Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids?
Abstract
Simple Summary
Abstract
1. Introduction
2. Nanotechnology
2.1. Current Trends and Potentials of Nanomedicines
2.2. Application of Nanotechnology in Drug Delivery
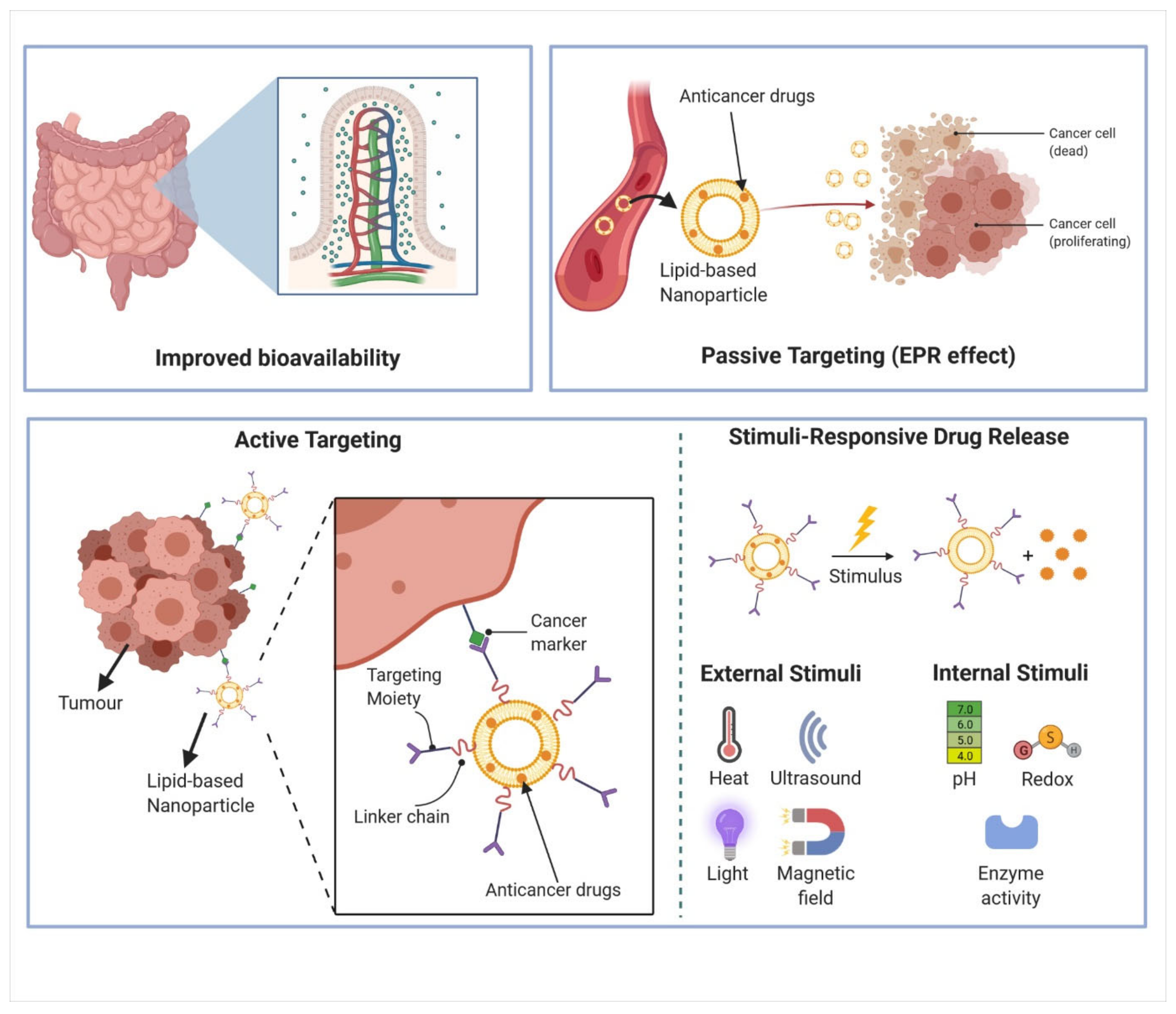
2.2.1. Improved Bioavailability
2.2.2. Passive and Active Targeting
3. Alkaloid
3.1. Anti-Cancer Properties of Alkaloids
3.2. Limitations of Current Alkaloid Anticancer Drugs Formulation
3.2.1. Taxanes
3.2.2. Vinca Alkaloids
3.2.3. Topoisomerase I Inhibitors
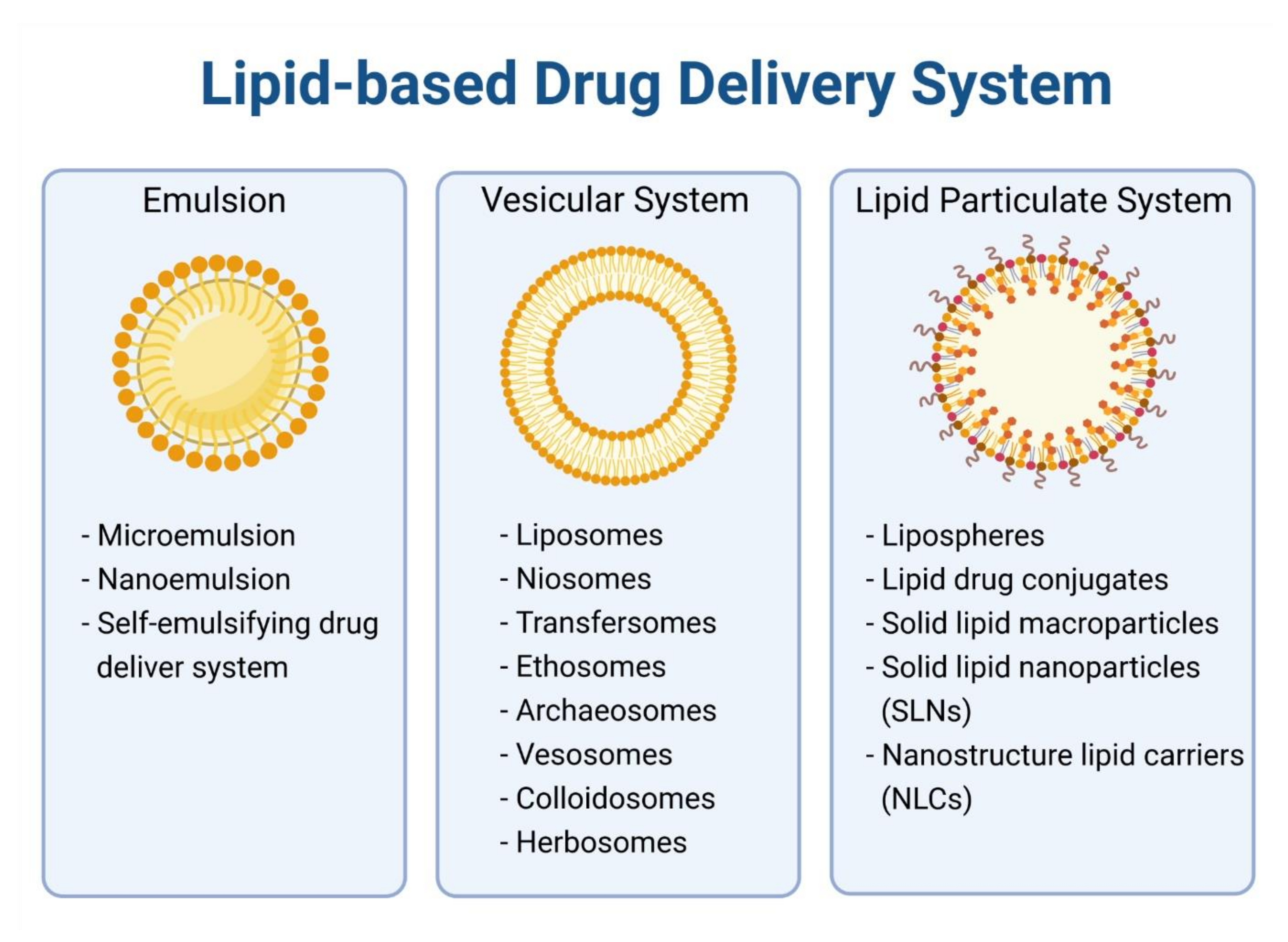
4. Lipid-Based Nanoparticles for Encapsulation of Anticancer Alkaloids
4.1. Liposome
4.2. Micelles
4.3. Solid Lipid Nanoparticles
4.4. Nanostructured Lipid Carriers
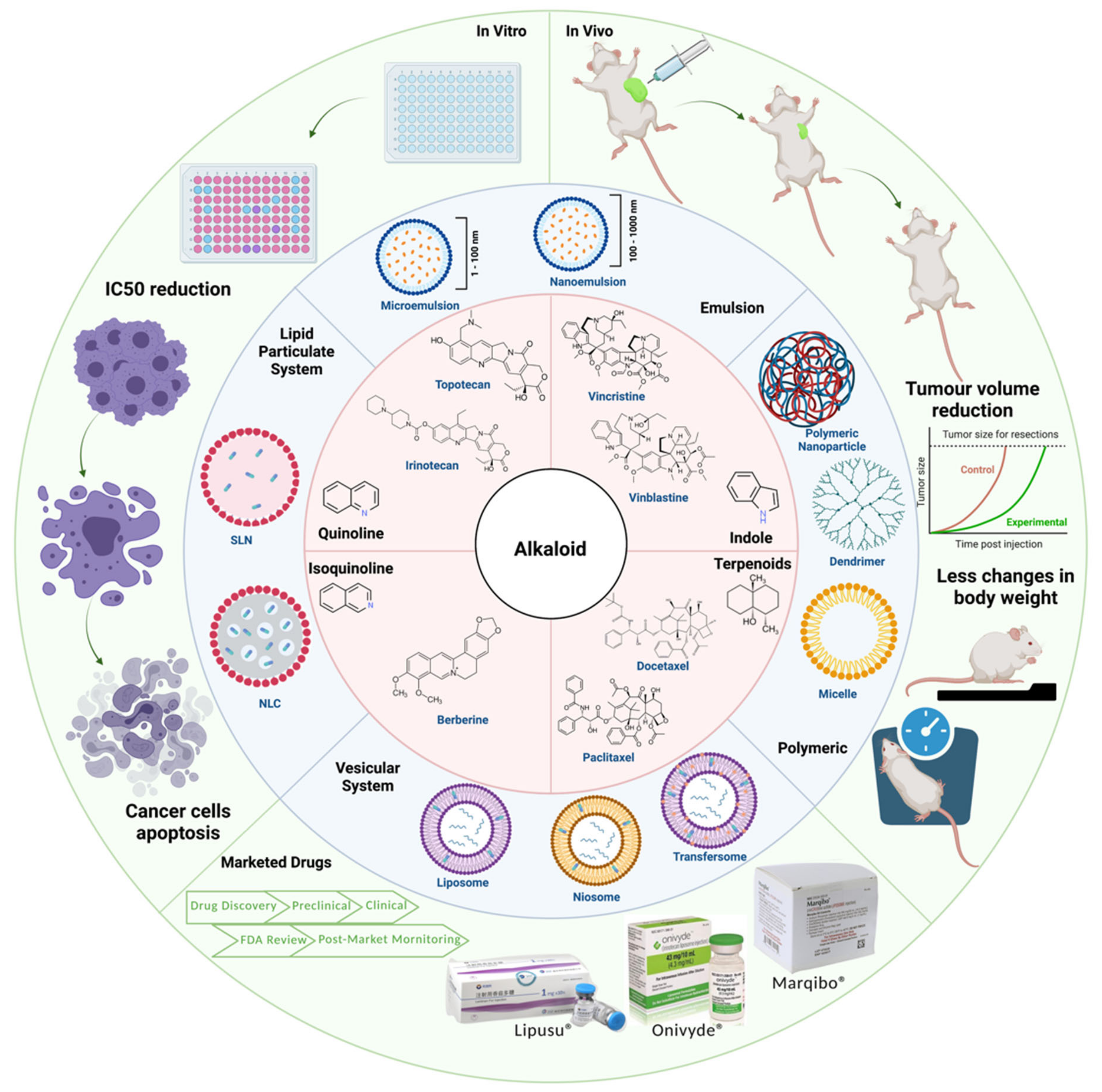
5. Integration of Lipid-Based Nanoparticles Improves Efficacy and Safety of Alkaloids
5.1. In Vitro Efficacy of Alkaloid Encapsulated in Lipid-Based Nanoparticles
5.2. In Vivo Efficacy and Toxicity of Alkaloids in Lipid-Based Nanoparticles
5.3. From Bench to Bedside
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 0-D | No Dimension |
| 1-D | One Dimension |
| 2-D | Two-Dimension |
| ABC | ATP-Binding Cassette |
| ABCB1 | ABC Sub-Family B Member 1 |
| ABCC1 | ABC transporter C1 |
| ABCG2 | ABC transporter G2 |
| ATM | Ataxia-Telangiectasia Mutated |
| ATR | Ataxia–Telangiectasia and RAD3-Related |
| AUC | Area Under The Curve |
| BAK | Bcl-2 Homologous Antagonist Killer |
| BAX | Bcl-2-Associated X Protein |
| BCL | B cell lymphoma |
| BCRP | Breast Cancer Resistance Protein |
| BH3 | Bcl-2 Homology 3 |
| BIND-014 | PSMA-Targeted Docetaxel Nanoparticles |
| CDK | Cyclin-Dependent Kinase |
| Chk2 | Checkpoint Kinase 2 |
| CL | Clearance |
| Cmax | Peak Drug Concentration |
| COVID-19 | Coronavirus Disease 2019 |
| CR | Complete Remission |
| CYP | Cytochrome P450 |
| DDR | DNA Damage Response |
| DISC | Death-Inducing Signaling Complex |
| DNA | Deoxyribonucleic Acid |
| DR3 | Death Receptor 3 |
| EGFR | Epidermal Growth Factor Receptor |
| EPR | Enhanced Permeability and Retention |
| FADD | Fas-Associated Protein With Death Domain |
| FDA | Food and Drug Administration |
| GDP | Guanosine 5′-Diphosphate |
| GTP | Guanosine 5′-Triphosphate |
| IC50 | Half-Maximal Inhibitory Concentration |
| ID | Injected Dose |
| ILs | Interleukins |
| Lys | Lysine |
| MCL-1 | Myeloid Cell Leukemia 1 |
| MDR | Multidrug Resistance |
| MOMP | Mitochondria Outer Membrane Permeabilization |
| MRD | Minimal Residual Disease |
| MRE11 | Meiotic Recombination 11 Homolog 1 |
| MRI | Magnetic Resonance Imaging |
| MRN | MRE11–RAD50–NBS1 |
| mRNA | Messenger Ribonucleic Acid |
| MTD | Maximum Tolerated Dose |
| NLC | Nanostructured Lipid Carriers |
| NNI | National Nanotechnology Initiative |
| Orn | Ornithine |
| pCR | Pathological Complete Response |
| PEG | Polyethylene Glycol |
| Phe | Phenylalanine |
| PSMA | Prostate Specific Membrane Antigen |
| RAD50 | Double Strand Break Repair Protein |
| RB | Retinoblastoma |
| RES | Reticuloendothelial System |
| SAC | Spindle Assembly Checkpoint |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| scFv | Single Chain Fragment Variable |
| SCLC | Small Cell Lung Cancer |
| SGT-53 | Tumor Suppressor Gene TP53/scL-p53 |
| SLN | Solid Lipid Nanoparticles |
| SN-38 | 7-Ethyl-10-Hydroxycamptothecin |
| SN-38G | SN-38 Glucuronide |
| t1/2 | Elimination half-life |
| TfR | Transferrin Receptor |
| TL1A | TNF-Like Cytokine 1A |
| TNF | Tumor Necrosis Factor |
| TOP1cc | Topoisomerase I-DNA Cleaved Complexes |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| UGT1A | Uridine Diphosphate Glucuronosyltransferase 1A |
| Vd | Volume of Distribution |
| VYXEOS | Liposomal Cytarabine/Daunorubicin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol. Biomark. Prev. 2017, 26, 963–970. [Google Scholar] [CrossRef]
- Perera, S.K.; Jacob, S.; Sullivan, R.; Barton, M. Evidence-based benchmarks for use of cancer surgery in high-income countries: A population-based analysis. Lancet Oncol. 2021, 22, 173–181. [Google Scholar] [CrossRef]
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin Chem Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, R.; Ono, N.; Hirai Morita, A.; Katsuragi, T.; Nakamura, S.; Huang, M.; Altaf-Ul-Amin, M.; Kanaya, S. Classification of alkaloids according to the starting substances of their biosynthetic pathways using graph convolutional neural networks. BMC Bioinform. 2019, 20, 380. [Google Scholar] [CrossRef] [PubMed]
- Ehrenworth, A.M.; Peralta-Yahya, P. Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering. Nat. Chem. Biol. 2017, 13, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S.; Mignani, S.; Vishwakarma, R.A. Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis. J. Med. Chem. 2018, 61, 10345–10374. [Google Scholar] [CrossRef]
- Reuvers, T.G.A.; Kanaar, R.; Nonnekens, J. DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage. Cancers 2020, 12, 2098. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies to Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Douer, D. Efficacy and Safety of Vincristine Sulfate Liposome Injection in the Treatment of Adult Acute Lymphocytic Leukemia. Oncologist 2016, 21, 840–847. [Google Scholar] [CrossRef]
- Innocenti, F.; Schilsky, R.L.; Ramirez, J.; Janisch, L.; Undevia, S.; House, L.K.; Das, S.; Wu, K.; Turcich, M.; Marsh, R.; et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J. Clin. Oncol. 2014, 32, 2328–2334. [Google Scholar] [CrossRef]
