Novel Treatment Strategies Utilizing Immune Reactions against Chronic Myelogenous Leukemia Stem Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Immune Status of CML Patients
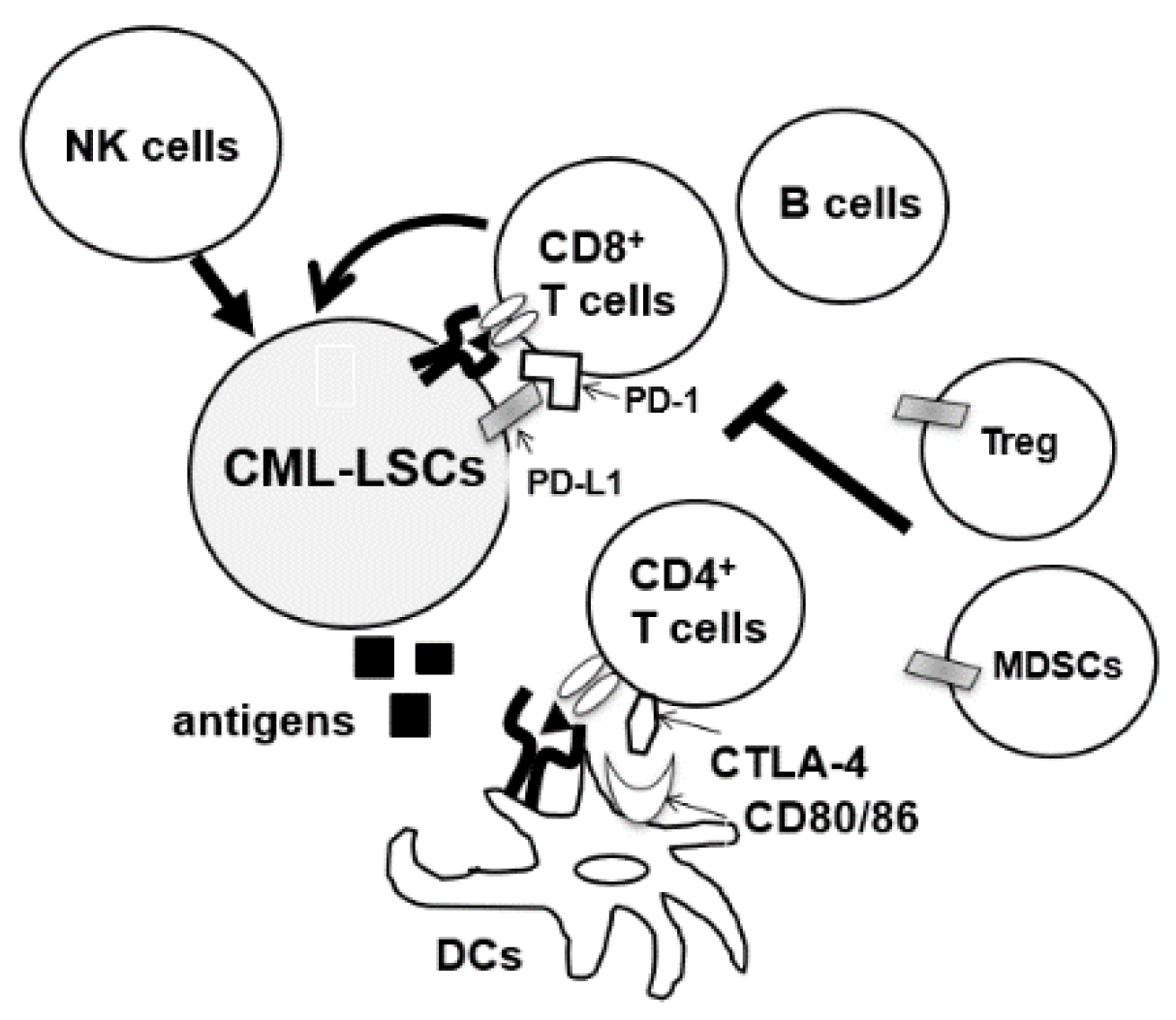
2.1. Immunogenecity of CML-LSCs
2.2. Immune status in CML Patients at Diagnosis
2.3. Immunomodulatory Effects of TKIs in CML
2.3.1. Imatinib
2.3.2. Dasatinib
2.3.3. Nilotinib
2.3.4. Bosutinib
2.3.5. Ponatinib
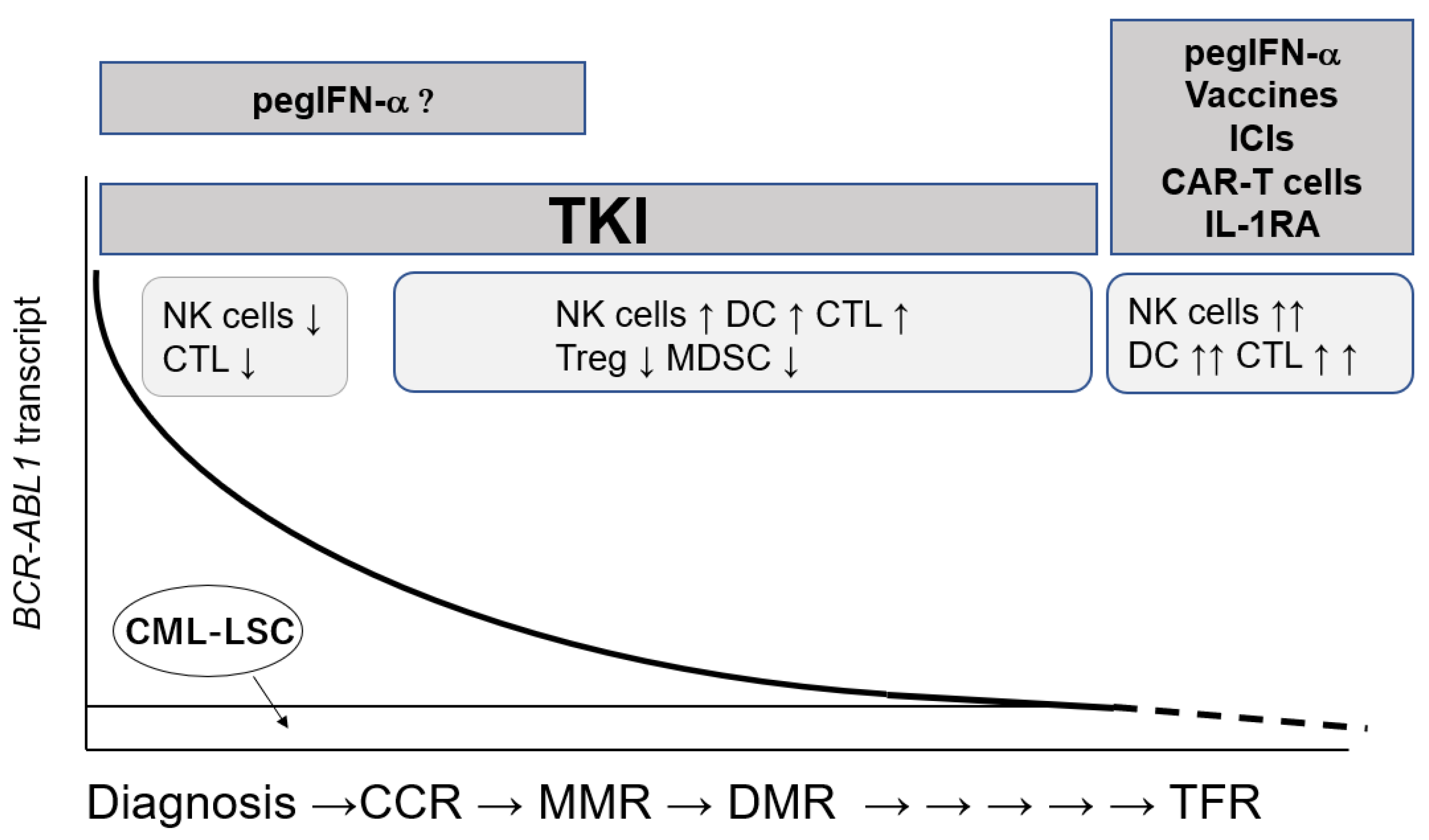
3. TFR and Immune Cells
3.1. TKI Discontinuation Trials
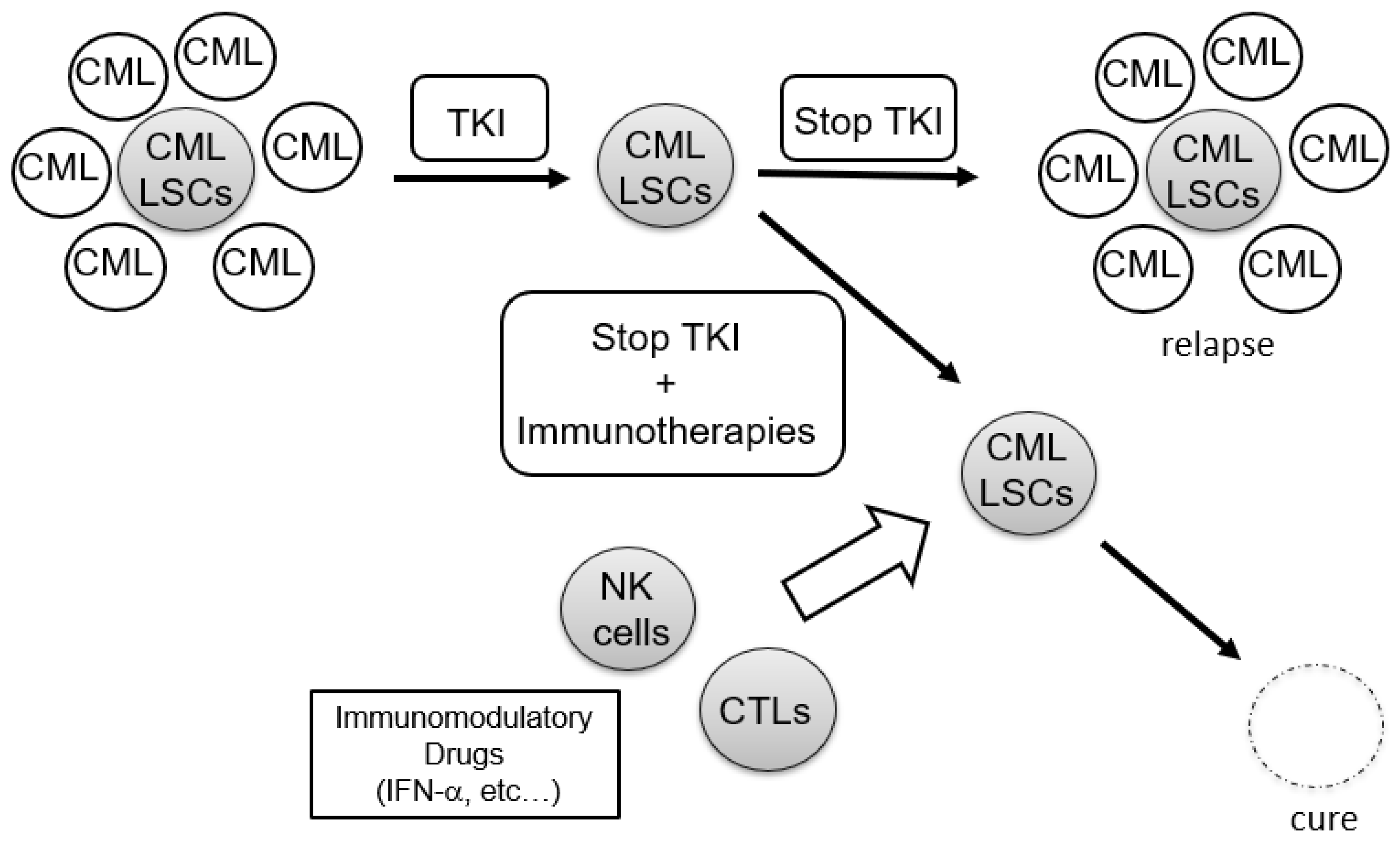
3.2. Role of Immune Reactions against CML-LSC in TFR
4. Immunotherapies against CML-LSCs
4.1. IFN α
4.2. Vaccination
4.2.1. BCR-ABL1peptides
4.2.2. Leukemia-Associated Antigens
4.2.3. Cellular Vaccines
4.3. Immune Checkpoint Inhibitors
4.4. Chimeric Antigen Receptor-T Therapy
4.5. Other Immunomodulations
5. Conclusions
Funding
Conflicts of Interest
References
- Goldman, J.M.; Melo, J.V. Targeting the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1084–1086. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.W.; Mauro, M.J.; Chuah, C.; Kim, D.-W.; Dyagil, I.; Glushko, N.; Milojkovic, D.; Le Coutre, P.; et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. J. Clin. Oncol. 2018, 36, 231–237. [Google Scholar] [CrossRef]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; Le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.; et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef]
- Cortés, J.E.; Kim, D.-W.; Kantarjian, H.M.; Brümmendorf, T.H.; Dyagil, I.; Griskevicius, L.; Malhotra, H.; Powell, C.; Gogat, K.; Countouriotis, A.M.; et al. Bosutinib Versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: Results from the BELA Trial. J. Clin. Oncol. 2012, 30, 3486–3492. [Google Scholar] [CrossRef]
- Chu, S.; McDonald, T.; Lin, A.; Chakraborty, S.; Huang, Q.; Snyder, D.S.; Bhatia, R. Persistence of leukemia stem cells in chronic my-elogenous leukemia patients in prolonged remission with imatinib treatment. Blood 2011, 118, 5565–5572. [Google Scholar] [CrossRef]
- Chomel, J.C.; Bonnet, M.-L.; Sorel, N.; Bertrand, A.; Meunier, M.-C.; Fichelson, S.; Melkus, M.; Griscelli, A.B.; Guilhot, F.; Turhan, A. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood 2011, 118, 3657–3660. [Google Scholar] [CrossRef]
- Corbin, A.S.; Agarwal, A.; Loriaux, M.; Cortes, J.; Deininger, M.W.; Druker, B.J. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Investig. 2011, 121, 396–409. [Google Scholar] [CrossRef]
- Hamilton, A.; Helgason, G.V.; Schemionek, M.; Zhang, B.; Mysina, S.; Allan, E.K.; Nicolini, F.E.; Mueller-Tidow, C.; Bhatia, R.; Brunton, V.G.; et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood 2012, 119, 1501–1510. [Google Scholar] [CrossRef]
- Graham, S.M.; Jørgensen, H.G.; Allan, E.; Pearson, C.; Alcorn, M.J.; Richmond, L.; Holyoake, T.L. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002, 99, 319–325. [Google Scholar] [CrossRef]
- Mu, H.; Zhu, X.; Jia, H.; Zhou, L.; Liu, H. Combination Therapies in Chronic Myeloid Leukemia for Potential Treatment-Free Remission: Focus on Leukemia Stem Cells and Immune Modulation. Front. Oncol. 2021, 11, 643382. [Google Scholar] [CrossRef]
- Mahon, F.X. Treatment-free remission in CML: Who, how, and why? Hematol. Am. Soc. Hematol. Educ. Program 2017, 1, 102–109. [Google Scholar] [CrossRef]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing chronic myeloid leukemia for treatment-free remission: A proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Kwaśnik, P.; Giannopoulos, K. Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia. J. Pers. Med. 2021, 11, 697. [Google Scholar] [CrossRef]
- Matsushita, M.; Ozawa, K.; Suzuki, T.; Nakamura, M.; Nakano, N.; Kanchi, S.; Ichikawa, D.; Matsuki, E.; Sakurai, M.; Karigane, D.; et al. CXorf48 is a potential therapeutic target for achieving treatment-free remission in CML patients. Blood Cancer J. 2017, 7, e601. [Google Scholar] [CrossRef]
