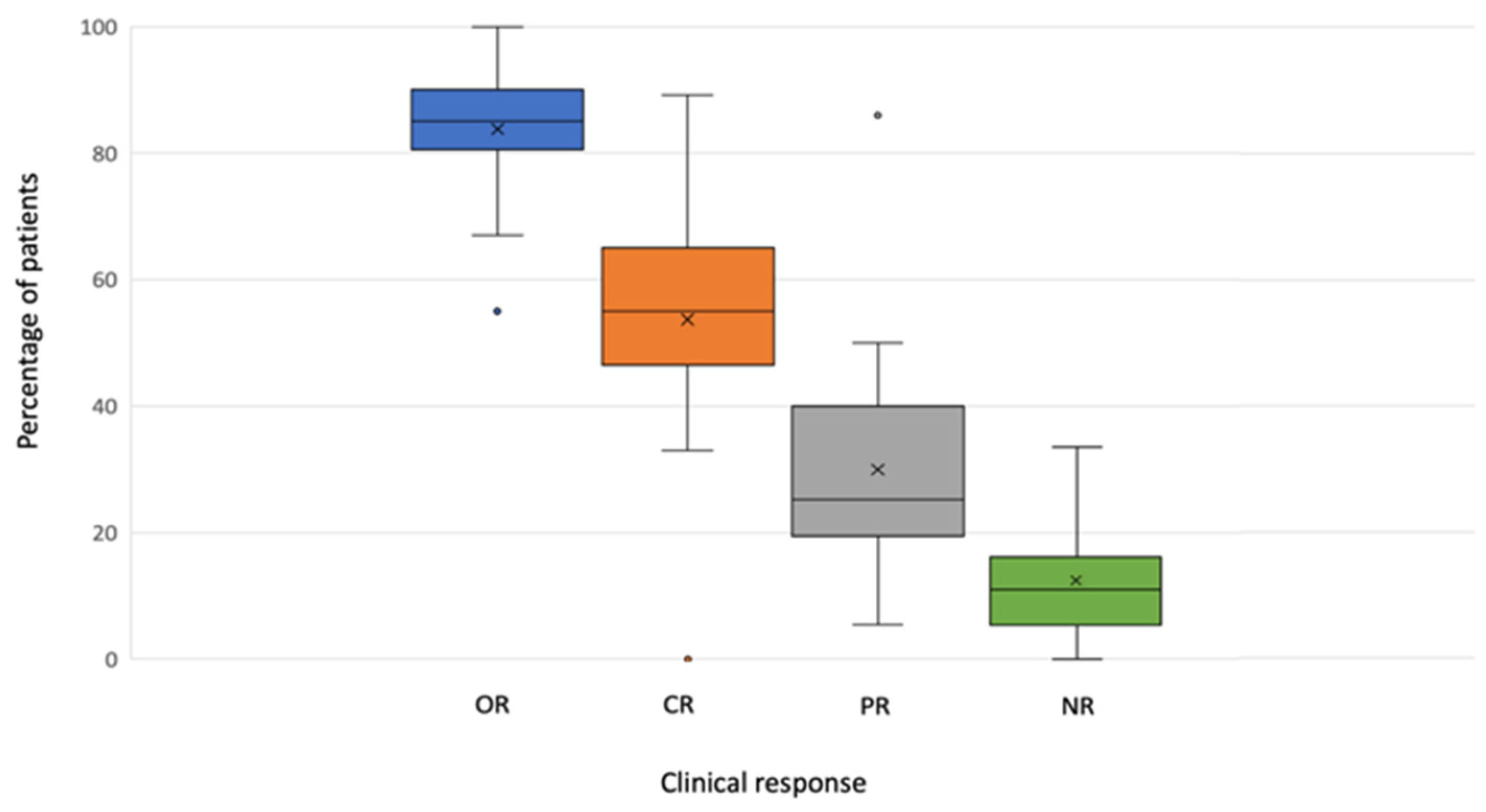
All studies provided data on the clinical response. A median OR of 85.00% (range 55.00–100.00) was reported, with a median CR of 58.5% (range 0.00–89.20%) (Table 2).

Table 2.
Clinical response to ILP in studies included in the systematic review.
The valid data on ILP efficacy with melphalan alone could be ascertained from seven studies that included 508 ILPs. They reported a median OR of 84.5% (range 79–90.6%), with a median CR of 57% (range 33–64%). Regarding the melphalan and TNF combination, twelve studies [21,22,24,26,27,31,34,35,37,41,42,43] (n = 855) reported valid data, with a median OR of 93.00% (range 67–100%) and a median CR of 61.5% (range 33–76%).
According to Rossi et al. [37], the complete response rate was higher among the patients who underwent isolated limb perfusion with TNF-α, with respect to those who had undergone isolated limb perfusion with only melphalan (60.3% versus 41.5%; p = 0.036). However, the aforementioned study failed to demonstrate significant differences between the melphalan monotherapy and the combination in the short-term response rate, with a complete response rate of 25% (14 of 58 patients) in the melphalan arm and 26% (15 of 58 patients) in the melphalan-plus-TNF-α arm (p = 0.435 and p = 0.890, respectively).
In general, in the studies with a greater number of patients included [21,27,33,34,35,37,41,43,45]. Nevertheless, other therapies were also included in the literature. Specifically, two studies included the combination of melphalan with D-actinomycin (n = 243; OR 87.65% [range 80.70–96.60%]; CR 76% [range 62.8–89.20%]); and one included the combination of melphalan with dacarbazine (n = 100; OR 90.80%; CR 73.40%). In addition, a pilot trial [23] that included 17 patients used melphalan combined with L-19 TNF at different doses (325 µg and 650 µg) reported CR in half of the patients (5/10) who received the 650 µg dose, since none of the seven patients in the 325 µg dose cohort achieved this. Rossi et al. [22] compared the combination of melphalan, TNF-α and IFN α-2b versus melphalan and TNF-α. 50% responses (12/24) were observed in the first group and 53% (10/19) in the second group. Grunhagen et al. [41] reported that no significant difference in the CR-rate was found between patients receiving a melphalan-ILP with or without IFNγ (78% vs. 66%, respectively, p = 0.274). No study was able to establish clearly that the addition of TNF-α increased the rate of complete responses.