- Rosello, S.; Blasco, I.; Garcia Fabregat, L.; Cervantes, A.; Jordan, K.; Committee, E.G. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28, iv100–iv118. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. The long view of nanotechnology development: The National Nanotechnology Initiative at 10 years. J. Nanopart. Res. 2011, 13, 427–445. [Google Scholar] [CrossRef]
- Porter, A.L.; Youtie, J. How interdisciplinary is nanotechnology? J. Nanopart. Res. 2009, 11, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Pratt, E.C.; Shaffer, T.M.; Zhang, Q.; Drain, C.M.; Grimm, J. Nanoparticles as multimodal photon transducers of ionizing radiation. Nat. Nanotechnol. 2018, 13, 418–426. [Google Scholar] [CrossRef]
- Yaari, Z.; da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M.; et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef]
- Petersen, G.H.; Alzghari, S.K.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control Release 2016, 232, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kummel, S.; Hilfrich, J.; et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [CrossRef]
- Tardi, P.; Johnstone, S.; Harasym, N.; Xie, S.; Harasym, T.; Zisman, N.; Harvie, P.; Bermudes, D.; Mayer, L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 2009, 33, 129–139. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Nishida, K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Zheng, Y.; Zhao, Y.; Wang, Y.; Hao, J.; Zhao, X.; Yi, K.; Shi, L.; Kang, C.; et al. Virus-like nanoparticle as a co-delivery system to enhance efficacy of CRISPR/Cas9-based cancer immunotherapy. Biomaterials 2020, 258, 120275. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Cong, H.L.; Hu, H.; Xu, F.J. Rational design and latest advances of codelivery systems for cancer therapy. Mater. Today Bio. 2020, 7, 100056. [Google Scholar] [CrossRef]
- Song, Q.; Yin, Y.; Shang, L.; Wu, T.; Zhang, D.; Kong, M.; Zhao, Y.; He, Y.; Tan, S.; Guo, Y.; et al. Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy. Nano Lett. 2017, 17, 6366–6375. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021. [Google Scholar] [CrossRef]
- Liu, J.; Song, L.; Liu, S.; Zhao, S.; Jiang, Q.; Ding, B. A Tailored DNA Nanoplatform for Synergistic RNAi-/Chemotherapy of Multidrug-Resistant Tumors. Angew. Chem. Int. Ed. Engl. 2018, 57, 15486–15490. [Google Scholar] [CrossRef]
- Ortíz, R.; Quiñonero, F.; García-Pinel, B.; Fuel, M.; Mesas, C.; Cabeza, L.; Melguizo, C.; Prados, J. Nanomedicine to Overcome Multidrug Resistance Mechanisms in Colon and Pancreatic Cancer: Recent Progress. Cancers 2021, 13, 2058. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Kim, Y.S.; Belatsarkouski, I.; Gillenwater, A.M.; O’Neill, B.E.; Lapotko, D.O. Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nat. Nanotechnol. 2016, 11, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Dyett, B.P.; Li, M.; Zhao, H.; Zhang, X. Plasmonic Nanobubbles in “Armored” Surface Nanodroplets. J. Phys. Chem. C 2019, 123, 29866–29874. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control Release 2014, 190, 15–28. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Min Sen Oh, V.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.W.; Yoon, I.S.; Kim, D.D. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: Enhanced intestinal absorption and lymphatic uptake. Int. J. Nanomed. 2014, 9, 495–504. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, D.; Swarnakar, N.K.; Thanki, K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 2012, 33, 6758–6768. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Kulhari, H.; Kuncha, M.; Rachamalla, S.S.; Adams, D.J.; Bansal, V.; Sistla, R. Improving Efficacy, Oral Bioavailability, and Delivery of Paclitaxel Using Protein-Grafted Solid Lipid Nanoparticles. Mol. Pharm. 2016, 13, 3903–3912. [Google Scholar] [CrossRef]
- Seo, Y.G.; Kim, D.H.; Ramasamy, T.; Kim, J.H.; Marasini, N.; Oh, Y.K.; Kim, D.W.; Kim, J.K.; Yong, C.S.; Kim, J.O.; et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int. J. Pharm. 2013, 452, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Katare, O.P.; Beg, S.; Lohan, S.; Singh, B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf. B Biointerfaces 2016, 141, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef]
- Diwan, R.; Ravi, P.R.; Pathare, N.S.; Aggarwal, V. Pharmacodynamic, pharmacokinetic and physical characterization of cilnidipine loaded solid lipid nanoparticles for oral delivery optimized using the principles of design of experiments. Colloids Surf. B Biointerfaces 2020, 193, 111073. [Google Scholar] [CrossRef]
- Dening, T.J.; Rao, S.; Thomas, N.; Prestidge, C.A. Oral nanomedicine approaches for the treatment of psychiatric illnesses. J. Control Release 2016, 223, 137–156. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Omar, M.M.; Mahmoud, H.A.; Abou-Taleb, H.A. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Padera, T.P.; Kadambi, A.; di Tomaso, E.; Carreira, C.M.; Brown, E.B.; Boucher, Y.; Choi, N.C.; Mathisen, D.; Wain, J.; Mark, E.J.; et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002, 296, 1883–1886. [Google Scholar] [CrossRef]
- Schwartz, S., Jr. Unmet needs in developing nanoparticles for precision medicine. Nanomedicine 2017, 12, 271–274. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- McNeil, S.E. Evaluation of nanomedicines: Stick to the basics. Nat. Rev. Mater. 2016, 1, 16073. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Burgess, D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm. Sin. B 2020, 10, 2110–2124. [Google Scholar] [CrossRef]