- Chomel, J.C.; Bonnet, M.L.; Sorel, N.; Sloma, I.; Bennaceur-Griscelli, A.; Rea, D.; Legros, L.; Marfaing-Koka, A.; Bourhis, J.-H.; Ame, S.; et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget 2016, 7, 35293–35301. [Google Scholar] [CrossRef]
- Atallah, E.; Schiffer, C.A. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: When and for whom? Haematol. 2020, 105, 2738–2745. [Google Scholar] [CrossRef]
- Jo, T.; Noguchi, K.; Hayashi, S.; Irie, S.; Hayase, R.; Shioya, H.; Kaneko, Y.; Horio, K.; Taguchi, J. Long-lasting memory of cellular immunity in a chronic myeloid leukemia patient maintains molecular response 5 after cessation of dasatinib. Oncol. Lett. 2017, 15, 2935–2938. [Google Scholar] [CrossRef]
- Schütz, C.; Inselmann, S.; Saussele, S.; Dietz, C.T.; Mu Ller, M.C.; Eigendorff, E.; Brendel, C.A.; Metzelder, S.K.; Bru Mmendorf, T.H.; Waller, C.; et al. Expression of the CTLA-4 ligand CD86 on plasmacytoiddendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 2017, 31, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Saglio, G.; Gale, R.P. Prospects for achieving treatment-free remission in chronic myeloid leukaemia. Br. J. Haematol. 2020, 190, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, T.; Jiang, X.; Eaves, C.; Eaves, A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 1999, 94, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Simonetti, G.; Circosta, P.; Gaidano, V.; Cignetti, A.; Martinelli, G.; Saglio, G.; Gale, R.P. Chronic myeloid leukemia stem cells. Leukemia 2019, 33, 1543–1556. [Google Scholar] [CrossRef]
- Holyoake, T.L.; Vetrie, D. The chronic myeloid leukemia stem cell: Stemming the tide of persistence. Blood 2017, 129, 1595–1606. [Google Scholar] [CrossRef]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef]
- Tarafdar, A.; Hopcroft, L.E.M.; Gallipoli, P.; Pellicano, F.; Cassels, J.; Hair, A.; Korfi, K.; Jørgensen, H.G.; Vetrie, D.; Holyoake, T.L.; et al. CML cells actively evade host immune surveillance through cytokine-mediated downregulation of MHC-II expression. Blood 2017, 129, 199–208. [Google Scholar] [CrossRef]
- Levescot, A.; Flamant, S.; Basbous, S.; Jacomet, F.; Féraud, O.; Bourgeois, E.A.; Bonnet, M.-L.; Giraud, C.; Roy, L.; Barra, A.; et al. BCR-ABL–Induced Deregulation of the IL-33/ST2 Pathway in CD34(+) Progenitors from Chronic Myeloid Leukemia Patients. Cancer Res. 2014, 74, 2669–2676. [Google Scholar] [CrossRef]
- Dong, R.; Cwynarski, K.; Entwistle, A.; Marelli-Berg, F.; Dazzi, F.; Simpson, E.; Goldman, J.M.; Melo, J.V.; Lechler, R.I.; Bellantuono, I.; et al. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood 2003, 101, 3560–3567. [Google Scholar] [CrossRef]
- Mellqvist, U.H.; Hansson, M.; Brune, M.; Dahlgren, C.; Hermodsson, S.; Hellstrand, K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: Role of reactive oxygen species and regulation by histamine. Blood 2000, 96, 1961–1968. [Google Scholar] [CrossRef]
- Chen, C.I.-U.; Koschmieder, S.; Kerstiens, L.; Schemionek, M.; Altvater, B.; Pscherer, S.; Gerss, J.; Maecker, H.T.; Berdel, W.E.; Juergens, H.; et al. NK cells are dysfunctional in human chronic myelogenous leukemia before and on imatinib treatment and in BCR–ABL-positive mice. Leukemia 2011, 26, 465–474. [Google Scholar] [CrossRef]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.; Hughes, T.; Yong, A. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef]
- Brück, O.; Blom, S.; Dufva, O.; Turkki, R.; Chheda, H.; Ribeiro, A.; Kovanen, P.; Aittokallio, T.; Koskenvesa, P.; Kallioniemi, O.; et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia 2018, 32, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Shen, N.; Zhao, Z.; Cui, J.; Zhou, S.; Jiang, L.; Zhu, X.; Tang, L.; Liang, H.; et al. The interaction of tumor cells and myeloid-derived suppressor cells in chronic myelogenous leukemia. Leuk. Lymphoma 2019, 61, 128–137. [Google Scholar] [CrossRef]
