Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk
Simple Summary
Abstract
1. Introduction
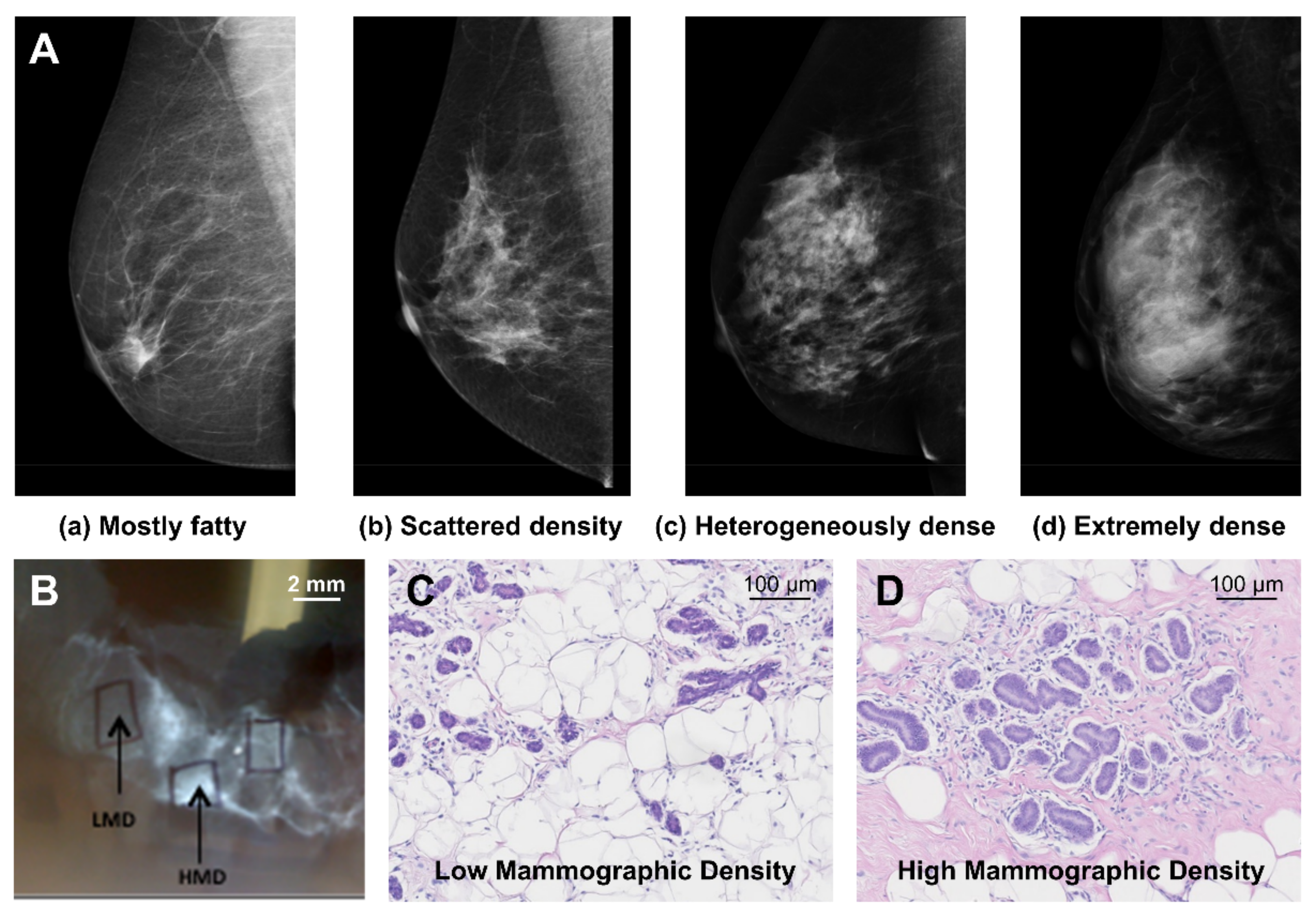
2. Mammographic Density and Breast Cancer Risk
3. Contribution of Genetic and Environmental Factors to Mammographic Density
4. Biological Mechanisms in Mammographic Density
4.1. Mammary Gland Epithelium
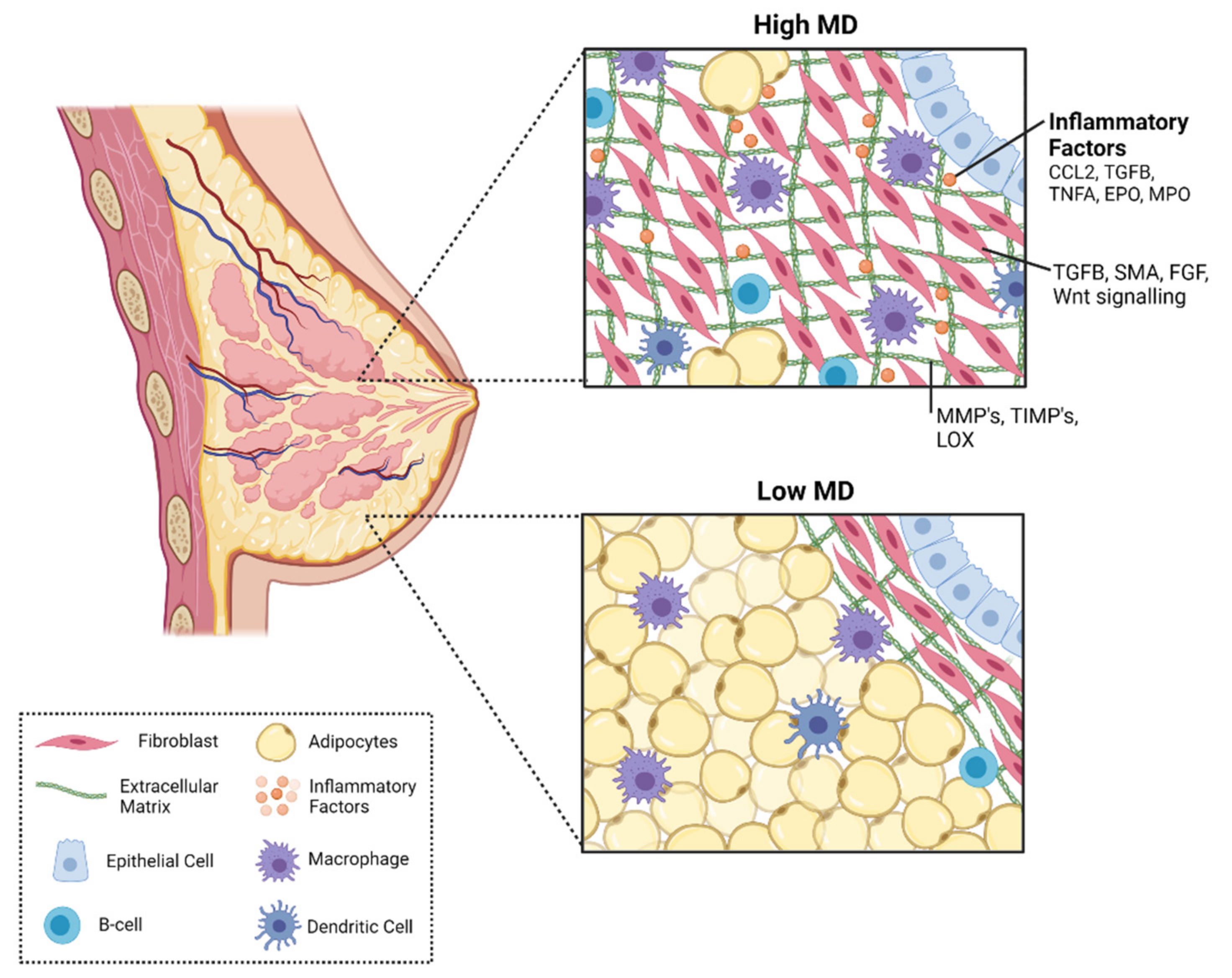
4.2. Mammary Gland Stroma
4.3. Collagen
4.4. Immune Cells
5. Immune Signaling Factors in Mammographic Density and Breast Cancer Risk
5.1. Monocyte Chemotactic Protein 1 (CCL2)
5.2. Transforming Growth Factor Beta 1 (TGFB1)
5.3. Peroxidase Enzymes
5.4. Tumour Necrosis Factor Alpha (TNFA)
6. Inflammation as a Target for Therapeutic Intervention
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Kotsopoulos, J. BRCA Mutations and Breast Cancer Prevention. Cancers 2018, 10, 524. [Google Scholar] [CrossRef]
- Melvin, J.C.; Wulaningsih, W.; Hana, Z.; Purushotham, A.D.; Pinder, S.E.; Fentiman, I.; Gillett, C.; Mera, A.; Holmberg, L.; Van Hemelrijck, M. Family history of breast cancer and its association with disease severity and mortality. Cancer Med. 2016, 5, 942–949. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; et al. Migration patterns and breast cancer risk in Asian-American women. J. Nat. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Engmann, N.J.; Golmakani, M.K.; Miglioretti, D.L.; Sprague, B.L.; Kerlikowske, K. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017, 3, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.W.; Chew, G.; Hill, P.; Huang, D.; Ingman, W.; Hodson, L.; Brown, K.A.; Magenau, A.; Allam, A.H.; McGhee, E.; et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.W.; Hill, P.; Chew, G.; Neeson, P.J.; Halse, H.; Williams, E.D.; Henderson, M.A.; Thompson, E.W.; Britt, K.L. High mammographic density in women is associated with protumor inflammation. Breast Cancer Res. 2018, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, C.G.; Courneya, K.S.; Boyd, N.F.; Yaffe, M.J.; McTiernan, A.; Brant, R.; Jones, C.A.; Stanczyk, F.Z.; Terry, T.; Cook, L.S.; et al. Association between sex hormones, glucose homeostasis, adipokines, and inflammatory markers and mammographic density among postmenopausal women. Breast Cancer Res. Treat. 2013, 139, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Lewis, M.T.; Hess, K.; Wong, H.; Tsimelzon, A.; Karadag, N.; Cairo, M.; Wei, C.; Meric-Bernstam, F.; Brown, P.; et al. Decreased TGFbeta signaling and increased COX2 expression in high risk women with increased mammographic breast density. Breast Cancer Res. Treat. 2010, 119, 305–314. [Google Scholar] [CrossRef]
- Johns, P.C.; Yaffe, M.J. X-ray characterisation of normal and neoplastic breast tissues. Phys. Med. Biol. 1987, 32, 675–695. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.J.; Zysk, A.; Dasari, P.; Britt, K.; Hopper, J.L.; Stone, J.; Thompson, E.W.; Ingman, W.V. InforMD: A new initiative to raise public awareness about breast density. Ecancermedicalscience 2018, 12, 807. [Google Scholar] [CrossRef]
- Boyd, N.F. Mammographic density and risk of breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e57–e62. [Google Scholar] [CrossRef]
- Ghadge, A.G.; Dasari, P.; Stone, J.; Thompson, E.W.; Robker, R.L.; Ingman, W.V. Pubertal mammary gland development is a key determinant of adult mammographic density. Semin. Cell Dev. Biol. 2021, 114, 143–158. [Google Scholar] [CrossRef]
- Timmers, J.M.; van Doorne-Nagtegaal, H.J.; Zonderland, H.M.; van Tinteren, H.; Visser, O.; Verbeek, A.L.; den Heeten, G.J.; Broeders, M.J. The Breast Imaging Reporting and Data System (BI-RADS) in the Dutch breast cancer screening programme: Its role as an assessment and stratification tool. Eur. Radiol. 2012, 22, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Nat. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, A.M.; Sieh, W.; Lipson, J.A.; Rothstein, J.H.; McGuire, V.; Whittemore, A.S.; Rubin, D.L. Breast Cancer Risk and Mammographic Density Assessed with Semiautomated and Fully Automated Methods and BI-RADS. Radiology 2017, 282, 348–355. [Google Scholar] [CrossRef]
- Astley, S.M.; Harkness, E.F.; Sergeant, J.C.; Warwick, J.; Stavrinos, P.; Warren, R.; Wilson, M.; Beetles, U.; Gadde, S.; Lim, Y.; et al. A comparison of five methods of measuring mammographic density: A case-control study. Breast Cancer Res. 2018, 20, 10. [Google Scholar] [CrossRef]
- Wolfe, J.N. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 1976, 37, 2486–2492. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Sun, L.; Guo, H.; Chiarelli, A.; Hislop, G.; Yaffe, M.; Minkin, S. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2086–2092. [Google Scholar] [CrossRef]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Vachon, C.M.; Brandt, K.R.; Ghosh, K.; Scott, C.G.; Maloney, S.D.; Carston, M.J.; Pankratz, V.S.; Sellers, T.A. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 43–49. [Google Scholar] [CrossRef]