- Perry, J.L.; Reuter, K.G.; Luft, J.C.; Pecot, C.V.; Zamboni, W.; DeSimone, J.M. Mediating Passive Tumor Accumulation through Particle Size, Tumor Type, and Location. Nano Lett. 2017, 17, 2879–2886. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery—Personal Remarks and Future Prospects. J. Pers. Med. 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N.; et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Korn, R.L.; Raghunand, N.; Sachdev, J.C.; Newbold, R.G.; Jameson, G.; Fetterly, G.J.; Prey, J.; Klinz, S.G.; Kim, J.; et al. Correlation between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients with Advanced Solid Tumors: A Pilot Study. Clin. Cancer Res. 2017, 23, 3638. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Abarghooi Kahaki, F.; Ahangarzadeh, S.; Yaghoobi, H.; Yarian, F.; Arezumand, R.; Ranjbari, J.; Mokhtarzadeh, A.; de la Guardia, M. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J. Control Release 2017, 268, 323–334. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Dorrance, A.M.; Neviani, P.; Ferenchak, G.J.; Huang, X.; Nicolet, D.; Maharry, K.S.; Ozer, H.G.; Hoellarbauer, P.; Khalife, J.; Hill, E.B.; et al. Targeting leukemia stem cells in vivo with antagomiR-126 nanoparticles in acute myeloid leukemia. Leukemia 2015, 29, 2143–2153. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Liang, B.; Shahbaz, M.; Wang, Y.; Gao, H.; Fang, R.; Niu, Z.; Liu, S.; Wang, B.; Sun, Q.; Niu, W.; et al. Integrinbeta6-targeted immunoliposomes mediate tumor-specific drug delivery and enhance therapeutic efficacy in colon carcinoma. Clin. Cancer Res. 2015, 21, 1183–1195. [Google Scholar] [CrossRef]
- Wei, Y.; Gu, X.; Sun, Y.; Meng, F.; Storm, G.; Zhong, Z. Transferrin-binding peptide functionalized polymersomes mediate targeted doxorubicin delivery to colorectal cancer in vivo. J. Control Release 2020, 319, 407–415. [Google Scholar] [CrossRef]
- Bar-Zeev, M.; Livney, Y.D.; Assaraf, Y.G. Targeted nanomedicine for cancer therapeutics: Towards precision medicine overcoming drug resistance. Drug Resist. Updates 2017, 31, 15–30. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, e1902604. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Opadele, A.E.; Onodera, Y.; Nam, J.M. Targeting Integrins in Cancer Nanomedicine: Applications in Cancer Diagnosis and Therapy. Cancers 2019, 11, 1783. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.-L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Pola, R.; Gremse, F.; Theek, B.; Ehling, J.; Moeckel, D.; Hermanns-Sachweh, B.; Pechar, M.; Ulbrich, K.; Hennink, W.E.; et al. Passive versus Active Tumor Targeting Using RGD- and NGR-Modified Polymeric Nanomedicines. Nano Lett. 2014, 14, 972–981. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C.W. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.A.; Dreicer, R.; Anderson, J.; Garcia, J.A.; Alva, A.; Hart, L.L.; Milowsky, M.I.; Posadas, E.M.; Ryan, C.J.; Graf, R.P.; et al. Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients With Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1344–1351. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; LoRusso, P.M.; Burris, H.A., III; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I Study of PSMA-Targeted Docetaxel-Containing Nanoparticle BIND-014 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Nemunaitis, J.; Leung, P.K.; Nunan, R.; Adams, J.; Chang, E.H. Safety and Efficacy in Advanced Solid Tumors of a Targeted Nanocomplex Carrying the p53 Gene Used in Combination with Docetaxel: A Phase 1b Study. Mol. Ther. 2016, 24, 1697–1706. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.; Zhang, X.Q.; Guo, C.C.; Shen, Y.; Pirollo, K.F.; Sabir, S.; Leung, C.; Leong-Wu, C.; Ling, C.M.; Chang, E.H.; et al. A Phase l Study of a Tumor-targeted Systemic Nanodelivery System, SGT-94, in Genitourinary Cancers. Mol. Ther. 2016, 24, 1484–1491. [Google Scholar] [CrossRef]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: A phase 1 dose-escalation study. Lancet Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef]
- Sanz-Ortega, L.; Rojas, J.M.; Barber, D.F. Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting. Pharmaceutics 2020, 12, 812. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Ryan, G.M.; Leung, A.W.Y.; Firmino, N.S.; Bennewith, K.L.; Bally, M.B. Liposomal Formulations to Modulate the Tumour Microenvironment and Antitumour Immune Response. Int. J. Mol. Sci. 2018, 19, 2922. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.; Yao, H.; Xu, J. Insights into drug discovery from natural products through structural modification. Fitoterapia 2015, 103, 231–241. [Google Scholar] [CrossRef]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, C.; Zhao, Q.; Bentouhami, E.; Djehal, A.; Nebigil, C.G.; Johnson, R.A.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; et al. Cancer wars: Natural products strike back. Front. Chem. 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Stockigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Cortinovis, D.; Bidoli, P.; Canova, S.; Colonese, F.; Gemelli, M.; Lavitrano, M.L.; Banna, G.L.; Liu, S.V.; Morabito, A. Novel Cytotoxic Chemotherapies in Small Cell Lung Carcinoma. Cancers 2021, 13, 1152. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illes, A.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Macheret, M.; Halazonetis, T.D. DNA replication stress as a hallmark of cancer. Annu. Rev. Pathol. 2015, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Rethinking the war on cancer. Lancet 2014, 383, 558–563. [Google Scholar] [CrossRef]