Two 12/24) studies compared the results of performing one ILP with repeated ILPs [25,27] (total n = 422 ILPs). In addition, Deroose et al. [26] and Grunhagen et al. [24] performed repeated ILPs and compared the results with those found in their center’s database based on just an initial ILP. None of them found statistically significant differences in the response rate after a first ILP or after repeated ILP.
Regarding temperature, all the studies were carried out in hyperthermia except those of Noorda, which also included ILPs carried out in normothermia. Only one study [29] analyzed the results reported separately using the data from using different temperatures at different durations (39–40 °C for 60 min; 39–40 °C for 90 min; 39–40 °C for 120 min or 41–41.5 °C for 120 min), finding a longer perfusion time (120 min) under mild hyperthermia (39–40 °C) as a predictive factor of CR.
Several studies analyzed the predictive factors of response. In the multivariate analysis, they found that the statistically significant predictive factors for the complete response were: a total number of metastases less than ten [27,29,33], a longer perfusion time (120 min) under mild hyperthermia (39–40 °C) [29], TNF dose [34,35], age < 65 years [34], and the absence of lymph node metastases [37,43] or at stage IIIB or less [34]. Disease stage was also a predictor of the complete response in the Deroose et al. [35]. No study was able to demonstrate that gender was included in the CR rate in multivariate analysis.
Data on clinical response are graphically represented in Figure 2.
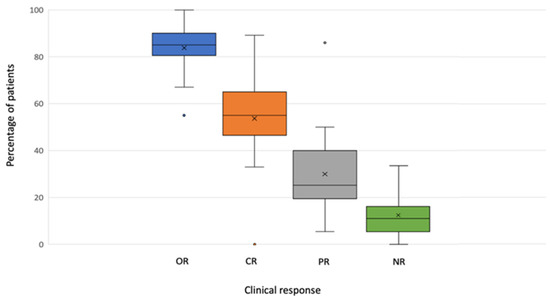
Figure 2.
Boxplot of response by variables.
3.2. Survival
Twenty-three studies (n = 2642) provided valid data on survival (Table 3). Of these, 17 studies (n = 2195) provided data on OS, reporting a median OS of 38 months (range: 17–56 months), as well as a median OS at 3, 5, and 10 years of 38%, 35%, and 16%, respectively.

Table 3.
Survival results of ILP for unresectable locally advanced melanoma.
Thirteen studies (n= 1060) [24,26,30,31,32,33,34,35,38,41,42,44] reported valid data on the rate of local progression-free survival (LPFS). A median of 56% (range: 46–63%) of the patients presented a local relapse with a median LPFS of 13 months (range: 6–17.4 months).
Regarding the impact on survival with the addition of TNF to melphalan therapy, six studies (n = 837) reported valid results [22,27,28,31,33,37]. None of them reported statistically significant differences between the treatment with melphalan + TNF vs. melphalan alone. The addition of TNF-α was also not identified as a predictive factor for survival in any study.
On the other hand, and considering the other drugs, Rossi et al. [22] included 31 patients in their pilot trial, comparing the combination with melphalan, TNF-α and IFN α-2b versus melphalan and TNF-α. A significant increase in PFS was demonstrated in the IFN α-2b group (median time to progression: 26 and 17 months, respectively; log-rank test p-value: 0.037). This survival benefit was confirmed by a multivariate analysis, where treatment was found to be an independent predictor of longer OS. Another parameter registered in five studies [30,31,33,35] was the melanoma-specific survival (MSS), with a median of 30 months (range 24–52 months).
The median time to local progression (TTLP) for patients with CR was 18 months (range: 10–23.8 months) [30,31,36,38,41,42], with a median of 5.7 months (range: 4–14 months) for patients with RP [30,31,37,41]. Both Madu et al., and Rossi et al., observed statistically significant differences for the LPFS of patients who presented with CR vs. those who did not (p < 0.001 and p < 0.0001, respectively). No study observed statistically significant differences in TTLP for the patients treated with melphalan alone vs. patients treated with melphalan plus TNF-α [31,34,37].
Five studies provided valid results on the time to systemic progression (TTSP) [24,36,38,39,40], with a median of 10.75 months (range: 7.5–19.7) for the general population. TTSP did not differ between patients receiving a single ILP (12 months) and multiple ILPs (15 months; p = 0.27) [24].
Belgrano et al. [27] and Grunhagen et al. [24] reported survival comparisons with an ILP and with repeated ILPs. Only Belgrano et al. (n = 380) reported differences in the overall survival, with a median OS of 34 months for the patients treated with one ILP, 41 months for patients treated with two ILPs, and 93 months for those who underwent three to five ILPs (p = 0.02). Grunhagen et al. [24] also did not observe significant differences in BPD after ILP, compared to re-ILP: the median time to local progression (TTLP) was 14 months for the repeated perfusion versus 16 months for the overall population and 18 months for single ILPs (p = 0.40).
Two studies compared the effectiveness of ILP based on the age of the patients: one of them stratified the patients at <75 and ≥75 years old [45] and another at ≤70 and >70 years old [30]. Noorda et al. [45]. found no significant differences in the rate of CR, recurrence, DFS, and OS between both groups. Madu et al. [30] reported a median MSS (45 months for patients ≤70 years of age, and 18 months for patients over 70 years of age) as the only difference found between the two groups (p = 0.038), showing that an age of over 70 years (p < 0.001, HR 3.86, 95% CI 1.94–7.71) increased the risk of death by melanoma.
Five studies [34,35,36,38,39] reported survival data stratified by the tumor stage. Stage IIIA (lymph node micrometastases in the previous AJCC staging system) was associated with a better survival in several studies. According to Deroose et al., 35 patients presenting with stage IIIA disease had 5- and 10-year, disease-specific survival rates of 47 and 31% with a median disease-specific survival of 58 months, compared with 12%, 4%, and 20 months in the stage IIIA-B group (p < 0.001). No patient with stage IV disease survived for more than 3 years.
The predictive survival factors were defined in 13 studies according to a multivariate analysis. The identified predictive factors of a higher survival were: a lower stage, smaller number of metastases, low Breslow index, an increased number of ILPs, CR, and a lower age. Additionally, Deroose et al. [34] identified sex as a prognostic factor for the time to systemic progression (TTSP). Alexander et al. [28] reported that the female sex was significantly and independently associated with prolonged in-field PFS, while only the female sex was shown to be associated with OS (p = 0.27). However, according to Rossi et al. [22], the only independent prognostic factor was treatment, with a risk reduction of 62% in favor of adding IFN to melphalan with a TNF-α therapy. Deroose et al. [34] analyzed BMI (body mass index) as a prognostic baseline factor, but did not reach a significant conclusion regarding the clinical outcome, nor for TTLP, TTSP, or OS.