- Bachy, E.; Bernaud, J.; Roy, P.; Rigal, D.; Nicolini, F.E. Quantitative and functional analyses of CD4+CD25+FoxP3+ regulatory T cells in chronic phase chronic myeloid leukaemia patients at diagnosis and on imatinib mesylate. Br. J. Haematol. 2011, 153, 139–143. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Costantini, A.; Viola, N.; Costantini, B.; Olivieri, J.; Butini, L.; Mancini, G.; Scortechini, I.; Chiarucci, M.; Poiani, M.; et al. Immunomodulatory Effects of Tyrosine Kinase Inhibitor In Vitro and In Vivo Study. Biol. Blood Marrow Transplant. 2018, 24, 267–275. [Google Scholar] [CrossRef]
- Seggewiss, R.; Loré, K.; Greiner, E.; Magnusson, M.K.; Price, D.A.; Douek, D.C.; Dunbar, C.E.; Wiestner, A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005, 105, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Schade, A.E.; Schieven, G.L.; Townsend, R.; Jankowska, A.M.; Susulic, V.; Zhang, R.; Szpurka, H.; Maciejewski, J.P. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008, 111, 1366–1377. [Google Scholar] [CrossRef]
- Tanaka, A.; Nishikawa, H.; Noguchi, S.; Sugiyama, D.; Morikawa, H.; Takeuchi, Y.; Ha, D.; Shigeta, N.; Kitawaki, T.; Maeda, Y.; et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J. Exp. Med. 2020, 217, 20191009. [Google Scholar] [CrossRef]
- Kreutzman, A.; Yadav, B.; Brummendorf, T.H.; Gjertsen, B.T.; Lee, M.H.; Janssen, J.; Kasanen, T.; Koskenvesa, P.; Lotfi, K.; Markevärn, B.; et al. Immunological monitoring of newly diagnosed CML patients treated with bosutinib or imatinib first-line. OncoImmunology 2019, 8, e1638210. [Google Scholar] [CrossRef]
- Mustjoki, S.; Ekblom, M.; Arstila, T.P.; Dybedal, I.; Epling-Burnette, P.K.; Guilhot, F.; Hjorth-Hansen, H.; Höglund, M.; Kovanen, P.; Laurinolli, T.; et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009, 23, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Kreutzman, A.; Juvonen, V.; Kairisto, V.; Ekblom, M.; Stenke, L.; Seggewiss, R.; Porkka, K.; Mustjoki, S. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010, 116, 772–782. [Google Scholar] [CrossRef]
- Chang, M.C.; Cheng, H.I.; Hsu, K.; Hsu, Y.N.; Kao, C.W.; Chang, Y.F.; Lim, K.H.; Chen, C.G. NKG2A Down-Regulation by Dasatinib Enhances Natural Killer Cytotoxicity and Accelerates Effective Treatment Responses in Patients With Chronic Myeloid Leukemia. Front. Immunol. 2019, 9, 3152. [Google Scholar] [CrossRef]
- Najima, Y.; Yoshida, C.; Iriyama, N.; Fujisawa, S.; Wakita, H.; Chiba, S.; Okamoto, S.; Kawakami, K.; Takezako, N.; Kumagai, T.; et al. Regulatory T cell inhibition by dasatinib is associated with natural killer cell differentiation and a favorable molecular response—The final results of the D-first study. Leuk. Res. 2018, 66, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Kitawaki, T.; Otsuka, Y.; Takaori-Kondo, A.; Kadowaki, N. Programmed cell death 1-expressing CD56-negative natural killer (NK) cell expansion is a hallmark of chronic NK cell activation during dasatinib treatment. Cancer Sci. 2021, 112, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; He, L.; Wang, X.; Lin, M.; Dai, J. Effects of dasatinib on CD8+T, Th1, and Treg cells in patients with chronic myeloid leukemia. J. Int. Med. Res. 2020, 48, 300060519877321. [Google Scholar] [CrossRef]
- Fei, F.; Yu, Y.; Schmitt, A.; Rojewski, M.T.; Chen, B.; Greiner, J.; Götz, M.; Bunjes, D.; Schmitt, M. Effects of nilotinib on regulatory T cells: The dose matters. Mol. Cancer 2010, 9, 22. [Google Scholar] [CrossRef]
- Dörfel, D.; Lechner, C.J.; Joas, S.; Funk, T.; Gutknecht, M.; Salih, J.; Geiger, J.; Kropp, K.N.; Maurer, S.; Müller, M.R.; et al. The BCR-ABL inhibitor nilotinib influences phenotype and function of monocyte-derived human dendritic cells. Cancer Immunol. Immunother. 2018, 67, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.T.; Kosaka, Y.; Malla, P.; LaTocha, D.; Lamble, A.; Hayes-Lattin, B.; Byrd, K.; Druker, B.J.; Tyner, J.W.; Chang, B.H.; et al. Concomitant use of a dual Src/ABL kinase inhibitor eliminates the in vitro efficacy of blinatumomab against Ph+ ALL. Blood 2021, 137, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Mauro, M.J.; Cortes, J.; Minami, H.; Rea, D.; DeAngelo, D.; Breccia, M.; Goh, Y.-T.; Talpaz, M.; Hochhaus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N. Engl. J. Med. 2019, 381, 2315–2326. [Google Scholar] [CrossRef]