- Barisic, M.; Silva e Sousa, R.; Tripathy, S.K.; Magiera, M.M.; Zaytsev, A.V.; Pereira, A.L.; Janke, C.; Grishchuk, E.L.; Maiato, H. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 2015, 348, 799–803. [Google Scholar] [CrossRef]
- Gui, M.; Ma, M.; Sze-Tu, E.; Wang, X.; Koh, F.; Zhong, E.D.; Berger, B.; Davis, J.H.; Dutcher, S.K.; Zhang, R.; et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 2021, 28, 29–37. [Google Scholar] [CrossRef]
- Kopf, A.; Renkawitz, J.; Hauschild, R.; Girkontaite, I.; Tedford, K.; Merrin, J.; Thorn-Seshold, O.; Trauner, D.; Hacker, H.; Fischer, K.D.; et al. Microtubules control cellular shape and coherence in amoeboid migrating cells. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Roeles, J.; Tsiavaliaris, G. Actin-microtubule interplay coordinates spindle assembly in human oocytes. Nat. Commun. 2019, 10, 4651. [Google Scholar] [CrossRef] [PubMed]
- Zenker, J.; White, M.D.; Templin, R.M.; Parton, R.G.; Thorn-Seshold, O.; Bissiere, S.; Plachta, N. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 2017, 357, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Basnet, N.; Nedozralova, H.; Crevenna, A.H.; Bodakuntla, S.; Schlichthaerle, T.; Taschner, M.; Cardone, G.; Janke, C.; Jungmann, R.; Magiera, M.M.; et al. Direct induction of microtubule branching by microtubule nucleation factor SSNA1. Nat. Cell Biol. 2018, 20, 1172–1180. [Google Scholar] [CrossRef]
- Lee, G.; Leech, G.; Rust, M.J.; Das, M.; McGorty, R.J.; Ross, J.L.; Robertson-Anderson, R.M. Myosin-driven actin-microtubule networks exhibit self-organized contractile dynamics. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharm. 2017, 83, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Du, L. Targeting and extending the eukaryotic druggable genome with natural products: Cytoskeletal targets of natural products. Nat. Prod. Rep. 2020, 37, 634–652. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, E.J.; Zhang, B.; Wu, C.; Pardeshi, L.; Shi, C.; Mou, P.K.; Liu, Y.; Tan, K.; Shim, J.S. Synthetic lethality of RB1 and aurora A is driven by stathmin-mediated disruption of microtubule dynamics. Nat. Commun. 2020, 11, 5105. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Diaz, J.F.; Altmann, K.H.; Steinmetz, M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.L.; Li, J.; Bennett, M.J.; Rohena, C.C.; Peng, J.; Schriemer, D.C.; Mooberry, S.L. Taccalonolide binding to tubulin imparts microtubule stability and potent in vivo activity. Cancer Res. 2013, 73, 6780–6792. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, X.; Shen, Z.; Chen, S.; Yu, H.; Williams, N.; Wang, G. Anti-mitotic chemotherapeutics promote apoptosis through TL1A-activated death receptor 3 in cancer cells. Cell Res. 2018, 28, 544–555. [Google Scholar] [CrossRef]
- Henriques, A.C.; Ribeiro, D.; Pedrosa, J.; Sarmento, B.; Silva, P.M.A.; Bousbaa, H. Mitosis inhibitors in anticancer therapy: When blocking the exit becomes a solution. Cancer Lett. 2019, 440–441, 64–81. [Google Scholar] [CrossRef]
- Dang, F.; Nie, L.; Wei, W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021, 28, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef]
- Haschka, M.D.; Soratroi, C.; Kirschnek, S.; Hacker, G.; Hilbe, R.; Geley, S.; Villunger, A.; Fava, L.L. The NOXA-MCL1-BIM axis defines lifespan on extended mitotic arrest. Nat. Commun. 2015, 6, 6891. [Google Scholar] [CrossRef]
- Topham, C.; Tighe, A.; Ly, P.; Bennett, A.; Sloss, O.; Nelson, L.; Ridgway, R.A.; Huels, D.; Littler, S.; Schandl, C.; et al. MYC Is a Major Determinant of Mitotic Cell Fate. Cancer Cell 2015, 28, 129–140. [Google Scholar] [CrossRef]
- Wang, P.; Lindsay, J.; Owens, T.W.; Mularczyk, E.J.; Warwood, S.; Foster, F.; Streuli, C.H.; Brennan, K.; Gilmore, A.P. Phosphorylation of the proapoptotic BH3-only protein bid primes mitochondria for apoptosis during mitotic arrest. Cell Rep. 2014, 7, 661–671. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pena-Blanco, A.; Haschka, M.D.; Jenner, A.; Zuleger, T.; Proikas-Cezanne, T.; Villunger, A.; Garcia-Saez, A.J. Drp1 modulates mitochondrial stress responses to mitotic arrest. Cell Death Differ. 2020, 27, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, C.; Cicchillitti, L.; Raspaglio, G.; Bartollino, S.; Cimitan, S.; Bertucci, C.; Mozzetti, S.; Gallo, D.; Persico, M.; Fattorusso, C.; et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009, 69, 6906–6914. [Google Scholar] [CrossRef]
- Henriques, A.C.; Silva, P.M.A.; Sarmento, B.; Bousbaa, H. Antagonizing the spindle assembly checkpoint silencing enhances paclitaxel and Navitoclax-mediated apoptosis with distinct mechanistic. Sci. Rep. 2021, 11, 4139. [Google Scholar] [CrossRef]
- Arnst, K.E.; Banerjee, S.; Chen, H.; Deng, S.; Hwang, D.J.; Li, W.; Miller, D.D. Current advances of tubulin inhibitors as dual acting small molecules for cancer therapy. Med. Res. Rev. 2019, 39, 1398–1426. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef]
- Alushin, G.M.; Lander, G.C.; Kellogg, E.H.; Zhang, R.; Baker, D.; Nogales, E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 2014, 157, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Manchado, E.; Guillamot, M.; Malumbres, M. Killing cells by targeting mitosis. Cell Death Differ. 2012, 19, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer 2014, 13, 275–284. [Google Scholar] [CrossRef]