3.3. Secondary Effectiveness Endpoints
The limb salvage rate (LSR) was analyzed in six studies, which included 474 patients in whom the only therapeutic alternative to ILP was amputation. They reported a median LSR of 94% with a median follow-up of 32 months (range 19–51 months).
3.4. Toxicity
3.4.1. Locoregional Toxicity
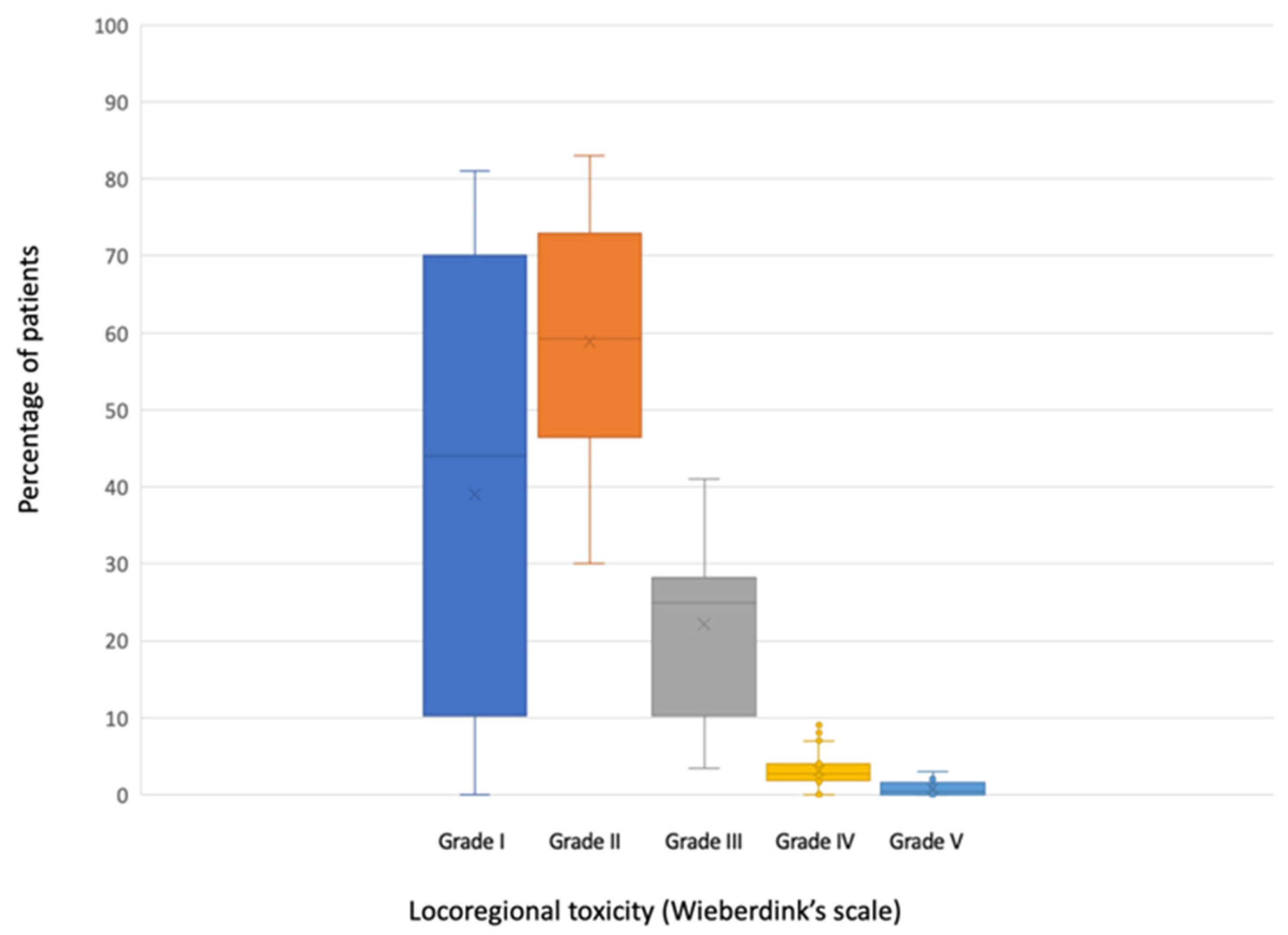
All the studies included in this review, except for one [28], reported valid data on locoregional toxicity (n = 2546) (Table 4). Three of them [26,27,34] grouped mild toxicities (Wieberdink grade I and II) when reporting their results. A median of 44.4% of the patients (range 0–81%) did not present any toxicity (Wieberdink grade I). Grade II occurred in a median of 56% (range 30–83%); grade III in a median of 24.4% (range 0–38.2) and grade IV in a median of 2.5% (range 0–7%). A median of 0.2% of patients (range 0–3%) required amputation due to toxicity produced by ILP (grade V).

Table 4.
Regional toxicity of ILP.
Two studies [21,43] reported valid data on locoregional toxicity comparing melphalan and melphalan plus TNF-α. Alexander et al. observed that the most significant systemic toxicities were associated with the use of TNF (transient hypotension was the most common). Cornett et al. [21] reported that grade 4 AEs were observed in 14 patients (11%), with 3 out of 64 patients (5%) in the melphalan-alone arm and 11 of 66 patients (17%) in the melphalan-plus-TNF arm (p = 0.028). However, Noorda et al. [43] found no difference in locoregional toxicity, complications or long-term morbidity. Rossi et al. [22] also did not observe a difference when adding IFN.
In relation to toxicity produced by other drugs, Papadia et al. [23] reported that the acute local toxicity of L19-TNF ILP was mild, most likely because TNF via L19-TNF was targeted directly to the tumor tissue using a much lower total TNF-α activity compared to TM-ILP. Rossi et al. [22] showed that a grade 2 toxicity was similar in the melphalan-plus-TNF group to that of the group with melphalan, TNF-α and IFN-γ (83% vs. 79%; p = 0.70).