- Eriksson, L.; Hall, P.; Czene, K.; Dos Santos Silva, I.; McCormack, V.; Bergh, J.; Bjohle, J.; Ploner, A. Mammographic density and molecular subtypes of breast cancer. Br. J. Cancer 2012, 107, 18–23. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Colditz, G.A.; Collins, L.C.; Schnitt, S.J.; Rosner, B.; Vachon, C.; Tamimi, R.M. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J. Nat. Cancer Inst. 2011, 103, 1179–1189. [Google Scholar] [CrossRef]
- Heusinger, K.; Jud, S.M.; Haberle, L.; Hack, C.C.; Adamietz, B.R.; Meier-Meitinger, M.; Lux, M.P.; Wittenberg, T.; Wagner, F.; Loehberg, C.R.; et al. Association of mammographic density with hormone receptors in invasive breast cancers: Results from a case-only study. Int. J. Cancer 2012, 131, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Warren, R.; Girling, A.; Thompson, D.; Easton, D. Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J. 2010, 16, 279–289. [Google Scholar] [CrossRef]
- Mokhtary, A.; Karakatsanis, A.; Valachis, A. Mammographic Density Changes over Time and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4805. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Tamimi, R.M.; Scott, C.G.; Jensen, M.R.; Pankratz, V.; Visscher, D.; Norman, A.; Couch, F.; Shepherd, J.; Fan, B.; et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013, 15, R104. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Czene, K.; Rosenberg, L.; Humphreys, K.; Hall, P. Possible influence of mammographic density on local and locoregional recurrence of breast cancer. Breast Cancer Res. 2013, 15, R56. [Google Scholar] [CrossRef]
- Maskarinec, G.; Pagano, I.S.; Little, M.A.; Conroy, S.M.; Park, S.Y.; Kolonel, L.N. Mammographic density as a predictor of breast cancer survival: The Multiethnic Cohort. Breast Cancer Res. 2013, 15, R7. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Ichikawa, L.; Kerlikowske, K.; Brinton, L.A.; Farhat, G.N.; Vacek, P.M.; Weaver, D.L.; Schairer, C.; Taplin, S.H.; Sherman, M.E. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J. Nat. Cancer Inst. 2012, 104, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Duffy, S.; Yen, A.M.; Tabar, L.; Smith, R.A.; Chen, H.H. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1219–1228. [Google Scholar] [CrossRef]
- Weigel, S.; Heindel, W.; Heidrich, J.; Hense, H.W.; Heidinger, O. Digital mammography screening: Sensitivity of the programme dependent on breast density. Eur. Radiol. 2017, 27, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.E.; Li, J.; Hall, P.; Hartman, M.; dos-Santos-Silva, I.; Humphreys, K.; Czene, K. Change of mammographic density predicts the risk of contralateral breast cancer--a case-control study. Breast Cancer Res. 2013, 15, R57. [Google Scholar] [CrossRef]
- Cil, T.; Fishell, E.; Hanna, W.; Sun, P.; Rawlinson, E.; Narod, S.A.; McCready, D.R. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer 2009, 115, 5780–5787. [Google Scholar] [CrossRef]
- Buist, D.S.; Abraham, L.A.; Barlow, W.E.; Krishnaraj, A.; Holdridge, R.C.; Sickles, E.A.; Carney, P.A.; Kerlikowske, K.; Geller, B.M. Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res. Treat. 2010, 124, 863–873. [Google Scholar] [CrossRef]
- Sung, H.; Ren, J.; Li, J.; Pfeiffer, R.M.; Wang, Y.; Guida, J.L.; Fang, Y.; Shi, J.; Zhang, K.; Li, N.; et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer 2018, 4, 3. [Google Scholar] [CrossRef]
- Moore, J.X.; Han, Y.; Appleton, C.; Colditz, G.; Toriola, A.T. Determinants of Mammographic Breast Density by Race Among a Large Screening Population. JNCI Cancer Spectr. 2020, 4, pkaa010. [Google Scholar] [CrossRef]
- Gierach, G.L.; Loud, J.T.; Chow, C.K.; Prindiville, S.A.; Eng-Wong, J.; Soballe, P.W.; Giambartolomei, C.; Mai, P.L.; Galbo, C.E.; Nichols, K.; et al. Mammographic density does not differ between unaffected BRCA1/2 mutation carriers and women at low-to-average risk of breast cancer. Breast Cancer Res. Treat. 2010, 123, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ramon, Y.C.T.; Chirivella, I.; Miranda, J.; Teule, A.; Izquierdo, A.; Balmana, J.; Sanchez-Heras, A.B.; Llort, G.; Fisas, D.; Lope, V.; et al. Mammographic density and breast cancer in women from high risk families. Breast Cancer Res. 2015, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.S.; Vachon, C.M.; Kuni, C.C.; King, R.A.; Arnett, D.K.; Grabrick, D.M.; Rich, S.S.; Anderson, V.E.; Sellers, T.A. Genetic analysis of mammographic breast density in adult women: Evidence of a gene effect. J. Nat. Cancer Inst. 1997, 89, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyd, N.F.; Dite, G.S.; Stone, J.; Gunasekara, A.; English, D.R.; McCredie, M.R.; Giles, G.G.; Tritchler, D.; Chiarelli, A.; Yaffe, M.J.; et al. Heritability of mammographic density, a risk factor for breast cancer. N. Engl. J. Med. 2002, 347, 886–894. [Google Scholar] [CrossRef]