- Baranello, L.; Wojtowicz, D.; Cui, K.; Devaiah, B.N.; Chung, H.J.; Chan-Salis, K.Y.; Guha, R.; Wilson, K.; Zhang, X.; Zhang, H.; et al. RNA Polymerase II Regulates Topoisomerase 1 Activity to Favor Efficient Transcription. Cell 2016, 165, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chan, N.L.; Hsieh, T.S. New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 2013, 82, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.C.; Chalmers, A.J.; El-Khamisy, S.F. Topoisomerase I inhibition in colorectal cancer: Biomarkers and therapeutic targets. Br. J. Cancer 2012, 106, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Tainer, J.A. The MRE11-RAD50-NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu. Rev. Biochem. 2018, 87, 263–294. [Google Scholar] [CrossRef] [PubMed]
- Lecona, E.; Fernandez-Capetillo, O. Targeting ATR in cancer. Nat. Rev. Cancer 2018, 18, 586–595. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Voigt, W.; Jordan, K. Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- Piao, X.; Yin, H.; Guo, S.; Wang, H.; Guo, P. RNA Nanotechnology to Solubilize Hydrophobic Antitumor Drug for Targeted Delivery. Adv. Sci. 2019, 6, 1900951. [Google Scholar] [CrossRef]
- Hennenfent, K.L.; Govindan, R. Novel formulations of taxanes: A review. Old wine in a new bottle? Ann. Oncol. 2006, 17, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; Baker, S.D.; Verweij, J. Paclitaxel repackaged in an albumin-stabilized nanoparticle: Handy or just a dandy? J. Clin. Oncol. 2005, 23, 7765–7767. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical Translation of Nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Van Zuylen, L.; Karlsson, M.O.; Verweij, J.; Brouwer, E.; de Bruijn, P.; Nooter, K.; Stoter, G.; Sparreboom, A. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother. Pharm. 2001, 47, 309–318. [Google Scholar] [CrossRef]
- Gardner, E.R.; Dahut, W.L.; Scripture, C.D.; Jones, J.; Aragon-Ching, J.B.; Desai, N.; Hawkins, M.J.; Sparreboom, A.; Figg, W.D. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin. Cancer Res. 2008, 14, 4200–4205. [Google Scholar] [CrossRef]
- Hormann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Engels, F.K.; Loos, W.J.; van der Bol, J.M.; de Bruijn, P.; Mathijssen, R.H.; Verweij, J.; Mathot, R.A. Therapeutic drug monitoring for the individualization of docetaxel dosing: A randomized pharmacokinetic study. Clin. Cancer Res. 2011, 17, 353–362. [Google Scholar] [CrossRef]
- Engels, F.K.; Sparreboom, A.; Mathot, R.A.; Verweij, J. Potential for improvement of docetaxel-based chemotherapy: A pharmacological review. Br. J. Cancer 2005, 93, 173–177. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control Release 2005, 104, 103–111. [Google Scholar] [CrossRef]
- Madsen, M.L.; Due, H.; Ejskjaer, N.; Jensen, P.; Madsen, J.; Dybkaer, K. Aspects of vincristine-induced neuropathy in hematologic malignancies: A systematic review. Cancer Chemother. Pharm. 2019, 84, 471–485. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Sacco, J.J.; Botten, J.; Macbeth, F.; Bagust, A.; Clark, P. The average body surface area of adult cancer patients in the UK: A multicentre retrospective study. PLoS ONE 2010, 5, e8933. [Google Scholar] [CrossRef]
- O’Brien, S.; Schiller, G.; Lister, J.; Damon, L.; Goldberg, S.; Aulitzky, W.; Ben-Yehuda, D.; Stock, W.; Coutre, S.; Douer, D.; et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 31, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Merchant, M.S.; Cole, D.E.; Jayaprakash, N.; Bernstein, D.; Delbrook, C.; Richards, K.; Widemann, B.C.; Wayne, A.S. Vincristine Sulfate Liposomes Injection (VSLI, Marqibo(R)): Results From a Phase I Study in Children, Adolescents, and Young Adults With Refractory Solid Tumors or Leukemias. Pediatr. Blood Cancer 2016, 63, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharm. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, Z.L.; Qian, Z.Z.; Hu, G.; Wang, H.Q.; Liu, W.H.; Cheng, G. Pharmacokinetic characteristics of vincristine sulfate liposomes in patients with advanced solid tumors. Acta Pharm. Sin. 2012, 33, 852–858. [Google Scholar] [CrossRef] [PubMed]
- De Man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharm. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, A.; Sun, X.; Liu, L.; Zhu, Y.; Zhang, T.; Jia, J.; Tan, S.; Wu, J.; Wang, X.; et al. Multicenter, Randomized, Phase III Trial of Neoadjuvant Chemoradiation With Capecitabine and Irinotecan Guided by UGT1A1 Status in Patients With Locally Advanced Rectal Cancer. J. Clin. Oncol. 2020, 38, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014, 74, 7003–7013. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control Release 2012, 163, 34–45. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hussain, A.; Hussain, M.S.; Mirza, M.A.; Iqbal, Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev. Ind. Pharm. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Tam, Y.K.; Leung, A.K.K.; Cullis, P.R. Production of limit size nanoliposomal systems with potential utility as ultra-small drug delivery agents. J. Liposome Res. 2016, 26, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharm. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharm. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Grenader, T. Pharmacological basis of pegylated liposomal doxorubicin: Impact on cancer therapy. Eur. J. Pharm. Sci. 2012, 45, 388–398. [Google Scholar] [CrossRef]