- Jain, P.; Kantarjian, H.; Boddu, P.C.; Nogueras-González, G.M.; Verstovsek, S.; Garcia-Manero, G.; Borthakur, G.; Sasaki, K.; Kadia, T.M.; Sam, P.; et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after frontline TKIs. Blood Adv. 2019, 3, 851–861. [Google Scholar] [CrossRef]
- Efficace, F.; Baccarani, M.; Breccia, M.; Alimena, G.; Rosti, G.; Cottone, F.; Deliliers, G.L.; Baratè, C.; Rossi, A.R.; Fioritoni, G.; et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011, 118, 4554–4560. [Google Scholar] [CrossRef]
- Dusetzina, S.B.; Winn, A.N.; Abel, G.A.; Huskamp, H.A.; Keating, N.L. Cost Sharing and Adherence to Tyrosine Kinase Inhibitors for Patients with Chronic Myeloid Leukemia. J. Clin. Oncol. 2014, 32, 306–311. [Google Scholar] [CrossRef]
- Cortes, J.; Rea, D.; Lipton, J.H. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am. J. Hematol. 2019, 94, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E. Tyrosine Kinase Inhibitor Therapy Discontinuation for Patients with Chronic Myeloid Leukaemia in Clinical Practice. Curr. Hematol. Malign Rep. 2019, 14, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.G.; Srivastava, R.; Jamil, M.O. Treatment-Free Remission: A New Therapeutic Goal in Chronic Myelogenous Leukemia. Curr. Oncol. Rep. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Radich, J.P.; Hochhaus, A.; Masszi, T.; Hellmann, A.; Stentoft, J.; Casares, M.T.G.; García-Gutiérrez, J.V.; Conneally, E.; le Coutre, P.D.; Gattermann, N.; et al. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia 2021, 35, 1344–1355. [Google Scholar] [CrossRef]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Shah, N.P.; García-Gutiérrez, V.; Jiménez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.-X.; Levy, M.Y.; Gómez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk. Lymphoma 2020, 61, 650–659. [Google Scholar] [CrossRef]
- Takahashi, N.; Kyo, T.; Maeda, Y.; Sugihara, T.; Usuki, K.; Kawaguchi, T.; Usui, N.; Okamoto, S.; Ohe, Y.; Ohtake, S.; et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica 2011, 97, 903–906. [Google Scholar] [CrossRef]
- Alikian, M.; Gale, R.P.; Apperley, J.F.; Foroni, L. Molecular techniques for the personalised management of patients with chronic myeloid leukaemia. Biomol. Detect. Quantif. 2017, 11, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Malagola, M.; Zanaglio, C.; Polverelli, N.; Eke, E.D.; D’Adda, M.; Farina, M.; Bucelli, C.; Scaffidi, L.; Toffoletti, E.; et al. Digital PCR improves the quantitation of DMR and the selection of CML candidates to TKIs discontinuation. Cancer Med. 2019, 8, 2041–2055. [Google Scholar] [CrossRef]
- Burchert, A.; Müller, M.C.; Kostrewa, P.; Erben, P.; Bostel, T.; Liebler, S.; Hehlmann, R.; Neubauer, A.; Hochhaus, A. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010, 28, 1429–1435. [Google Scholar] [CrossRef]
- Cayssials, E.; Jacomet, F.; Piccirilli, N.; Lefèvre, L.; Roy, L.; Guilhot, F.; Chomel, J.C.; Leleu, X.; Gombert, J.; Herbelin, A.; et al. Sustained treatment-free remission in chronic myeloid leukaemia is associated with an increased frequency of innate CD8(+) T-cells. Br. J. Haematol. 2019, 186, 54–59. [Google Scholar] [CrossRef]
- Irani, Y.D.; Hughes, A.; Clarson, J.; Kok, C.H.; Shanmuganathan, N.; White, D.L.; Yeung, D.T.; Ross, D.M.; Hughes, T.P.; Yong, A.S. Successful treatment-free remission in chronic myeloid leukaemia and its association with reduced immune suppressors and increased natural killer cells. Br. J. Haematol. 2020, 191, 433–441. [Google Scholar] [CrossRef]