Madu et al. [30] and Noorda et al. [45] observed that the incidence and severity of locoregional toxicity did not differ between age groups. Noorda et al. [45] did not find differences in the short- or long-term morbidity, or in other postoperative complications (seroma, local infection, etc.). Regarding the toxicities after the first ILP or after repeated ILP [25,26,27], no studies found significant differences (p = 0.664, p = 0.288, and p = 0.28, respectively). Katsarelias et al. [29] performed a multivariate analysis, comparing Wieberdink I–III versus IV–V and Wieberdink I-II versus III toxicities, and concluded that the perfusion at 41–41.5 °C for 120 min had a higher rate of severe toxicity (grade III–V), with an odds ratio of 3.9 (p = 0.04) and 2.59 (p = 0.05), respectively.
The predictive factors of toxicity were analyzed in four studies (n = 619) [21,29,33,40]. A more advanced age [33,40], longer perfusion time (120 vs. 90 min), higher perfusion temperature (41 °C vs. 40 °C) [29,33] and the female sex were identified as predictive factors of toxicity [40].
Data on locoregional toxicity are graphically represented in Figure 3.
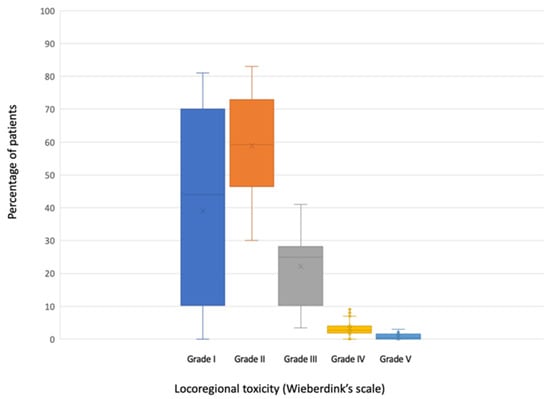
Figure 3.
Boxplot of locoregional toxicity according to Wieberdink’s scale.
3.4.2. Systemic Toxicity
The systemic toxicity was reported in twelve studies [22,23,28,30,33,34,35,36,37,39,41] (Table 5) in a very heterogeneous manner; severe toxicities (myocardial ischemia, pulmonary embolism) were rare. The most commonly reported adverse effects were hematological (especially leukopenia, as well as thrombocytopenia and anemia), fatigue, fever, hypotension (this was transitory and treated with vasopressors, associated in some studies with TNF-α leaks), and the mild elevation of myoglobin. Four deaths were described at some point during hospital admission after ILP: three of a cardiac cause and one of a respiratory cause.

Table 5.
Systemic toxicity of ILP.
Finally, Belgrano et al. [27]. did not find differences in the systemic toxicity between the first and subsequent ILP (p = 0.54).
3.5. Complimentary Data
Seven studies reported the percentage of leakage measured during ILP [22,24,28,30,34,35,41]. The median was 0% (range 0–0%), with a median maximum leakage of 20.5 (range 7.4–32%).
Noorda et al. evaluated hospital stays in two studies [25,45]. In their 2016 study with re-ILP, they reported a median of 23 days (range 9–65 days), which was not significantly longer than after the first procedure (20 days, p > 0.05). In 2002 it was observed that patients older than 75 years of age stayed significantly longer in the hospital than younger patients. Additionally, the female sex, wound infection and more severe limb toxicity were risk factors for a longer hospital stay.
4. Discussion
In this review, we confirm that ILP still offers a high efficacy, with response results comparable to those reported in the review by Moreno-Ramirez et al. [46], with a median global response of 90% (64–100%) and median complete responses of 58% (25–89%). The most widely used and tested drugs are melphalan and TNF-α. Others, such as dacarbazine or dactonomycin, have been used in few centers and their efficacy is less well-established. Higher overall and complete response rates are obtained with the melphalan-TNF combination than with melphalan alone. However, it appears that the addition of TNF to ILP is associated with a greater toxicity and there is no survival benefit. In general, studies agree that the longer the time interval between the treatment of the primary tumor and the development of ITM and the lower the tumor burden, the better the MSS and overall survival. Thus, there is some consensus in most melanoma referral centers that the true indication for TM-ILP is the presence of a bulky disease or the event of relapse after previous M-ILP [35].
Performing more than one ILP for the same patient is safe and does not appear to increase locoregional toxicity. Even a higher number of re ILPs was described as a predictive factor for survival in the multivariate analysis performed by Belgrano et al. [27]. For recurrent metastases in transit, re-ILP still plays a role, especially if the patient responds after the first treatment.
ILP in the elderly offers response rates similar to those obtained in young patients and is safe, with no evidence of increased short-term morbidity or a higher incidence of postoperative complications.
Another factor described as a predictor of better survival was the treatment with IFN alpha 2b. In fact, in our center and others, interferon alfa 2b was administered to some patients in an effort to consolidate the antitumor effect of ILP, based on the results of the pilot study carried out by Rossi et al. [22] as justification. This clinical trial tested the hypothesis that the systemic administration of low-dose interferon alfa 2b could increase the duration of progression-free survival in patients undergoing TNF-based ILP. A statistically significant difference in progression-free survival of 26 vs. 17 months favoring the IFN group (P = 0.037) was observed. In addition, this survival benefit was confirmed at multivariate analysis, where treatment was the only prognostic factor retained by the prediction model and the analysis of the risk of disease progression over time suggested that this survival benefit appeared to vanish after IFN discontinuation.
At low doses, IFNα, the only drug that was used in adjuvant melanoma until the approval of targeted therapies and immunotherapy in recent years, appears to inhibit tumor angiogenesis by directly inhibiting endothelial cell proliferation and negatively regulating the expression of proangiogenic factors (e.g., VEGF, b-FGF, IL-8, and matrix metalloproteinases) [47].