- Steffes, V.M.; Zhang, Z.; MacDonald, S.; Crowe, J.; Ewert, K.K.; Carragher, B.; Potter, C.S.; Safinya, C.R. PEGylation of Paclitaxel-Loaded Cationic Liposomes Drives Steric Stabilization of Bicelles and Vesicles thereby Enhancing Delivery and Cytotoxicity to Human Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, L.; Jiang, S. Superhydrophilic Zwitterionic Polymers Stabilize Liposomes. Langmuir 2012, 28, 11625–11632. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Lewrick, F.; Süss, R. Remote loading of anthracyclines into liposomes. Methods Mol. Biol. 2010, 605, 139–145. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control Release 2014, 174, 98–108. [Google Scholar] [CrossRef]
- Deshpande, P.; Jhaveri, A.; Pattni, B.; Biswas, S.; Torchilin, V. Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018, 25, 517–532. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kawabata, S.; Tashita, H.; Shimizu, N. Ultrasound-mediated drug delivery using liposomes modified with a thermosensitive polymer. Ultrason. Sonochem. 2014, 21, 310–316. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Porav, S.; Banciu, M.; Porfire, A. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv. Transl. Res. 2019, 9, 260–272. [Google Scholar] [CrossRef]
- Legut, M.; Lipka, D.; Filipczak, N.; Piwoni, A.; Kozubek, A.; Gubernator, J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines - in vitro studies. Int. J. Nanomed. 2014, 9, 653–668. [Google Scholar] [CrossRef][Green Version]
- Lakkadwala, S.; Singh, J. Dual Functionalized 5-Fluorouracil Liposomes as Highly Efficient Nanomedicine for Glioblastoma Treatment as Assessed in an In Vitro Brain Tumor Model. J. Pharm. Sci. 2018, 107, 2902–2913. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 291–302. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019, 29, 103–113. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharm. 2015, 73, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomedicine 2018, 14, 2023–2050. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.Y.; Rubinstein, I.; Onyüksel, H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm. Res. 2011, 28, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, A.; Rubinstein, I.; Önyüksel, H. Sterically Stabilized Phospholipid Mixed Micelles: In Vitro Evaluation as a Novel Carrier for Water-Insoluble Drugs. Pharm. Res. 2003, 20, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Du, G.; Wang, N.; Liu, C.; Hang, H.; Liang, W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J. Natl. Cancer Inst. 2007, 99, 1004–1015. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Peter, W. The Lipid Bilayer. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target 2012, 20, 813–830. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2018, 19, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, R.; Rezayat, S.M.; Faridi-Majidi, R.; Sadri, K.; Jaafari, M.R. Optimization of Docetaxel Loading Conditions in Liposomes: Proposing potential products for metastatic breast carcinoma chemotherapy. Sci. Rep. 2020, 10, 5569. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Winters, G.; Srinivasulu, M.; Crawford, J.; Wong, M.; Amankwa, L.; Waterhouse, D.; Masin, D.; Webb, M.; Harasym, N.; et al. Development of a weak-base docetaxel derivative that can be loaded into lipid nanoparticles. J. Control Release 2010, 144, 332–340. [Google Scholar] [CrossRef]
- Chang, M.; Lu, S.; Zhang, F.; Zuo, T.; Guan, Y.; Wei, T.; Shao, W.; Lin, G. RGD-modified pH-sensitive liposomes for docetaxel tumor targeting. Colloids Surf. B Biointerfaces 2015, 129, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Liu, D.; Guo, W.; Wang, M.; Wu, C.; Guo, M.; Ai, X.; Wang, Y.; He, Z. Docetaxel prodrug liposomes for tumor therapy: Characterization, in vitro and in vivo evaluation. Drug Deliv. 2016, 23, 1272–1281. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, B.; Tao, W.; Li, J.; Kou, L.; Lian, H.; Che, X.; He, Z.; Sun, J. ATB(0,+) transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater. Sci. 2017, 5, 295–304. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, Q.; Shi, Y.; Huang, Y.; Zhang, J.; Zhang, X.; Lin, G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control Release 2018, 286, 369–380. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, Y.; Feng, S.S. Formulation of Docetaxel by folic acid-conjugated d-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials 2011, 32, 4058–4066. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Z.; Wang, F.; Ji, X.; Zhao, Z.; Luan, Y. Docetaxel-loaded PEO-PPO-PCL/TPGS mixed micelles for overcoming multidrug resistance and enhancing antitumor efficacy. J. Mater. Chem. B 2015, 3, 4259–4271. [Google Scholar] [CrossRef]
- Liu, H.; Tu, L.; Zhou, Y.; Dang, Z.; Wang, L.; Du, J.; Feng, J.; Hu, K. Improved Bioavailability and Antitumor Effect of Docetaxel by TPGS Modified Proniosomes: In Vitro and In Vivo Evaluations. Sci. Rep. 2017, 7, 43372. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ravar, F.; Saadat, E.; Gholami, M.; Dehghankelishadi, P.; Mahdavi, M.; Azami, S.; Dorkoosh, F.A. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J. Control Release 2016, 229, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, J.; Liu, J.; He, H.; Zhang, W.; Li, Z. Tumor targeting effects of a novel modified paclitaxel-loaded discoidal mimic high density lipoproteins. Drug Deliv. 2013, 20, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.Q.; Chen, M.S.; Zhou, X.; Guo, L.M.; Zhu, J.J.; Wang, R.; Zhang, X.X.; Gan, Y. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharm. Sin. 2021. [Google Scholar] [CrossRef]