- Reeves, K.W.; Stone, R.A.; Modugno, F.; Ness, R.B.; Vogel, V.G.; Weissfeld, J.L.; Habel, L.A.; Sternfeld, B.; Cauley, J.A. Longitudinal association of anthropometry with mammographic breast density in the Study of Women’s Health Across the Nation. Int. J. Cancer 2009, 124, 1169–1177. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Byrne, C.; Colditz, G.A.; Hankinson, S.E. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J. Nat. Cancer Inst. 2007, 99, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Lockwood, G.A.; Byng, J.W.; Little, L.E.; Yaffe, M.J.; Tritchler, D.L. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br. J. Cancer 1998, 78, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Hjerkind, K.V.; Ellingjord-Dale, M.; Johansson, A.L.V.; Aase, H.S.; Hoff, S.R.; Hofvind, S.; Fagerheim, S.; Dos-Santos-Silva, I.; Ursin, G. Volumetric Mammographic Density, Age-Related Decline, and Breast Cancer Risk Factors in a National Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1065–1074. [Google Scholar] [CrossRef]
- Checka, C.M.; Chun, J.E.; Schnabel, F.R.; Lee, J.; Toth, H. The relationship of mammographic density and age: Implications for breast cancer screening. AJR Am. J. Roentgenol. 2012, 198, W292–W295. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Maskarinec, G.; Perez-Gomez, B.; Vachon, C.; Miao, H.; Lajous, M.; López-Ridaura, R.; Rice, M.; Pereira, A.; Garmendia, M.L.; et al. Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLOS Med. 2017, 14, e1002335. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Breast Cancer in Australia: An Overview. Canberra AIHW 2012, 71, 1–202. [Google Scholar]
- Nguyen, T.L.; Schmidt, D.F.; Makalic, E.; Dite, G.S.; Stone, J.; Apicella, C.; Bui, M.; Macinnis, R.J.; Odefrey, F.; Cawson, J.N.; et al. Explaining variance in the cumulus mammographic measures that predict breast cancer risk: A twins and sisters study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2395–2403. [Google Scholar] [CrossRef]
- Woolcott, C.G.; Koga, K.; Conroy, S.M.; Byrne, C.; Nagata, C.; Ursin, G.; Vachon, C.M.; Yaffe, M.J.; Pagano, I.; Maskarinec, G. Mammographic density, parity and age at first birth, and risk of breast cancer: An analysis of four case-control studies. Breast Cancer Res. Treat. 2012, 132, 1163–1171. [Google Scholar] [CrossRef]
- Lope, V.; Pérez-Gómez, B.; Sánchez-Contador, C.; Santamariña, M.C.; Moreo, P.; Vidal, C.; Laso, M.S.; Ederra, M.; Pedraz-Pingarrón, C.; González-Román, I.; et al. Obstetric history and mammographic density: A population-based cross-sectional study in Spain (DDM-Spain). Breast Cancer Res. Treat. 2012, 132, 1137–1146. [Google Scholar] [CrossRef]
- Bremnes, Y.; Ursin, G.; Bjurstam, N.; Lund, E.; Gram, I.T. Different types of postmenopausal hormone therapy and mammographic density in Norwegian women. Int. J. Cancer 2007, 120, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Qureshi, S.A.; Hofvind, S.; Hilsen, M.; Aase, H.; Skaane, P.; Vatten, L.; Ursin, G. Hormone therapy use and mammographic density in postmenopausal Norwegian women. Breast Cancer Res. Treat. 2012, 132, 297–305. [Google Scholar] [CrossRef]
- Lowry, S.J.; Aiello Bowles, E.J.; Anderson, M.L.; Buist, D.S. Predictors of breast density change after hormone therapy cessation: Results from a randomized trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Lange, T.; Huynh, S.; Aro, A.R.; von Euler-Chelpin, M.; Vejborg, I.; Tjonneland, A.; Lynge, E.; Andersen, Z.J. Hormone replacement therapy, mammographic density, and breast cancer risk: A cohort study. Cancer Causes Control. 2018, 29, 495–505. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Cook, A.J.; Buist, D.S.; Cummings, S.R.; Vachon, C.; Vacek, P.; Miglioretti, D.L. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J. Clin. Oncol. 2010, 28, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, F.J.; Peeters, P.H.; Warren, R.M.; Bingham, S.A.; van Noord, P.A.; Monninkhof, E.M.; Grobbee, D.E.; van Gils, C.H. Postmenopausal hormone therapy and changes in mammographic density. J. Clin. Oncol. 2007, 25, 1323–1328. [Google Scholar] [CrossRef]
- Cecchini, R.S.; Costantino, J.P.; Cauley, J.A.; Cronin, W.M.; Wickerham, D.L.; Bandos, H.; Weissfeld, J.L.; Wolmark, N. Baseline mammographic breast density and the risk of invasive breast cancer in postmenopausal women participating in the NSABP study of tamoxifen and raloxifene (STAR). Cancer Prev. Res. 2012, 5, 1321–1329. [Google Scholar] [CrossRef]
- Cuzick, J.; Warwick, J.; Pinney, E.; Duffy, S.W.; Cawthorn, S.; Howell, A.; Forbes, J.F.; Warren, R.M. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J. Nat. Cancer Inst. 2011, 103, 744–752. [Google Scholar] [CrossRef]
- Cuzick, J.; Warwick, J.; Pinney, E.; Warren, R.M.; Duffy, S.W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Nat. Cancer Inst. 2004, 96, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Venzon, D.; Jones, E.C.; Premkumar, A.; O’Shaughnessy, J.; Zujewski, J. Effect of tamoxifen on mammographic density. Cancer Epidemiol. Biomark. Prev. 2000, 9, 917–921. [Google Scholar]
- Li, J.; Humphreys, K.; Eriksson, L.; Edgren, G.; Czene, K.; Hall, P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J. Clin. Oncol. 2013, 31, 2249–2256. [Google Scholar] [CrossRef]
- Henry, N.L.; Chan, H.P.; Dantzer, J.; Goswami, C.P.; Li, L.; Skaar, T.C.; Rae, J.M.; Desta, Z.; Khouri, N.; Pinsky, R.; et al. Aromatase inhibitor-induced modulation of breast density: Clinical and genetic effects. Br. J. Cancer 2013, 109, 2331–2339. [Google Scholar] [CrossRef]