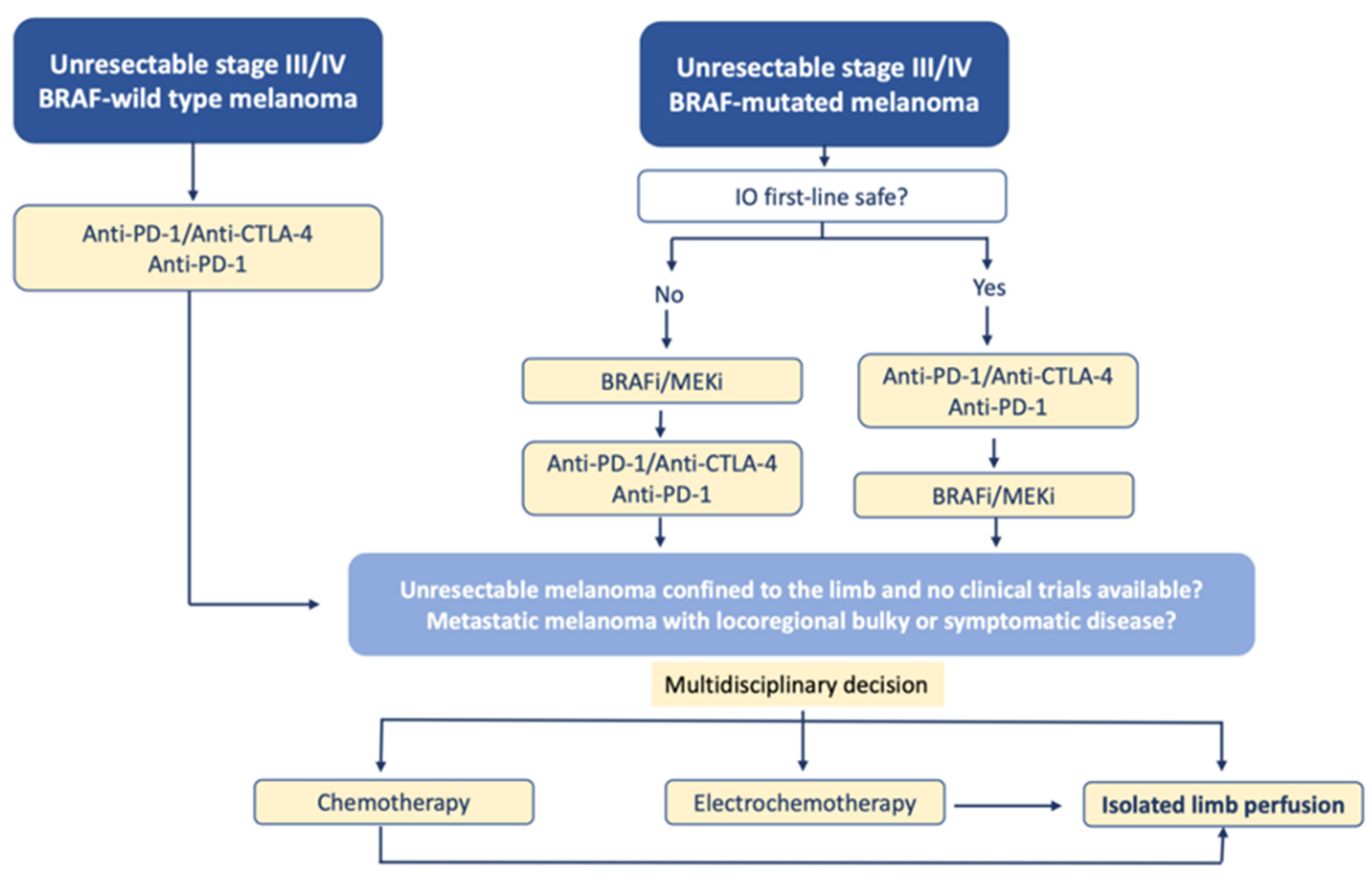
Although the presence of disseminated disease was not a contraindication of ILP for in-transit melanoma, the current treatment of these patients is undoubtedly immunotherapy and targeted therapies, as shown by the following therapeutic algorithm that we propose (Figure 4):
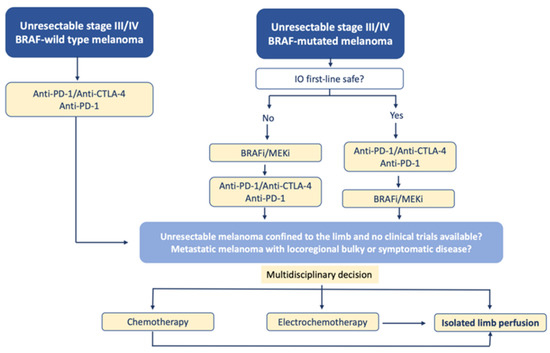
Figure 4.
Proposal for a therapeutic algorithm for the management of patients with unresectable melanoma of the limbs including ILP (modified from ESMO guidelines).
Electrochemotherapy, as indicated in the previous algorithm, is a valid alternative to ILP in case of cutaneous and subcutaneous metastases of melanoma.
It is based on the phenomenon of reversible electroporation. In this method, by applying an electric current to the tissue, we induce a temporary increase in permeability of the cell membrane, thus enabling a free flow of large molecules into the cell, including cytostatics that, at baseline, are not transported to the cytosol. As a result, their potential toxicity increases considerably [48].
The studies published in relation to electrochemotherapy in melanoma, similar to the studies on ILP, are heterogeneous and generally include few patients. In a review of the literature carried out by Wichtowski [49], which included 12 publications, an OR of 74% (CR rate of 40.1% and PR rate of 34%), slightly lower than those described in our review was recorded, so this option could be chosen in centers where ILP is not available or when the patient is not a candidate for it.
The development of new systemic drugs in the last decade has radically changed the treatment of melanoma. The introduction of BRAF/MEK inhibitors and immune checkpoint inhibitors offers new hope for patients with stage IV melanoma. The specific response rates with these drugs for in-transit metastases have not been reported to date, but the results in terms of the overall survival are superior to those demonstrated in this review.
Immunotherapy studies report a 3-year OS rate from 50–58% and a 5-year OS rate from 34–44% with anti-PD1 as single therapies and 52% with the combination antiPD1 + anti CTLA 4 [50,51]. The targeted therapy studies show a median overall survival of 22.3 to 33.6 months [52,53]. Additionally, the updated results for dabrafenib-trametinib have recently been published [54] with a 5-year OS of 34%. It must be taken into account that the majority of patients included in these clinical trials had stage IV melanoma, while most of the patients in our review had stage III, that is, they presented an earlier stage of the disease.