- Zhang, X.G.; Miao, J.; Dai, Y.Q.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int. J. Pharm. 2008, 361, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes(R): Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.J.; Ke, X.Y.; Huang, Y.; Chen, X.M.; Zhao, X.; Zhao, B.X.; Lu, W.L.; Lou, J.N.; Zhang, X.; Zhang, Q. The antiangiogenic efficacy of NGR-modified PEG-DSPE micelles containing paclitaxel (NGR-M-PTX) for the treatment of glioma in rats. J. Drug Target. 2011, 19, 382–390. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, S.; Jiang, Y.; Gu, L.Y.; Xiao, Y.; Wang, X.; Cheng, L.; Li, X.T. Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG2000-transferrin in treatment of brain glioma. Eur. J. Pharm. Sci. 2017, 96, 129–140. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Yang, X.; Wang, F.; Lu, W. Folic acid-conjugated liposomal vincristine for multidrug resistant cancer therapy. Asian J. Pharm. Sci. 2013, 8, 118–127. [Google Scholar] [CrossRef]
- Aboutaleb, E.; Atyabi, F.; Khoshayand, M.R.; Vatanara, A.R.; Ostad, S.N.; Kobarfard, F.; Dinarvand, R. Improved brain delivery of vincristine using dextran sulfate complex solid lipid nanoparticles: Optimization and in vivo evaluation. J. Biomed. Mater. Res. A 2014, 102, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Xu, Q.; Huang, Z.; Wang, Y.; Shen, Q. Hyaluronic acid-coated cationic nanostructured lipid carriers for oral vincristine sulfate delivery. Drug Dev. Ind. Pharm. 2017, 43, 661–667. [Google Scholar] [CrossRef]
- Li, X.T.; He, M.L.; Zhou, Z.Y.; Jiang, Y.; Cheng, L. The antitumor activity of PNA modified vinblastine cationic liposomes on Lewis lung tumor cells: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Ahmadvand, H.; Farhadi, A.; Najmafshar, A.; Chiani, M.; Norouzian, D. Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells. Drug Dev. Ind. Pharm. 2018, 44, 1371–1376. [Google Scholar] [CrossRef]
- Bahadori, F.; Topcu, G.; Eroglu, M.S.; Onyuksel, H. A new lipid-based nano formulation of vinorelbine. AAPS Pharm. Sci. Tech. 2014, 15, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, S.; Vali, A.M.; Rezaie, M. The effect of PEG coating on in vitro cytotoxicity and in vivo disposition of topotecan loaded liposomes in rats. Int. J. Pharm. 2008, 353, 251–259. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.H.; Zhang, L.; Yao, H.J.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Zhou, J.; Li, N.; et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 2012, 33, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.G.; Silva, E.J.; Martins, A.L.; Mota, M.F.; Braga, R.C.; Lima, E.M.; Valadares, M.C.; Taveira, S.F.; Marreto, R.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196. [Google Scholar] [CrossRef]
- Hattori, Y.; Shi, L.; Ding, W.; Koga, K.; Kawano, K.; Hakoshima, M.; Maitani, Y. Novel irinotecan-loaded liposome using phytic acid with high therapeutic efficacy for colon tumors. J. Control Release 2009, 136, 30–37. [Google Scholar] [CrossRef]
- Din, F.U.; Choi, J.Y.; Kim, D.W.; Mustapha, O.; Kim, D.S.; Thapa, R.K.; Ku, S.K.; Youn, Y.S.; Oh, K.T.; Yong, C.S.; et al. Irinotecan-encapsulated double-reverse thermosensitive nanocarrier system for rectal administration. Drug Deliv. 2017, 24, 502–510. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kuo, J.Y.; Hsu, C.C.; Tsai, W.C.; Li, W.C.; Yu, M.C.; Wen, H.W. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int. J. Pharm. 2013, 441, 381–388. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, J.; Zhang, C.X.; Li, X.Y.; Li, N.; Ju, R.J.; Shi, J.F.; Sun, M.G.; Zhao, W.Y.; Mu, L.M.; et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials 2013, 34, 4452–4465. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Choi, Y.J.; Han, J.W.; Reza, A.; Kim, J.H. Nanoceria-mediated delivery of doxorubicin enhances the anti-tumour efficiency in ovarian cancer cells via apoptosis. Sci. Rep. 2017, 7, 9513. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; Garcia, I.; Liz-Marzan, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. ACC Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Zeng, X.; Morgenstern, R.; Nystrom, A.M. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials 2014, 35, 1227–1239. [Google Scholar] [CrossRef]
- Dong, X.; Wang, W.; Qu, H.; Han, D.; Zheng, J.; Sun, G. Targeted delivery of doxorubicin and vincristine to lymph cancer: Evaluation of novel nanostructured lipid carriers in vitro and in vivo. Drug Deliv. 2016, 23, 1374–1378. [Google Scholar] [CrossRef]
- Ganoth, A.; Merimi, K.C.; Peer, D. Overcoming multidrug resistance with nanomedicines. Expert Opin. Drug Deliv. 2015, 12, 223–238. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updates 2017, 32, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, R.H.; Sparreboom, A.; Verweij, J. Determining the optimal dose in the development of anticancer agents. Nat. Rev. Clin. Oncol. 2014, 11, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hayes, M.E.; Connolly-Ingram, C.; Gabriel, B.S.; Hann, B.; Liu, B.; Park, J.W.; Hong, K.; et al. Development of a highly stable and targetable nanoliposomal formulation of topotecan. J. Control Release 2010, 141, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Akhter, S.; Greig, N.H.; Kamal, M.A.; Midoux, P.; Pichon, C. Engineered Nanoparticles Against MDR in Cancer: The State of the Art and its Prospective. Curr. Pharm. Des. 2016, 22, 4360–4373. [Google Scholar] [CrossRef]