- Kim, J.; Han, W.; Moon, H.G.; Ahn, S.; Shin, H.C.; You, J.M.; Han, S.W.; Im, S.A.; Kim, T.Y.; Koo, H.; et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012, 14, R102. [Google Scholar] [CrossRef]
- Vachon, C.M.; Suman, V.J.; Brandt, K.R.; Kosel, M.L.; Buzdar, A.U.; Olson, J.E.; Wu, F.F.; Flickinger, L.M.; Ursin, G.; Elliott, C.R.; et al. Mammographic Breast Density Response to Aromatase Inhibition. Clin. Cancer Res. 2013, 19, 2144–2153. [Google Scholar] [CrossRef]
- Azam, S.; Sjolander, A.; Eriksson, M.; Gabrielson, M.; Czene, K.; Hall, P. Determinants of Mammographic Density Change. JNCI Cancer Spectr. 2019, 3, pkz004. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Gandini, S.; Bonanni, B.; Mariette, F.; Guerrieri-Gonzaga, A.; Serrano, D.; Cassano, E.; Ramazzotto, F.; Baglietto, L.; Sandri, M.T.; et al. Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res. Treat. 2008, 108, 57–67. [Google Scholar] [CrossRef]
- Richert, M.M.; Schwertfeger, K.L.; Ryder, J.W.; Anderson, S.M. An atlas of mouse mammary gland development. J. Mammary Gland Biol. Neoplasia 2000, 5, 227–241. [Google Scholar] [CrossRef]
- Russo, J.; Hu, Y.-F.; Yang, X.; Russo, I.H. Chapter 1: Developmental, Cellular, and Molecular Basis of Human Breast Cancer. JNCI Monogr. 2000, 2000, 17–37. [Google Scholar] [CrossRef]
- Hovey, R.C.; Trott, J.F.; Vonderhaar, B.K. Establishing a framework for the functional mammary gland: From endocrinology to morphology. J. Mammary Gland Biol. Neoplasia 2002, 7, 17–38. [Google Scholar] [CrossRef]
- Polyak, K.; Kalluri, R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003244. [Google Scholar] [CrossRef]
- Wiseman, B.S.; Werb, Z. Stromal effects on mammary gland development and breast cancer. Science 2002, 296, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Mebius, R.E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 2011, 29, 23–43. [Google Scholar] [CrossRef]
- Boyd, N.F.; Jensen, H.M.; Cooke, G.; Han, H.L. Relationship between mammographic and histological risk factors for breast cancer. J. Nat. Cancer Inst. 1992, 84, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.A.; Morrison, A.S.; Brisson, J.; Burstein, N.A.; Sadowsky, N.S.; Kopans, D.B.; Meyer, J.E. Relationship between mammographic and histologic features of breast tissue in women with benign biopsies. Cancer 1988, 61, 266–271. [Google Scholar] [CrossRef]
- Bartow, S.A.; Pathak, D.R.; Mettler, F.A.; Key, C.R.; Pike, M.C. Breast Mammographic Pattern: A Concatenation of Confounding and Breast Cancer Risk Factors. Am. J. Epidemiol. 1995, 142, 813–819. [Google Scholar] [CrossRef]
- Li, T.; Sun, L.; Miller, N.; Nicklee, T.; Woo, J.; Hulse-Smith, L.; Tsao, M.S.; Khokha, R.; Martin, L.; Boyd, N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Cawson, J.; Hill, P.; Haviv, I.; Jenkins, M.; Hopper, J.L.; Southey, M.C.; Campbell, I.G.; Thompson, E.W. Image-guided sampling reveals increased stroma and lower glandular complexity in mammographically dense breast tissue. Breast Cancer Res. Treat. 2011, 128, 505–516. [Google Scholar] [CrossRef]
- Ghosh, K.; Brandt, K.R.; Reynolds, C.; Scott, C.G.; Pankratz, V.S.; Riehle, D.L.; Lingle, W.L.; Odogwu, T.; Radisky, D.C.; Visscher, D.W.; et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res. Treat. 2012, 131, 267–275. [Google Scholar] [CrossRef][Green Version]
- Khan, Q.J.; Kimler, B.F.; O’Dea, A.P.; Zalles, C.M.; Sharma, P.; Fabian, C.J. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007, 9, R35. [Google Scholar] [CrossRef]
- Conklin, M.W.; Keely, P.J. Why the stroma matters in breast cancer: Insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Alowami, S.; Troup, S.; Al-Haddad, S.; Kirkpatrick, I.; Watson, P.H. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003, 5, R129–R135. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Lühr, I.; Friedl, A.; Overath, T.; Tholey, A.; Kunze, T.; Hilpert, F.; Sebens, S.; Arnold, N.; Rösel, F.; Oberg, H.H.; et al. Mammary fibroblasts regulate morphogenesis of normal and tumorigenic breast epithelial cells by mechanical and paracrine signals. Cancer Lett. 2012, 325, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Jonkers, J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018, 37, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Raz, Y.; Erez, N. An inflammatory vicious cycle: Fibroblasts and immune cell recruitment in cancer. Exp. Cell Res. 2013, 319, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Y.; Jia, T.; Sun, Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol. 2015, 36, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- De Wever, O.; Nguyen, Q.D.; Van Hoorde, L.; Bracke, M.; Bruyneel, E.; Gespach, C.; Mareel, M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004, 18, 1016–1018. [Google Scholar] [CrossRef]