Guadagni et al. [55] evaluated the current role of melphalan, hypoxic, pelvic perfusion in patients with advanced pelvic melanoma retrospectively. The overall median survival time (MST) stratified for variables, including the BRAF V600E mutation and eligibility for treatments with new immunotherapy drugs, was assessed in 41 patients with pelvic melanoma loco regional metastases who received a total of 175 treatments with melphalan hypoxic perfusion and cytoreductive excision. The first treatment resulted in a 97.5% response-rate in the full cohort and a 100% response-rate in the 22 wild-type BRAF patients. MST spent 18 months in the full sample, 20 months for the 22 wild-type BRAF patients and 21 months for the 11 wild-type BRAF patients not eligible for immunotherapy. Guadagni et al. conclude that Melphalan hypoxic perfusion is a potentially effective treatment for patients with locoregional metastases of pelvic melanoma and propose to determine if Melphalan pelvic perfusion under conditions of hypoxia may generate an immune response that could be augmented by systemic immunotherapy with anti-programmed cell death-ligand protein 1 (PD-L1) antibodies [56].
In this regard, the study by Ariyan CE et al. [57] combines isolated limb infusion (ILI) and ipilimumab and shows a positive synergistic effect. In this study, 26 patients with advanced melanoma were treated locally by ILI with the nitrogen mustard-alkylating agent melphalan, followed by the systemic administration of CTLA-4 blocking the antibody (ipilimumab) in a phase II trial. This combination of local chemotherapy with a systemic checkpoint blockade inhibitor resulted in a response rate of 85% at 3 months (62% complete and 23% partial response rate) and a 58% progression-free survival at 1 year. The clinical response was associated with an increased T-cell infiltration, similar to that seen in the murine models. All together, these findings suggest that local chemotherapy combined with checkpoint blockade-based immunotherapy may synergize and induce a durable response to cancer therapy.
It would also be interesting to analyze the role of ILP in some subtypes of melanomas with a worse response to immunotherapy (such as acral or mucosal melanomas). Although there are also no clinical trials in this setting, their role would be similar to that of other melanomas, that is, it could be assessed as a palliative treatment after the progression to immunotherapy or targeted therapies.
Finally, a major limitation of our review is the age and the great heterogeneity between the included studies (most of them with a small number of patients and carried out retrospectively), which makes it difficult to transfer their results to the current era.
5. Conclusions
ILP, with its low incidence of regional and systemic toxicity, is a valuable palliative treatment not only for patients with a disease confined to the limbs, but also for patients with metastatic melanoma with bulky or symptomatic diseases to improve their quality of life. Therefore, we believe that this procedure should still be considered when the rest of the highly effective systemic therapies available at the present time have failed, especially in cases where local disease morbidity (ulceration, painful and bleeding lesions, and others) is a major challenge, and other locoregional strategies, such as electrochemotherapy are not indicated or are ineffective. Above all, ILP must always be considered in cases where amputation could eventually be indicated.
The clinical trials that combine ILP, intralesional and systemic therapies are underway and the first preliminary results seem encouraging. Hopefully, the emerging new data from these combinatorial strategies could clarify the future role of ILP in the global management of locoregional melanoma disease.
Author Contributions
L.S.-O.: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing original draft, writing review and editing; L.F.-P.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, project administration, resources, writing original draft; N.P.-C.: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing original draft, writing review and editing; M.d.C.Á.d.l.G.: conceptualization, data curation, Investigation; R.d.T.-S.: conceptualization, data curation, investigation; J.G.-M.: methodology, resources, investigation, writing—original draft; J.A.M.-R.: data curation, software, resources; A.A.M.: methodology, resources, investigation, writing original draft; O.A.-T.: methodology, resources, investigation, writing—original draft; M.C.C.-M.: methodology, resources, investigation, writing—original draft; J.M.B.-A.: methodology, resources, investigation, writing—original draft; A.R.F.-L.: Methodology, resources, investigation, writing—original draft; J.M.J.-B.: investigation, resources; J.C.S.-J.: investigation, resources; D.M.-R.: conceptualization, data curationm formal analysism investigation, methodology, project administration, resources, supervision, validation, writing original draft; L.d.l.C.-M.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing original draft, writing review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GLOBOCAN. 2020. Available online: https://gco.iarc.fr/ (accessed on 10 May 2021).
- Pawlik, T.M.; Ross, M.I.; Johnson, M.M.; Schacherer, C.W.; McClain, D.M.; Mansfield, P.F.; Lee, J.E.; Cormier, J.N.; Gershenwald, J.E. Predictors and Natural History of In-Transit Melanoma After Sentinel Lymphadenectomy. Ann. Surg. Oncol. 2005, 12, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ekenberg, M.; Wesslau, H.; Bagge, R.O.; Engström, M. Patient experiences with isolated limb perfusion for malignant melanoma —A qualitative study. Eur. J. Oncol. Nurs. 2019, 43, 101672. [Google Scholar] [CrossRef] [PubMed]
- Creech, O.; Krementz, E.T.; Ryan, R.F.; Winblad, J.N. Chemotherapy of Cancer. Ann. Surg. 1958, 148, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Benckhuijsen, C.; Kroon, B.B.; van Geel, A.N.; Wieberdink, J. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur. J. Surg. Oncol. 1988, 14, 157–163. [Google Scholar] [PubMed]
- Creech, O.M.D., Jr.; Robert, F.; Ryan, M.D.; Edward, T.; Krementz, M.D. Treatment of malignant melanoma by isolation perfusion technique. J. Am. Med. Assoc. 1959, 169, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Stehlin, J.S., Jr. Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg. Gynecol. Obs. 1969, 129, 305–308. [Google Scholar]
- Eggermont, A.M. The success of TNF alpha in isolated limb perfusion for irresectable extremity soft tissue sarcomas, melanoma and carcinomas: Observations in patients and preclinical perfusion models. Gan Kagaku Ryoho. 1996, 23, 1357–1370. [Google Scholar]
- Thompson, J.F.; Waugh, R.C.; Saw, R.P.; Kam, P.C. Isolated limb infusion (ILI) with melphalan for recurrent limb melanoma. Reg. Cancer Treat 1993, 6, 51–52. [Google Scholar]
- Liénard, D.; Eggermont, A.M.; Koops, H.S.; Kroon, B.B.; Rosenkaimer, F.; Autier, P.; Lejeune, F.J. Isolated perfusion of the limb with high-dose tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and melphalan for melanoma stage III. Results of a multi-centre pilot study. Melanoma Res. 1994, 1, 21–26. [Google Scholar]
- Vaglini, M.; Belli, F.; Ammatuna, M.; Inglese, M.G.; Manzi, R.; Prada, A.; Persiani, L.; Santinami, M.; Santoro, N.; Cascinelli, N. Treatment of primary or relapsing limb cancer by isolation perfusion with high-dose alpha-tumor necrosis factor, gamma-interferon, and melphalan. Cancer 1994, 73, 483–492. [Google Scholar] [CrossRef]
- Fraker, D.L.; Alexander, H.R.; Andrich, M.; Rosenberg, S.A. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: Results of a tumor necrosis factor dose-escalation study. J. Clin. Oncol. 1996, 14, 479–489. [Google Scholar] [CrossRef]
- Liénard, D.; Eggermont, A.M.M.; Koops, H.S.; Kroon, B.; Towse, G.; Hiemstra, S.; Schmitz, P.; Clarke, J.; Steinmann, G.; Rosenkaimer, F.; et al. Isolated limb perfusion with tumour necrosis factor-α and melphalan with or without interferon-γ; for the treatment of in-transit melanoma metastases: A multicentre randomized phase II study. Melanoma Res. 1999, 9, 491–502. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Re- search and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Wieberdink, J.; Benckhuysen, C.; Braat, R.; Van Slooten, E.; Olthuis, G. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur. J. Cancer Clin. Oncol. 1982, 18, 905–910. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE); NCI, NIH, DHHS; DCTD: Bethesda, MD, USA, 2003; pp. 1–72. [Google Scholar]
- Common Terminology Criteria for Adverse Events, Version 4.0 (CTCAE). Publised: 28 May 2009 (v4.03: 14 June 2010) by National Cancer Institute. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 10 May 2021).