- Dumas, P.; Bérard, E.; Bréal, C.; Dulucq, S.; Réa, D.; Nicolini, F.; Forcade, E.; Dufossée, M.; Pasquet, J.; Turcq, B.; et al. Killer immunoglobulin-like receptor genotypes and chronic myeloid leukemia outcomes after imatinib cessation for treatment-free remission. Cancer Med. 2019, 8, 4976–4985. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, G.; Caocci, G.; Littera, R.; Atzeni, S.; Vacca, A.; Mulas, O.; Langiu, M.; Greco, M.; Orrù, S.; Orrù, N.; et al. Homozygosity for killer immunoglobin-like receptor haplotype A predicts complete molecular response to treatment with tyrosine kinase inhibitors in chronic myeloid leukemia patients. Exp. Hematol. 2013, 41, 424–431. [Google Scholar] [CrossRef]
- Yeung, D.T.; Tang, C.; Vidovic, L.; White, D.L.; Branford, S.; Hughes, T.P.; Yong, A.S. KIR2DL5B genotype predicts outcomes in CML patients treated with response-directed sequential imatinib/nilotinib strategy. Blood 2015, 126, 2720–2723. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Liu, Y.; Hu, Z.-H.; Williams, K.M.; Lazarus, H.M.; Vij, R.; Kharfan-Dabaja, M.A.; Ortí, G.; Wiernik, P.H.; Weisdorf, D.; et al. The Role of Donor Lymphocyte Infusion (DLI) in Post-Hematopoietic Cell Transplant (HCT) Relapse for Chronic Myeloid Leukemia (CML) in the Tyrosine Kinase Inhibitor (TKI) Era. Biol. Blood Marrow Transplant. 2020, 26, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Chalandon, Y.; Passweg, J.R.; Schmid, C.; Olavarria, E.; Dazzi, F.; Simula, M.P.; Ljungman, P.; Schattenberg, A.; de Witte, T.; Lenhoff, S.; et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: A study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2010, 45, 558–564. [Google Scholar] [CrossRef]
- Italian Cooperative Study Group on Chronic Myeloid Leukemia; Tura, S.; Baccarani, M.; Zuffa, E.; Russo, D.; Fanin, R.; Zaccaria, A.; Fiacchini, M. Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. N. Engl. J. Med. 1994, 330, 820–825. [Google Scholar] [CrossRef]
- Fuchs, S.Y. Hope and Fear for Interferon: The Receptor-Centric Outlook on the Future of Interferon Therapy. J. Interf. Cytokine Res. 2013, 33, 211–225. [Google Scholar] [CrossRef]
- Selleri, C.; Sato, T.; Del Vecchio, L.; Luciano, L.; Barrett, A.J.; Rotoli, B.; Young, N.S.; Maciejewski, J.P. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-alpha in chronic myelogenous leukemia. Blood 1997, 89, 957–964. [Google Scholar] [CrossRef]
- Kwaa, A.K.R.; Talana, C.A.G.; Blankson, J.N. Interferon Alpha Enhances NK Cell Function and the Suppressive Capacity of HIV-Specific CD8 + T Cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Paquette, R.L.; Hsu, N.; Said, J.; Mohammed, M.; Rao, N.P.; Shih, G.; Schiller, G.; Sawyers, C.; Glaspy, A.J. Interferon-α induces dendritic cell differentiation of CML mononuclear cells in vitro and in vivo. Leukemia 2002, 16, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Hakem, R.; Le Bouteiller, P.; Barad, M.; Trujillo, M.; Mercier, P.; Wietzerbin, J.; Lemonnier, A.F. IFN-mediated differential regulation of the expression of HLA-B7 and HLA-A3 class I genes. J. Immunol. 1989, 142, 297–305. [Google Scholar] [PubMed]
- Polivkova, V.; Rohon, P.; Klamova, H.; Cerna, O.; Divoka, M.; Curik, N.; Zach, J.; Novak, M.; Marinov, I.; Soverini, S.; et al. Interferon-α Revisited:Individualized Treatment Management Eased the Selective Pressure of Tyrosine Kinase Inhibitors on BCR-ABL1 Mutations Resulting in a Molecular Response in High-Risk CML Patients. PLoS ONE. 2016, 11, e0155959. [Google Scholar] [CrossRef] [PubMed]
- Talpaz, M.; Mercer, J.; Hehlmann, R. The interferon-alpha revival in CML. Ann. Hematol. 2015, 94, 195–207. [Google Scholar] [CrossRef]