- O’Connell, J.T.; Sugimoto, H.; Cooke, V.G.; MacDonald, B.A.; Mehta, A.I.; LeBleu, V.S.; Dewar, R.; Rocha, R.M.; Brentani, R.R.; Resnick, M.B.; et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA 2011, 108, 16002–16007. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.J.; Moreira, J.; Lukanidin, E.M.; Ambartsumian, N.S. Human mammary fibroblasts stimulate invasion of breast cancer cells in a three-dimensional culture and increase stroma development in mouse xenografts. BMC Cancer 2010, 10, 444. [Google Scholar] [CrossRef]
- Hemalatha, S.K.; Sengodan, S.K.; Nadhan, R.; Dev, J.; Sushama, R.R.; Somasundaram, V.; Thankappan, R.; Rajan, A.; Latha, N.R.; Varghese, G.R.; et al. Brcal Defective Breast Cancer Cells Induce in vitro Transformation of Cancer Associated Fibroblasts (CAFs) to Metastasis Associated Fibroblasts (MAF). Sci. Rep. 2018, 8, 13903. [Google Scholar] [CrossRef]
- Calon, A.; Tauriello, D.V.; Batlle, E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 2014, 25, 15–22. [Google Scholar] [CrossRef]
- Rasanen, K.; Vaheri, A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Last, J.A.; Reiser, K.M. Collagen biosynthesis. Environ. Health Perspect. 1984, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Insua-Rodriguez, J.; Oskarsson, T. The extracellular matrix in breast cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef]
- Uitto, J.; Kouba, D. Cytokine modulation of extracellular matrix gene expression: Relevance to fibrotic skin diseases. J. Dermatol. Sci. 2000, 24 (Suppl. 1), S60–S69. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. TGF-beta and TNF-alpha: Antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004, 16, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, C.M.; Bissell, M.J. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: Insights from imaging. Histochem. Cell Biol. 2008, 130, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Nat. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Fan, X.; Hao, M.; Wang, J.; Zhou, X.; Sun, X. Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Mol. Cancer 2016, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Steude, J.S.; Maskarinec, G.; Erber, E.; Verheus, M.; Hernandez, B.Y.; Killeen, J.; Cline, J.M. Mammographic density and matrix metalloproteinases in breast tissue. Cancer Microenviron. 2010, 3, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front. Biosci. 2015, 20, 1144–1163. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Mamessier, E.; Pradel, L.C.; Thibult, M.L.; Drevet, C.; Zouine, A.; Jacquemier, J.; Houvenaeghel, G.; Bertucci, F.; Birnbaum, D.; Olive, D. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J. Immunol. 2013, 190, 2424–2436. [Google Scholar] [CrossRef]
- Iwamoto, M.; Shinohara, H.; Miyamoto, A.; Okuzawa, M.; Mabuchi, H.; Nohara, T.; Gon, G.; Toyoda, M.; Tanigawa, N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int. J. Cancer 2003, 104, 92–97. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Coussens, L.M. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007, 9, 212. [Google Scholar] [CrossRef]
- Hussein, M.R.; Hassan, H.I. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: Preliminary observations. J. Clin. Pathol. 2006, 59, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Sato, M.; Kaneko, R.; Itoh, M.; Sato, S.; Takeuchi, S. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol. Immunother. 1999, 48, 435–442. [Google Scholar] [CrossRef]
- Chua, A.C.; Hodson, L.J.; Moldenhauer, L.M.; Robertson, S.A.; Ingman, W.V. Dual roles for macrophages in ovarian cycle-associated development and remodelling of the mammary gland epithelium. Development 2010, 137, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Nseyo, O.; Campbell, M.J.; Esserman, L.J. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev. Mol. Diagn. 2011, 11, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Agudelo, J.S.; Camara, N.O. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Dziki, J.L.; Siu, B.F.; Medberry, C.J.; Dearth, C.L.; Badylak, S.F. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 2014, 35, 8605–8612. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.D.; Berliner, J.A.; Valente, A.J.; Territo, M.C.; Navab, M.; Parhami, F.; Gerrity, R.; Schwartz, C.J.; Fogelman, A.M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5134–5138. [Google Scholar] [CrossRef]
- Standiford, T.J.; Kunkel, S.L.; Phan, S.H.; Rollins, B.J.; Strieter, R.M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 1991, 266, 9912–9918. [Google Scholar] [CrossRef]
- Carulli, M.T.; Ong, V.H.; Ponticos, M.; Shiwen, X.; Abraham, D.J.; Black, C.M.; Denton, C.P. Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: Evidence for autocrine regulation of myofibroblast differentiation. Arthritis Rheum. 2005, 52, 3772–3782. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Glynn, D.J.; Hodson, L.J.; Huo, C.; Britt, K.; Thompson, E.W.; Woolford, L.; Evdokiou, A.; Pollard, J.W.; Robertson, S.A.; et al. CCL2-driven inflammation increases mammary gland stromal density and cancer susceptibility in a transgenic mouse model. Breast Cancer Res. 2017, 19, 4. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 2009, 284, 29087–29096. [Google Scholar] [CrossRef]