- Common Terminology Criteria for Adverse Events, Version 5.0 (CTCAE). Publised: 27 November 2017 by National Cancer Institute. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 10 May 2021).
- World Health Organisation. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organisation: Geneva, Switzerland, 1979. [Google Scholar]
- Cornett, W.R.; McCall, L.M.; Petersen, R.P.; Ross, M.I.; Briele, H.A.; Noyes, R.D.; Sussman, J.J.; Kraybill, W.G.; Kane, J.M.; Alexander, H.R.; et al. Randomized Multicenter Trial of Hyperthermic Isolated Limb Perfusion With Melphalan Alone Compared With Melphalan Plus Tumor Necrosis Factor: American College of Surgeons Oncology Group Trial Z0020. J. Clin. Oncol. 2006, 24, 4196–4201. [Google Scholar] [CrossRef]
- Rossi, C.R.; Russano, F.; Mocellin, S.; Chiarion-Sileni, V.; Foletto, M.; Pilati, P.; Campana, L.G.; Zanon, A.; Picchi, G.F.; Lise, M.; et al. TNF-Based Isolated Limb Perfusion Followed by Consolidation Biotherapy with Systemic Low-dose Interferon Alpha 2b in Patients with In-transit Melanoma Metastases: A Pilot Trial. Ann. Surg. Oncol. 2008, 15, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Papadia, F.; Basso, V.; Patuzzo, R.; Maurichi, A.; Sc, A.D.F.; Zardi, L.; Ventura, E.; González-Iglesias, R.; Lovato, V.; Giovannoni, L.; et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J. Surg. Oncol. 2013, 107, 173–179. [Google Scholar] [CrossRef]
- Grünhagen, D.J.; Van Etten, B.; Brunstein, F.; Graveland, W.J.; Van Geel, A.N.; De Wilt, J.H.W.; Eggermont, A.M.M. Efficacy of Repeat Isolated Limb Perfusions With Tumor Necrosis Factor α and Melphalan for Multiple In-Transit Metastases in Patients with Prior Isolated Limb Perfusion Failure. Ann. Surg. Oncol. 2005, 12, 609–615. [Google Scholar] [CrossRef]
- Noorda, E.; Vrouenraets, B.; Nieweg, O.; van Geel, A.; Eggermont, A.; Kroon, B. Repeat isolated limb perfusion with TNFα and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur. J. Surg. Oncol. 2006, 32, 318–324. [Google Scholar] [CrossRef]
- Deroose, J.P.; Grünhagen, D.J.; Eggermont, A.M.; Verhoef, C. Repeated isolated limb perfusion in melanoma patients with recurrent in-transit metastases. Melanoma Res. 2015, 25, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Belgrano, V.; Pettersson, J.; Nilsson, J.A.; Mattsson, J.; Katsarelias, D.; Bagge, R.O. Response and Toxicity of Repeated Isolated Limb Perfusion (re-ILP) for Patients With In-Transit Metastases of Malignant Melanoma. Ann. Surg. Oncol. 2019, 26, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Jr, H.R.A.; Fraker, D.L.; Bartlett, D.L.; Libutti, S.K.; Steinberg, S.M.; Soriano, P.; Beresnev, T. Analysis of Factors Influencing Outcome in Patients With In-Transit Malignant Melanoma Undergoing Isolated Limb Perfusion Using Modern Treatment Parameters. J. Clin. Oncol. 2010, 28, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Katsarelias, D.; Rådbo, E.; Ben-Shabat, I.; Mattsson, J.; Bagge, R.O. The Effect of Temperature and Perfusion Time on Response, Toxicity, and Survival in Patients with In-transit Melanoma Metastases Treated with Isolated Limb Perfusion. Ann. Surg. Oncol. 2018, 25, 1836–1842. [Google Scholar] [CrossRef] [Green Version]
- Madu, M.F.; Deken, M.M.; Van Der Hage, J.A.; Jóźwiak, K.; Wouters, M.; Van Akkooi, A.C.J. Isolated Limb Perfusion for Melanoma is Safe and Effective in Elderly Patients. Ann. Surg. Oncol. 2017, 24, 1997–2005. [Google Scholar] [CrossRef]
- Hoekstra, H.J.; Veerman, K.; Van Ginkel, R.J. Isolated limb perfusion for in-transit melanoma metastases: Melphalan or TNF-melphalan perfusion? J. Surg. Oncol. 2014, 109, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, I.F.; Chakera, A.H.; Drejøe, J.B.; Klyver, H.; Dahlstrøm, K.; Oturai, P.S.; Mortensen, J.; Hesse, B.; Schmidt, G.; Drzewiecki, K. Tumour response after hyperthermic isolated limb perfusion for locally advanced melanoma. Dan. Med. J. 2014, 61, A4741. [Google Scholar] [PubMed]
- Olofsson, R.; Mattsson, J.; Lindnér, P.; Bagge, R.O. Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int. J. Hyperth. 2013, 29, 551–557. [Google Scholar] [CrossRef]
- Deroose, J.P.; Eggermont, A.M.M.; Van Geel, A.N.; De Wilt, J.H.W.; Burger, J.W.A.; Verhoef, C. 20 Years Experience of TNF-Based Isolated Limb Perfusion for In-Transit Melanoma Metastases: TNF Dose Matters. Ann. Surg. Oncol. 2012, 19, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deroose, J.P.; Grünhagen, D.J.; van Geel, A.N.; de Wilt, J.H.W.; Eggermont, A.M.M.; Verhoef, C. Long-term outcome of isolated limb perfusion with tumour necrosis factor-α for patients with melanoma in-transit metastases. BJS 2011, 98, 1573–1580. [Google Scholar] [CrossRef]