- Preudhomme, C.; Guilhot, J.; Nicolini, F.; Guerci-Bresler, A.; Huguet, F.; Maloisel, F.; Coiteux, V.; Gardembas, M.; Berthou, C.; Vekhoff, A.; et al. Imatinib plus pegylated interferon-alpha2a in chronic myeloid leukemia. N. Engl. J. Med. 2010, 363, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F.; Rigal-Huguet, F.; Guilhot, J.; Guerci-Bresler, A.-P.; Maloisel, F.; Rea, D.; Coiteux, V.; Gardembas, M.; Berthou, C.; Vekhoff, A.; et al. Long-term outcome of imatinib 400 mg compared to imatinib 600 mg or imatinib 400 mg daily in combination with cytarabine or pegylated interferon alpha 2a for chronic myeloid leukaemia: Results from the French SPIRIT phase III randomised trial. Leukemia 2021, 35, 2332–2345. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R.; for the SAKK and the German CML Study Group; Lauseker, M.; Saußele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017, 31, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Dimou, M.; Panayiotidis, P. Tyrosine kinase inhibitors and interferon. Mediterr J. Hematol. Infect Dis. 2014, 6, e2014006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hjorth-Hansen, H.; for the Nordic CML Study Group (NCMLSG); Stentoft, J.; Richter, J.; Koskenvesa, P.; Hoglund, M.; Dreimane, A.; Porkka, K.; Gedde-Dahl, T.; Gjertsen, B.T.; et al. Safety and efficacy of the combination of pegylated interferon-α2b and dasatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Leukemia 2016, 30, 1853–1860. [Google Scholar] [CrossRef]
- Flygt, H.; Söderlund, S.; Stentoft, J.; Richter, J.; Koskenvesa, P.; Mustjoki, S.; Majeed, W.; Lübking, A.; Dreimane, A.; Markevärn, B.; et al. Long-term tolerability and efficacy after initial PegIFN-α addition to dasatinib in CML-CP - five-year follow-up of the NordCML007 study. Eur. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Etienne, G.; Dubruille, V.; Roy, L.; Huguet, F.; Legros, L.; Giraudier, S.; Coiteux, V.; Guerci-Bresler, A.; Lenain, P.; et al. Nilotinib and pegylated interferon alfa 2a for newly diagnosed chronic phase chronic myeloid leukaemia patients. Results of amulticentric phase II study. Lancet Haematol. 2015, 2, e37–e46. [Google Scholar] [CrossRef]
- Held, S.A.E.; Heine, A.; Mayer, K.T.; Kapelle, M.; Wolf, D.G.F.; Brossart, P. Advances in Immunotherapy of Chronic Myeloid Leukemia CML. Curr. Cancer Drug Targets 2013, 13, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Jing, W.; Yi, K.; Wu, S.; Zhou, F. Wilms’ tumor 1 gene in hematopoietic malignancies: Clinical implications and future directions. Leuk. Lymphoma 2020, 61, 2059–2067. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Thiede, C.; Platzbecker, U.; Kiani, A.; Oelschlaegel, U.; Babatz, J.; Lehmann, D.; Hölig, K.; Radke, J.; Tuve, S.; et al. Prophylactic transfer of BCR-ABL–, PR1-, and WT1-reactive donor T cells after T cell–depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood 2011, 117, 7174–7184. [Google Scholar] [CrossRef]
- Matsushita, M.; Ikeda, H.; Kizaki, M.; Okamoto, S.; Ogasawara, M.; Ikeda, Y.; Kawakami, Y. Quantitative monitoring of the PRAME gene for the detection of minimal residual disease in leukaemia. Br. J. Haematol. 2001, 112, 916–926. [Google Scholar] [CrossRef]
- Molldrem, J.J.; Lee, P.P.; Wang, C.; Felio, K.; Kantarjian, H.M.; Champlin, R.E.; Davis, M.M. Evidence that specific T ly mphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000, 6, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Bocchia, M.; Wentworth, P.; Southwood, S.; Sidney, J.; McGraw, K.; Scheinberg, D.; Sette, A. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood 1995, 85, 2680–2684. [Google Scholar] [CrossRef]
- Pinilla-Ibarz, J.; Korontsvit, T.; Zakhaleva, V.; Roberts, W.; Scheinberg, A.D. Synthetic peptide analogs derived from bcr/abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematol. 2005, 90, 1324–1332. [Google Scholar]
- Jain, N.; Reuben, J.M.; Kantarjian, H.; Li, C.; Gao, H.; Lee, B.N.; Cohen, E.N.; Ebarb, T.; Scheinberg, D.A.; Cortes, J. Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: A phase 2 trial. Cancer 2009, 115, 3924–3934. [Google Scholar] [CrossRef] [PubMed]
- Bocchia, M.; Gentili, S.; Abruzzese, E.; Fanelli, A.; Iuliano, F.; Tabilio, A.; Amabile, M.; Forconi, F.; Gozzetti, A.; Raspadori, D.; et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: A multicentre observational trial. Lancet 2005, 365, 657–662. [Google Scholar] [CrossRef]