- Yoshimura, T.; Howard, O.M.; Ito, T.; Kuwabara, M.; Matsukawa, A.; Chen, K.; Liu, Y.; Liu, M.; Oppenheim, J.J.; Wang, J.M. Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PLoS ONE 2013, 8, e58791. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef]
- Valkovic, T.; Lucin, K.; Krstulja, M.; Dobi-Babic, R.; Jonjic, N. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol. Res. Pract. 1998, 194, 335–340. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sangai, T.; Ishii, G.; Ikehara, A.; Nagashima, T.; Miyazaki, M.; Ochiai, A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int. J. Cancer 2009, 125, 1276–1284. [Google Scholar] [CrossRef]
- Ueno, T.; Toi, M.; Saji, H.; Muta, M.; Bando, H.; Kuroi, K.; Koike, M.; Inadera, H.; Matsushima, K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000, 6, 3282–3289. [Google Scholar]
- Goede, V.; Brogelli, L.; Ziche, M.; Augustin, H.G. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer 1999, 82, 765–770. [Google Scholar] [CrossRef]
- Lanca, T.; Costa, M.F.; Goncalves-Sousa, N.; Rei, M.; Grosso, A.R.; Penido, C.; Silva-Santos, B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic gammadelta T lymphocytes to tumor beds. J. Immunol. 2013, 190, 6673–6680. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Knight, D.A.; Snyder, L.A.; Smyth, M.J.; Stewart, T.J. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology 2013, 2, e25474. [Google Scholar] [CrossRef]
- Moore, B.B.; Paine, R., 3rd; Christensen, P.J.; Moore, T.A.; Sitterding, S.; Ngan, R.; Wilke, C.A.; Kuziel, W.A.; Toews, G.B. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 2001, 167, 4368–4377. [Google Scholar] [CrossRef]
- Smith, R.E.; Strieter, R.M.; Zhang, K.; Phan, S.H.; Standiford, T.J.; Lukacs, N.W.; Kunkel, S.L. A role for C-C chemokines in fibrotic lung disease. J. Leukoc. Biol. 1995, 57, 782–787. [Google Scholar] [CrossRef]
- Inoshima, I.; Kuwano, K.; Hamada, N.; Hagimoto, N.; Yoshimi, M.; Maeyama, T.; Takeshita, A.; Kitamoto, S.; Egashira, K.; Hara, N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L1038–L1044. [Google Scholar] [CrossRef]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; Denholm, E.M.; Phan, S.H. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J. Biol. Chem. 1996, 271, 17779–17784. [Google Scholar] [CrossRef]
- Lyons, R.M.; Gentry, L.E.; Purchio, A.F.; Moses, H.L. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J. Cell Biol. 1990, 110, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Jurukovski, V.; Chen, Y.; Fontana, L.; Dabovic, B.; Rifkin, D.B. Latent TGF-beta binding proteins. Int. J. Biochem. Cell Biol. 2005, 37, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.F., Jr.; Gorska, A.E.; Chytil, A.; Meise, K.S.; Page, D.L.; Coffey, R.J., Jr.; Moses, H.L. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc. Natl. Acad. Sci. USA 1995, 92, 4254–4258. [Google Scholar] [CrossRef]
- Gorska, A.E.; Jensen, R.A.; Shyr, Y.; Aakre, M.E.; Bhowmick, N.A.; Moses, H.L. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am. J. Pathol. 2003, 163, 1539–1549. [Google Scholar] [CrossRef]
- Sun, X.; Bernhardt, S.M.; Glynn, D.J.; Hodson, L.J.; Woolford, L.; Evdokiou, A.; Yan, C.; Du, H.; Robertson, S.A.; Ingman, W.V. Attenuated TGFB signalling in macrophages decreases susceptibility to DMBA-induced mammary cancer in mice. Breast Cancer Res. 2021, 23, 39. [Google Scholar] [CrossRef]
- Bachman, K.E.; Park, B.H. Duel nature of TGF-beta signaling: Tumor suppressor vs. tumor promoter. Curr. Opin. Oncol. 2005, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Moses, H.; Barcellos-Hoff, M.H. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb. Perspect. Biol. 2011, 3, a003277. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef]
- Heldin, C.H.; Landstrom, M.; Moustakas, A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009, 21, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.F.; Ma, H.Z.; Xin, H.L.; Feng, J.; Wen, A.D.; Li, Y. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. Int. J. Mol. Sci. 2013, 14, 15655–15668. [Google Scholar] [CrossRef]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Muraoka-Cook, R.S.; Kurokawa, H.; Koh, Y.; Forbes, J.T.; Roebuck, L.R.; Barcellos-Hoff, M.H.; Moody, S.E.; Chodosh, L.A.; Arteaga, C.L. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004, 64, 9002–9011. [Google Scholar] [CrossRef]
- Parvani, J.G.; Galliher-Beckley, A.J.; Schiemann, B.J.; Schiemann, W.P. Targeted inactivation of beta1 integrin induces beta3 integrin switching, which drives breast cancer metastasis by TGF-beta. Mol. Biol. Cell 2013, 24, 3449–3459. [Google Scholar] [CrossRef]
- Miyazono, K.; Ehata, S.; Koinuma, D. Tumor-promoting functions of transforming growth factor-beta in progression of cancer. Ups. J. Med. Sci. 2012, 117, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, B.; Bernabeu, C.; Kumar, S. Angiogenesis in breast cancer: The role of transforming growth factor beta and CD105. Microsc. Res. Tech. 2001, 52, 437–449. [Google Scholar] [CrossRef]