- Pace, M.; Gattai, R.; Mascitelli, E.M.; Millanta, L. Results of isolated lower limb perfusion for loco-regional advanced/recurrent melanoma using borderline true hyperthermia plus additional bolus of melphalan. A critical analysis of homogeneous cases. J. Surg. Oncol. 2011, 104, 718–723. [Google Scholar] [CrossRef]
- Rossi, C.R.; Pasquali, S.; Mocellin, S.; Vecchiato, A.; Campana, L.G.; Pilati, P.; Zanon, A.; Nitti, N. Long-Term Results of Melphalan-Based Isolated Limb Perfusion With or Without Low-Dose TNF for In-Transit Melanoma Metastases. Ann. Surg. Oncol. 2010, 17, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.E.; Meyer, T.; Waschke, L.; Merkel, S.; Goehl, J.; Hohenberger, W.; Knorr, C. Long-term outcome of hyperthermic isolated limb perfusion (HILP) in the treatment of locoregionally metastasised malignant melanoma of the extremities. Int. J. Hyperth. 2010, 26, 16–20. [Google Scholar] [CrossRef]
- Knorr, C.; Meyer, T.; Janssen, T.; Goehl, J.; Hohenberger, W. Hyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patients. Eur. J. Surg. Oncol. 2006, 32, 224–227. [Google Scholar] [CrossRef]
- Aloia, T.A.; Grubbs, E.; Onaitis, M.; Mosca, P.J.; Cheng, T.; Seigler, H.; Tyler, D.S. Predictors of outcome after hyperthermic isolated limb perfusion: Role of tumor response. Arch. Surg. 2005, 140, 1115–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünhagen, D.J.; Brunstein, F.; Graveland, W.J.; van Geel, A.N.; de Wilt, J.H.; Eggermont, A.M. One Hundred Consecutive Isolated Limb Perfusions With TNF-α and Melphalan in Melanoma Patients With Multiple In-Transit Metastases. Ann. Surg. 2004, 240, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.R.; Foletto, M.; Mocellin, S.; Pilati, P.; Lise, M. Hyperthermic Isolated Limb Perfusion With Low-Dose Tumor Necrosis Factor-α and Melphalan for Bulky In-Transit Melanoma Metastases. Ann. Surg. Oncol. 2004, 11, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Noorda, E.M. Isolated Limb Perfusion for Unresectable Melanoma of the Extremities. Arch. Surg. 2004, 139, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Noorda, E.M.; Takkenberg, B.; Vrouenraets, B.C.; Nieweg, O.E.; Van Geel, B.N.; Eggermont, A.M.M.; Hart, G.A.M.; Kroon, B.B.R. Isolated Limb Perfusion Prolongs the Limb Recurrence-Free Interval After Several Episodes of Excisional Surgery for Locoregional Recurrent Melanoma. Ann. Surg. Oncol. 2004, 11, 491–499. [Google Scholar] [CrossRef]
- Noorda, E.M.; Vrouenraets, B.C.; Nieweg, O.E.; Van Geel, A.N.; Eggermont, A.M.M.; Kroon, B.B.R. Safety and Efficacy of Isolated Limb Perfusion in Elderly Melanoma Patients. Ann. Surg. Oncol. 2002, 9, 968–974. [Google Scholar] [CrossRef]
- Moreno-Ramirez, D.; Cruz-Merino, L.; Ferrandiz, L.; Villegas-Portero, R.; Nieto-Garcia, M.-A.; De La Cruz-Merino, L. Isolated Limb Perfusion for Malignant Melanoma: Systematic Review on Effectiveness and Safety. Oncologist 2010, 15, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Marschall, Z.; Scholz, A.; Cramer, T.; Schäfer, G.; Schirner, M.; Oöcker, M.; Rosewicz, S. Effects of Interferon Alpha on Vascular Endothelial Growth Factor Gene Transcription and Tumor Angiogenesis. J. Natl. Cancer Inst. 2003, 95, 437–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavčič, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Wichtowski, M.; Murawa, D. Electrochemotherapy in the treatment of melanoma. Contemp. Oncol. 2018, 22, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Schachter, J.; Ribas, A.; Long, G.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Sileni, V.C.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. New Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Guadagni, S.; Fiorentini, G.; Clementi, M.; Palumbo, G.; Palumbo, P.; Chiominto, A.; Baldoni, S.; Masedu, F.; Valenti, M.; Di Tommaso, A.; et al. Does Locoregional Chemotherapy Still Matter in the Treatment of Advanced Pelvic Melanoma? Int. J. Mol. Sci. 2017, 18, 2382. [Google Scholar] [CrossRef] [Green Version]
- Guadagni, S.; Fiorentini, G.; Clementi, M.; Palumbo, G.; Chiominto, A.; Cappelli, S.; Masedu, F.; Valenti, M. Melphalan hypoxic perfusion with hemofiltration for melanoma locoregional metastases in the pelvis. J. Surg. Res. 2017, 215, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ariyan, C.E.; Brady, M.S.; Siegelbaum, R.H.; Hu, J.; Bello, D.M.; Rand, J.; Fisher, C.; Lefkowitz, R.A.; Panageas, K.S.; Pulitzer, M.; et al. Robust Antitumor Responses Result from Local Chemotherapy and CTLA-4 Blockade. Cancer Immunol. Res. 2018, 6, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).