- Oka, Y.; Tsuboi, A.; Elisseeva, O.; Udaka, K.; Sugiyama, H. WT1 as a Novel Target Antigen for Cancer Immunotherapy. Curr. Cancer Drug Targets 2002, 2, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Oka, Y.; Nishida, S.; Tsuboi, A.; Kawakami, M.; Shirakata, T.; Takahashi, K.; Murao, A.; Nakajima, H.; Narita, M.; et al. WT1 peptide vaccine induces reduction in minimal residual disease in an Imatinib-treated CML patient. Eur. J. Haematol. 2010, 85, 358–360. [Google Scholar] [CrossRef]
- Qazilbash, M.H.; Wieder, E.; Thall, P.F.; Wang, X.; Rios, R.; Lu, S.; Kanodia, S.; Ruisaard, K.E.; Giralt, S.A.; Estey, E.H.; et al. PR1 peptide vaccine induces specific immunity withAR-T clinical responses in myeloid malignancies. Leukemia 2017, 31, 697–704. [Google Scholar] [CrossRef]
- Weinstock, M.; Rosenblatt, J.; Avigan, D. Dendritic Cell Therapies for Hematologic Malignancies. Mol. Ther. Methods Clin. Dev. 2017, 5, 66–75. [Google Scholar] [CrossRef]
- Brusic, A.; Hainz, U.; Wadleigh, M.; Neuberg, D.; Su, M.; Canning, C.M.; Deangelo, D.J.; Stone, R.M.; Lee, J.S.; Mulligan, R.C.; et al. Detecting T-cell reactivity to whole cell vaccines: Proof of concept analysis of T-cell response to K562 cell antigens in CML patients. Oncoimmunology 2012, 1, 1095–1103. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non–Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef]
- Tolba, M.F. Revolutionizing the landscape of colorectal cancer treatment: The potential role of immune checkpoint inhibitors. Int. J. Cancer 2020, 147, 2996–3006. [Google Scholar] [CrossRef]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Hatic, H.; Sampat, D.; Goyal, G. Immune checkpoint inhibitors in lymphoma: Challenges and opportunities. Ann. Transl. Med. 2021, 9, 1037. [Google Scholar] [CrossRef]
- Mumprecht, S.; Schürch, C.; Schwaller, J.; Solenthaler, M.; Ochsenbein, A. Programmed death 1 signaling on chronic myeloid leukemia–specific T cells results in T-cell exhaustion and disease progression. Blood 2009, 114, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Bavaro, L.; De Benedittis, C.; Martelli, M.; Iurlo, A.; Orofino, N.; Sica, S.; Sorà, F.; Lunghi, F.; Ciceri, F.; et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: The NEXT-in-CML study. Blood 2020, 135, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Adnan-Awad, S.; Kankainen, M.; Mustjoki, S. Mutational landscape of chronic myeloid leukemia: More than a single oncogene leukemia. Leuk. Lymphoma 2021, 62, 2064–2078. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Chow, V.A.; Shadman, M.; Gopal, A.K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018, 132, 777–781. [Google Scholar] [CrossRef]
- Sadelain, M. CD19 CAR T Cells. Cell 2017, 171, 1471. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Deng, H.; Liu, M.; Yuan, T.; Zhang, H.; Cui, R.; Li, J.; Yuan, J.; Wang, X.; Wang, Y.; Deng, Q. Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients with and without Extramedullary Disease. Front. Immunol. 2021, 12, 720571. [Google Scholar] [CrossRef]
- Zhao, K.; Yin, L.-L.; Zhao, N.-M.; Pan, B.; Chen, W.; Cao, J.; Cheng, H.; Li, Z.-Y.; Li, D.-P.; Sang, W.; et al. IL1RAP as a surface marker for leukemia stem cells is related to clinical phase of chronic myeloid leukemia patients. Int. J. Clin. Exp. Med. 2014, 7, 4787–4798. [Google Scholar]
- Warda, W.; LaRosa, F.; Da Rocha, M.N.; Trad, R.; Deconinck, E.; Fajloun, Z.; Faure, C.; Caillot, D.; Moldovan, M.; Valmary-Degano, S.; et al. CML Hematopoietic Stem Cells Expressing IL1RAP Can Be Targeted by Chimeric Antigen Receptor–Engineered T Cells. Cancer Res. 2018, 79, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Sadovnik, I.; Cerny-Reiterer, S.; Rülicke, T.; Stefanzl, G.; Willmann, M.; Hoermann, G.; Bilban, M.; Blatt, K.; Herndlhofer, S.; et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 2014, 123, 3951–3962. [Google Scholar] [CrossRef]