- Sime, P.J.; Xing, Z.; Graham, F.L.; Csaky, K.G.; Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Investig. 1997, 100, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, N.; Factor, V.; Nagy, P.; Kopp, J.; Kondaiah, P.; Wakefield, L.; Roberts, A.B.; Sporn, M.B.; Thorgeirsson, S.S. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA 1995, 92, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H.; Yamamoto, T.; Suzuki, H.; Togawa, A.; Ohashi, N.; Fujigaki, Y.; Uchida, C.; Aoki, M.; Hosono, M.; Kitagawa, M.; et al. Treatment with anti-TGF-beta antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGF-beta signaling. Kidney Int. 2004, 65, 63–74. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakata, R.; Ueno, T.; Sata, M.; Ueno, H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology 2000, 32, 247–255. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Eminaga, S.; Toka, O.; Alcalai, R.; Wang, L.; Wakimoto, H.; Nayor, M.; Konno, T.; Gorham, J.M.; Wolf, C.M.; et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J. Clin. Investig. 2010, 120, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Abraham, D.J. Transforming growth factor-beta and connective tissue growth factor: Key cytokines in scleroderma pathogenesis. Curr. Opin. Rheumatol. 2001, 13, 505–511. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef]
- Lee, E.; Van den Berg, D.; Hsu, C.; Ursin, G.; Koh, W.P.; Yuan, J.M.; Stram, D.O.; Yu, M.C.; Wu, A.H. Genetic variation in Transforming Growth Factor beta 1 and mammographic density in Singapore Chinese women. Cancer Res. 2013, 73, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vliet, A.; Nguyen, M.N.; Shigenaga, M.K.; Eiserich, J.P.; Marelich, G.P.; Cross, C.E. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L537–L546. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Ali, M.; Iwamoto, Y.; Zeller, M.W.; Schob, S.; Linnoila, J.J.; Chen, J.W. Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid. Redox. Signal. 2015, 23, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Colon, S.; Luan, H.; Liu, Y.; Meyer, C.; Gewin, L.; Bhave, G. Peroxidasin and eosinophil peroxidase, but not myeloperoxidase, contribute to renal fibrosis in the murine unilateral ureteral obstruction model. Am. J. Physiol. Renal. Physiol. 2019, 316, F360–F371. [Google Scholar] [CrossRef]
- Koller, D.Y.; Nilsson, M.; Enander, I.; Venge, P.; Eichler, I. Serum eosinophil cationic protein, eosinophil protein X and eosinophil peroxidase in relation to pulmonary function in cystic fibrosis. Clin. Exp. Allergy 1998, 28, 241–248. [Google Scholar] [CrossRef] [PubMed]
- DeNichilo, M.O.; Panagopoulos, V.; Rayner, T.E.; Borowicz, R.A.; Greenwood, J.E.; Evdokiou, A. Peroxidase enzymes regulate collagen extracellular matrix biosynthesis. Am. J. Pathol. 2015, 185, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Samoszuk, M.K.; Nguyen, V.; Gluzman, I.; Pham, J.H. Occult deposition of eosinophil peroxidase in a subset of human breast carcinomas. Am. J. Pathol. 1996, 148, 701–706. [Google Scholar] [PubMed]
- Hennigan, K.; Conroy, P.J.; Walsh, M.T.; Amin, M.; O’Kennedy, R.; Ramasamy, P.; Gleich, G.J.; Siddiqui, Z.; Glynn, S.; McCabe, O.; et al. Eosinophil peroxidase activates cells by HER2 receptor engagement and β1-integrin clustering with downstream MAPK cell signaling. Clin. Immunol. 2016, 171, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.T.; Connell, K.; Sheahan, A.M.; Gleich, G.J.; Costello, R.W. Eosinophil peroxidase signals via epidermal growth factor-2 to induce cell proliferation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 946–952. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Bozcuk, H.; Uslu, G.; Samur, M.; Yildiz, M.; Ozben, T.; Ozdogan, M.; Artac, M.; Altunbas, H.; Akan, I.; Savas, B. Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine 2004, 27, 58–65. [Google Scholar] [CrossRef]
- Tripsianis, G.; Papadopoulou, E.; Anagnostopoulos, K.; Botaitis, S.; Katotomichelakis, M.; Romanidis, K.; Kontomanolis, E.; Tentes, I.; Kortsaris, A. Coexpression of IL-6 and TNF-alpha: Prognostic significance on breast cancer outcome. Neoplasma 2014, 61, 205–212. [Google Scholar] [CrossRef]
- Cai, X.; Cao, C.; Li, J.; Chen, F.; Zhang, S.; Liu, B.; Zhang, W.; Zhang, X.; Ye, L. Inflammatory factor TNF-α promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-κB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017, 8, 58338–58352. [Google Scholar] [CrossRef] [PubMed]
- Wolczyk, D.; Zaremba-Czogalla, M.; Hryniewicz-Jankowska, A.; Tabola, R.; Grabowski, K.; Sikorski, A.F.; Augoff, K. TNF-alpha promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell Oncol. 2016, 39, 353–363. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.H.; Kim, J.B.; Nam, S.J.; Yang, J.H.; Kim, J.H.; Lee, J.E. Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 2008, 13, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Pirianov, G.; Colston, K.W. Interactions of vitamin D analogue CB1093, TNFalpha and ceramide on breast cancer cell apoptosis. Mol. Cell Endocrinol. 2001, 172, 69–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, G.; Yan, Y.; Li, X.; Hu, Y.; Wang, J.; Yin, B.; Wu, Y.; Li, Z.; Yang, X.P. Transmembrane TNF-alpha promotes chemoresistance in breast cancer cells. Oncogene 2018, 37, 3456–3470. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.-Y.; Jiang, M.; Zhi, Q.; Bian, X.; Xu, M.-D.; Gong, F.-R.; Hou, J.; Tao, M.; Shou, L.-M.; et al. TNF-α sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.W.; Weissfeld, J.L.; Modugno, F.; Diergaarde, B. Circulating levels of inflammatory markers and mammographic density among postmenopausal women. Breast Cancer Res. Treat. 2011, 127, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Dang, H.X.; Hagemann, I.S.; Appleton, C.M.; Colditz, G.A.; Luo, J.; Maher, C.A. Increased breast tissue receptor activator of nuclear factor-kappaB ligand (RANKL) gene expression is associated with higher mammographic density in premenopausal women. Oncotarget 2017, 8, 73787–73792. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.; Schett, G.; Gay, S.; Distler, O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum. 2008, 58, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, J.; Palombella, V.J.; Henriksen-DeStefano, D.; Swenson, C.; Feinman, R.; Hirai, M.; Tsujimoto, M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J. Exp. Med. 1986, 163, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L.; Simmons, J.G.; Jobin, C.; Lund, P.K. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 2005, 280, 36099–36109. [Google Scholar] [CrossRef]
- Greenwel, P.; Tanaka, S.; Penkov, D.; Zhang, W.; Olive, M.; Moll, J.; Vinson, C.; Di Liberto, M.; Ramirez, F. Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol. Cell Biol. 2000, 20, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- Cuzick, J.; Otto, F.; Baron, J.A.; Brown, P.H.; Burn, J.; Greenwald, P.; Jankowski, J.; La Vecchia, C.; Meyskens, F.; Senn, H.J.; et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009, 10, 501–507. [Google Scholar] [CrossRef]
- Harris, R.E.; Beebe-Donk, J.; Doss, H.; Burr Doss, D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncol. Rep. 2005, 13, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.L.; Huang, D.; Huo, C.W.; Blick, T.; Hill, P.; Cawson, J.; Frazer, H.; Southey, M.D.; Hopper, J.L.; Henderson, M.A.; et al. Dynamic changes in high and low mammographic density human breast tissues maintained in murine tissue engineering chambers during various murine peripartum states and over time. Breast Cancer Res. Treat. 2013, 140, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Ozhand, A.; Lee, E.; Wu, A.H.; Ellingjord-Dale, M.; Akslen, L.A.; McKean-Cowdin, R.; Ursin, G. Variation in inflammatory cytokine/growth-factor genes and mammographic density in premenopausal women aged 50–55. PLoS ONE 2013, 8, e65313. [Google Scholar] [CrossRef]
- Esbona, K.; Inman, D.; Saha, S.; Jeffery, J.; Schedin, P.; Wilke, L.; Keely, P. COX-2 modulates mammary tumor progression in response to collagen density. Breast Cancer Res. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Wang, C.Y.; Sorensen, B.; Xiao, L.; Buist, D.S.; Aiello Bowles, E.J.; White, E.; Rossing, M.A.; Potter, J.; Urban, N. No effect of aspirin on mammographic density in a randomized controlled clinical trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1524–1530. [Google Scholar] [CrossRef]
- Stone, J.; Willenberg, L.; Apicella, C.; Treloar, S.; Hopper, J. The association between mammographic density measures and aspirin or other NSAID use. Breast Cancer Res. Treat. 2012, 132, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Buist, D.S.; Trentham-Dietz, A.; James-Todd, T.M.; Liao, Y. Nonsteroidal anti-inflammatory drugs and change in mammographic density: A cohort study using pharmacy records on over 29,000 postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1088–1095. [Google Scholar] [CrossRef]
- Wood, M.E.; Sprague, B.L.; Oustimov, A.; Synnstvedt, M.B.; Cuke, M.; Conant, E.F.; Kontos, D. Aspirin use is associated with lower mammographic density in a large screening cohort. Breast Cancer Res. Treat. 2017, 162, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hopper, J.L.; Nguyen, T.L.; Li, S. RE: Chemopreventive Agents to Reduce Mammographic Breast Density in Premenopausal Women: A Systematic Review of Clinical Trials. JNCI Cancer Spectr. 2021, 5, pkab051. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archer, M.; Dasari, P.; Evdokiou, A.; Ingman, W.V. Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk. Cancers 2021, 13, 5391. https://doi.org/10.3390/cancers13215391
Archer M, Dasari P, Evdokiou A, Ingman WV. Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk. Cancers. 2021; 13(21):5391. https://doi.org/10.3390/cancers13215391
Chicago/Turabian StyleArcher, Maddison, Pallave Dasari, Andreas Evdokiou, and Wendy V. Ingman. 2021. "Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk" Cancers 13, no. 21: 5391. https://doi.org/10.3390/cancers13215391
APA StyleArcher, M., Dasari, P., Evdokiou, A., & Ingman, W. V. (2021). Biological Mechanisms and Therapeutic Opportunities in Mammographic Density and Breast Cancer Risk. Cancers, 13(21), 5391. https://doi.org/10.3390/cancers13215391