- Warfvinge, R.; Geironson, L.; Sommarin, M.N.E.; Lang, S.; Karlsson, C.; Roschupkina, T.; Stenke, L.; Stentoft, J.; Olsson-Strömberg, U.; Hjorth-Hansen, H.; et al. Single-cell molecular analysis defines therapy response and immunophenotype of stem cell subpopulations in CML. Blood 2017, 129, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, W.; Xiao, Y.; Zhu, X.; Zhong, Z.; Li, Q.; Cheng, F.; Zou, P.; You, Y.; Zhu, X. A novel chimeric antigen receptor redirecting T-cell specificity towards CD26+ cancer cells. Leukemia 2021, 35, 119–129. [Google Scholar] [CrossRef]
- Harrison, C.; Kiladjian, J.-J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK Inhibition with Ruxolitinib versus Best Available Therapy for Myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chu, S.; Agarwal, P.; Campbell, V.L.; Hopcroft, L.; Jørgensen, H.G.; Lin, A.; Gaal, K.; Holyoake, T.L.; Bhatia, R. Inhibition of interleukin-1 signaling enhances elimination of tyrosine kinase inhibitor–treated CML stem cells. Blood 2016, 128, 2671–2682. [Google Scholar] [CrossRef]
- Javidi-Sharifi, N.; Hobbs, G. Future Directions in Chronic Phase CML Treatment. Curr. Hematol. Malign Rep. 2021, 1–9. [Google Scholar] [CrossRef]
- Gof, D.J.; Court Recart, A.; Sadarangani, A.; Chun, H.-J.; Barrett, C.L.; Krajewska, M.; Leu, H.; Low-Marchelli, J.; Ma, W.; Shih, A.Y.; et al. A Pan-BCL2 inhibitor renders bonemarrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell 2013, 12, 316–328. [Google Scholar] [CrossRef]
- Lee, J.B.; Khan, D.H.; Hurren, R.; Xu, M.; Na, Y.; Kang, H.; Mirali, S.; Wang, X.; Gronda, M.; Jitkova, Y.; et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood 2021, 138, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Prost, S.; Relouzat, F.; Spentchian, M.; Ouzegdouh, Y.; Saliba, J.; Massonnet, G.; Beressi, J.-P.; Verhoeyen, E.; Raggueneau, V.; Maneglier, B.; et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nat. Cell Biol. 2015, 525, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; He, K.; Tian, C.; Sun, H.; Zhu, C.; Bai, S.; Liu, J.; Wu, Q.; Xie, D.; Yue, T.; et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
| TKIs | Immune-Modulatory Effects |
|---|---|
| Imatinib | Treg ↓, effector and memoriy CD8+T cells ↑, exausted T cells ↓, NK cells ↑ |
| Dasatinib | LGL (CD8+T cells or NK cells) ↑,Treg ↓ |
| Nilotinib | Treg ↓DC (differentiation, IL-12 production) ↓ |
| Bosutinib | No change in T cells, monocyte, and granulocytes |
| Ponatinib | CD8+T cells (proliferation IFN-g production) ↓ |
| Immunotherapies | Targets | Mode of Actions |
|---|---|---|
| IFN-α | CTL, NK cells CML-LSC | Activation of immune cells Upregulation of HLA class I |
| Vaccines | CML-specific antigens (BCR-ABL) CML-associated antigens (PR1, WT1, PRAME) CML cells (K562-GM-CSF) | Activation of CML-specific CTLs |
| Immune checkpoint inhibitors | PD-1 on CTLs and NK cells PD-L1 on CML-LSCs | Reactivation of exhausted immune cells |
| Chimeric antigen receptor-T cells | CML-LSC surface antigens IL1RAP, CD26, CD25, CD33, CD44, CD123 | Target-dependent lysis of CML-LSCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushita, M. Novel Treatment Strategies Utilizing Immune Reactions against Chronic Myelogenous Leukemia Stem Cells. Cancers 2021, 13, 5435. https://doi.org/10.3390/cancers13215435
Matsushita M. Novel Treatment Strategies Utilizing Immune Reactions against Chronic Myelogenous Leukemia Stem Cells. Cancers. 2021; 13(21):5435. https://doi.org/10.3390/cancers13215435
Chicago/Turabian StyleMatsushita, Maiko. 2021. "Novel Treatment Strategies Utilizing Immune Reactions against Chronic Myelogenous Leukemia Stem Cells" Cancers 13, no. 21: 5435. https://doi.org/10.3390/cancers13215435
APA StyleMatsushita, M. (2021). Novel Treatment Strategies Utilizing Immune Reactions against Chronic Myelogenous Leukemia Stem Cells. Cancers, 13(21), 5435. https://doi.org/10.3390/cancers13215435