- Auria-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Vinuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. NANO Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Tavares, A.J.; Poon, W.; Zhang, Y.N.; Dai, Q.; Besla, R.; Ding, D.; Ouyang, B.; Li, A.; Chen, J.; Zheng, G.; et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl. Acad. Sci. USA 2017, 114, E10871–E10880. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. ACC Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, W.S.; Dugast, A.-S.; Suchy, J.J.; Grabow, S.; Fulton, R.B.; Sampson, J.F.; Luus, L.; Santiago, M.; Koshkaryev, A.; Sun, G.; et al. Synergy between EphA2-ILs-DTXp, a Novel EphA2-Targeted Nanoliposomal Taxane, and PD-1 Inhibitors in Preclinical Tumor Models. Mol. Cancer Ther. 2020, 19, 270. [Google Scholar] [CrossRef]
- Hafner, A.; Lovric, J.; Lakos, G.P.; Pepic, I. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, X.E.; Gao, L.L. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed. Pharm. 2009, 63, 603–607. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Xu, H.Q.; Huang, X.E.; Qian, Y.D.; Xiang, J. Clinical comparison between paclitaxel liposome (Lipusu(R)) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2591–2594. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhang, H.; Yang, Y.; Xie, X.-Y.; Yang, Y.-F.; Li, Z.; Li, Y.; Gong, W.; Yu, F.-L.; Yang, Z.; et al. Preparation, characterization, and efficacy of thermosensitive liposomes containing paclitaxel. Drug Deliv. 2016, 23, 1222–1231. [Google Scholar] [CrossRef]
- Peng, J.Q.; Fumoto, S.; Suga, T.; Miyamoto, H.; Kuroda, N.; Kawakami, S.; Nishida, K. Targeted co-delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J. Control Release 2019, 302, 42–53. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Wang, T.; He, W.; Zhou, W.; Li, M.; Yao, C.; Li, X. Improved Antitumor Activity of Novel Redox-Responsive Paclitaxel-Encapsulated Liposomes Based on Disulfide Phosphatidylcholine. Mol. Pharm. 2020, 17, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.S.; Veening-Griffioen, D.H.; Boon, W.P.C.; Moors, E.H.M.; Gispen-de Wied, C.C.; Schellekens, H.; van Meer, P.J.K. A standardised framework to identify optimal animal models for efficacy assessment in drug development. PLoS ONE 2019, 14, e0218014. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, L.I.; Johnston, A.P.R. It’s what’s on the inside that counts: Techniques for investigating the uptake and recycling of nanoparticles and proteins in cells. J. Colloid. Interface Sci. 2021, 587, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Poon, W.; Zhang, Y.N.; Lin, Z.P.; Kingston, B.R.; Tavares, A.J.; Zhang, Y.; Chen, J.; Valic, M.S.; Syed, A.M.; et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 2020, 19, 1362–1371. [Google Scholar] [CrossRef]
| Class | Drugs | Molecular Formula | Origin | Indication/Uses |
|---|---|---|---|---|
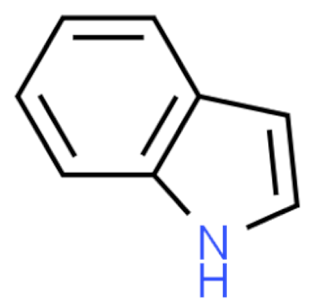 Indole | Vincristine 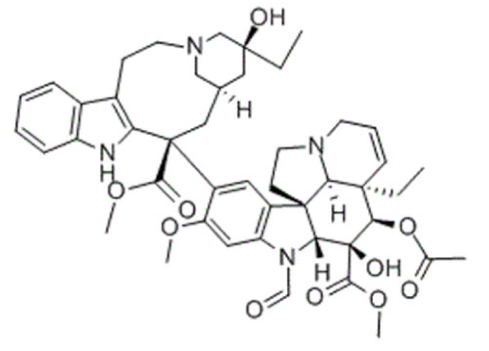 | C46H56N4O10 | Catharanthus roseus | Anticancer |
Vinblastine 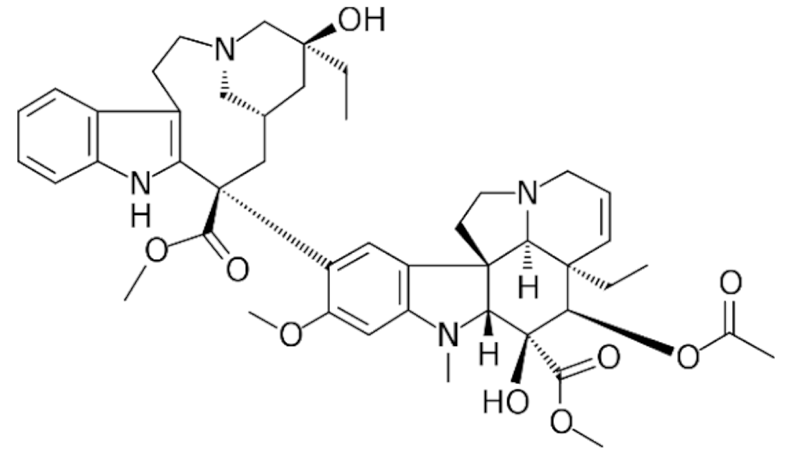 | C46H58N4O9 | |||
Vinorelbine 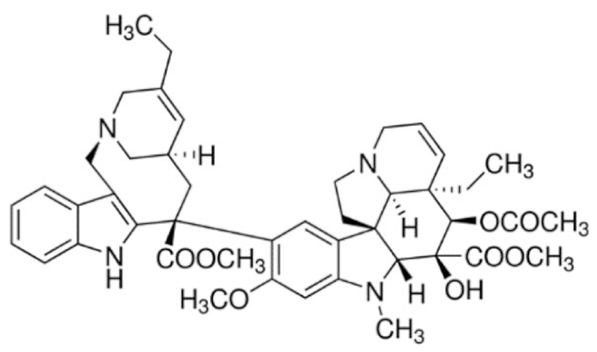 | C45H54N4O8 | |||
Vincamine 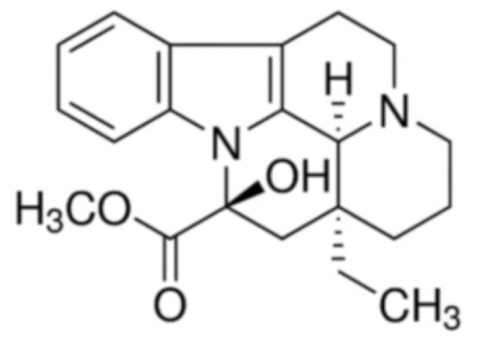 | C21H26N2O3 | Vinca minor | Primary degenerative and vascular dementia | |
Physostigmine 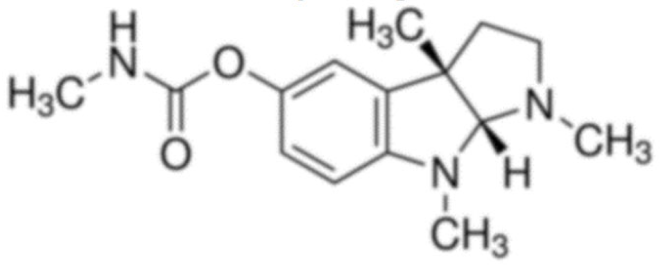 | C15H21N3O2 | Physostigma venenosum | Glaucoma | |
Ajmaline 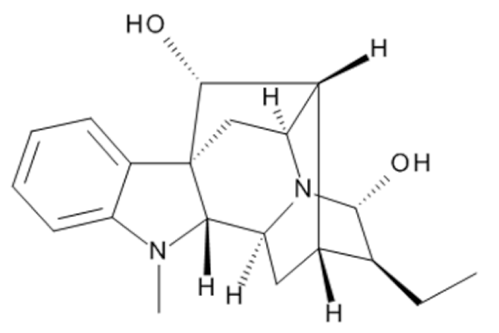 | C20H26N2O2 | Rauvolfia serpentina | Anti-arrhythmic | |
Ajmalicine 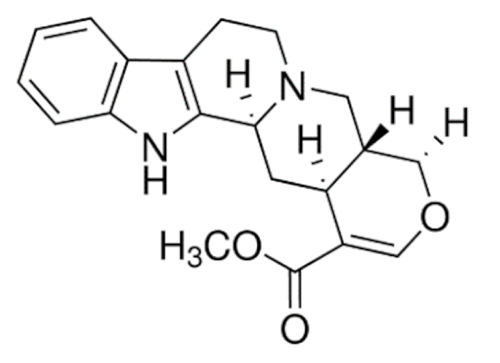 | C21H24N2O3 | Anti-hypertensive | ||
Reserpine 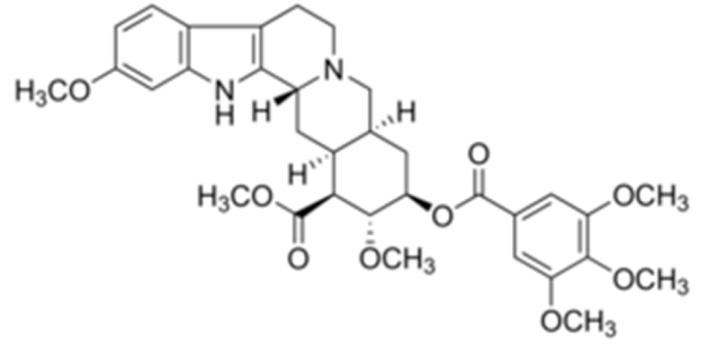 | C33H40N2O9 | Rauvolfia serpentina | Anti-hypertensive | |
Yohimbine 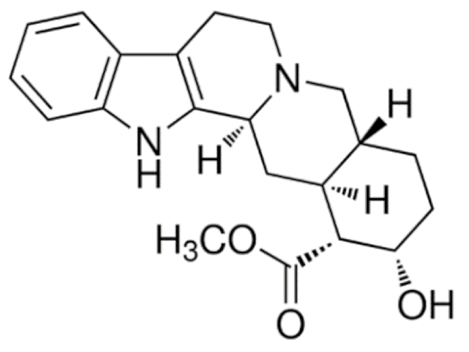 | C21H26N2O3 | Erectile dysfunction | ||
Strychnine 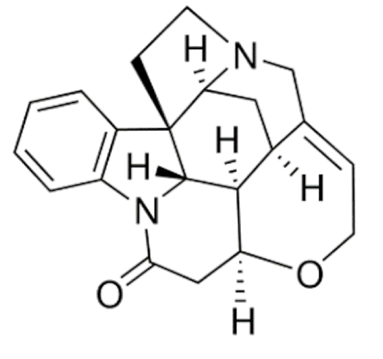 | C21H22N2O2 | Strychnos nux-vomica | Convulsant | |
Mitragynine 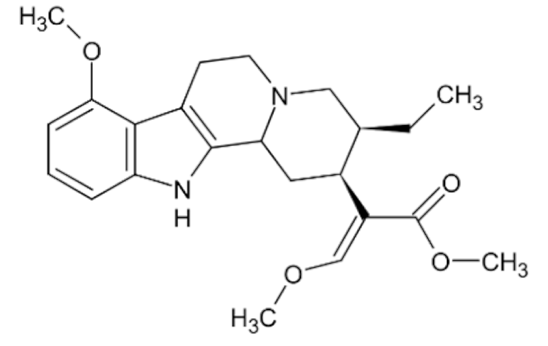 | C23H30N2O4 | Mitragyna speciosa | Stimulant, analgesic | |
Psilocin 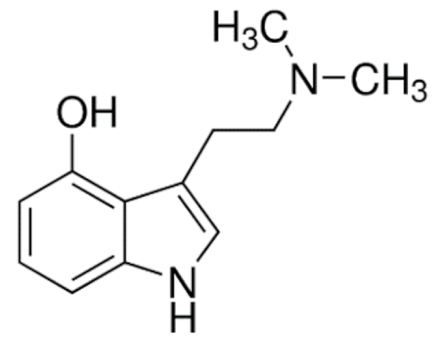 | C12H16N2O | Psilocybe cubensis | Hallucinogen | |
Psilocybin 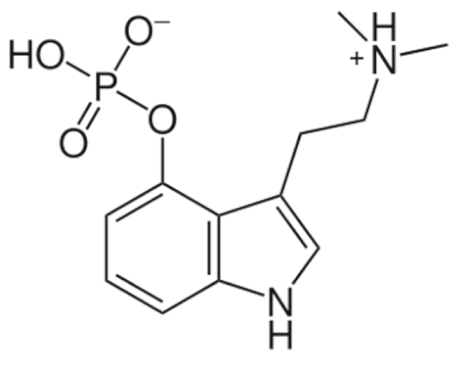 | C12H17N2O4P | |||
Ephedrine 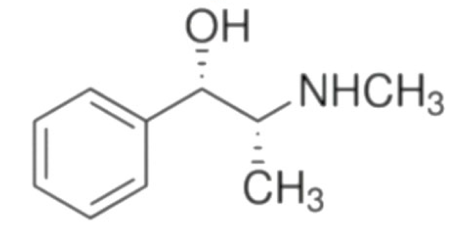 | C6H5CH(OH)CH (CH3)NHCH3 | Ephedra sinica | Bronchial asthma | |
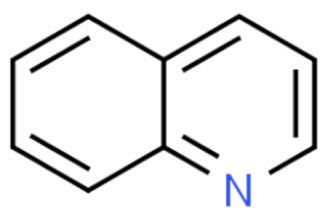 Quinoline | Irinotecan 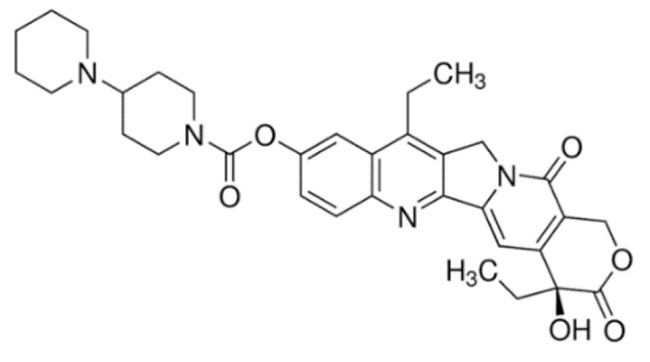 | C33H38N4O6 | Catharanthus roseus | Anticancer |
Topotecan 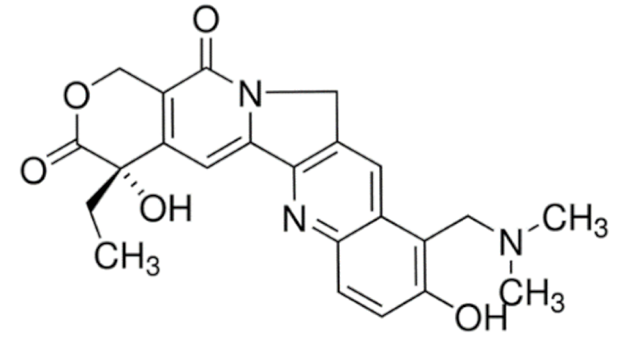 | C23H23N3O5 | |||
Colchicine 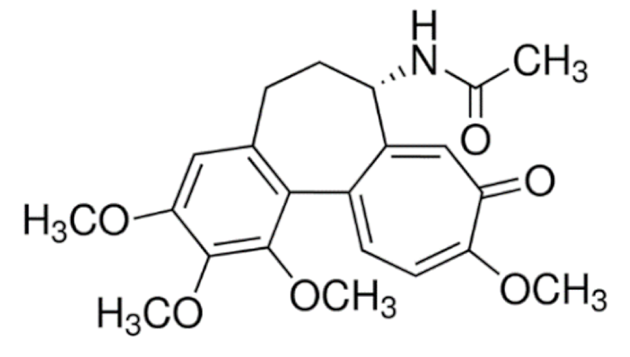 | C22H25NO6 | Colchicum autumnale | Gout | |
Quinidine 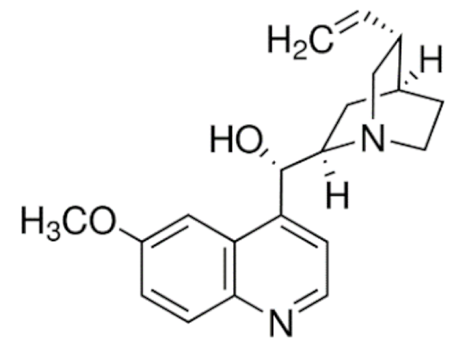 | C20H24N2O2 | Cinchona officinalis | Anti-arrhythmic | |
Quinine 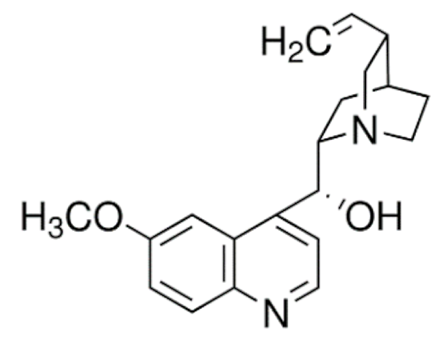 | C20H24N2O2 | Anti-malarial | ||
Cinchonine 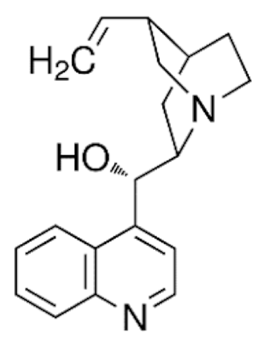 | C19H22N2O | |||
Cinchonidine 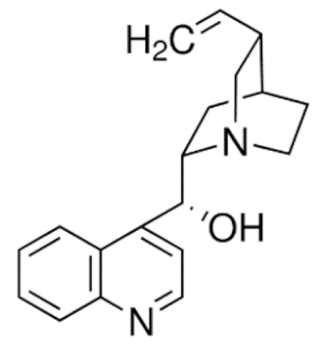 | C19H22N2O | |||
 Isoquinolines | Morphine  | C34H40N2O10S | Papaver somniferum | Analgesic |
Codeine 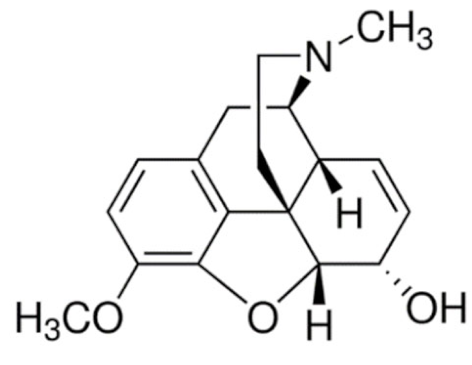 | C18H21NO3 | |||
Heroine 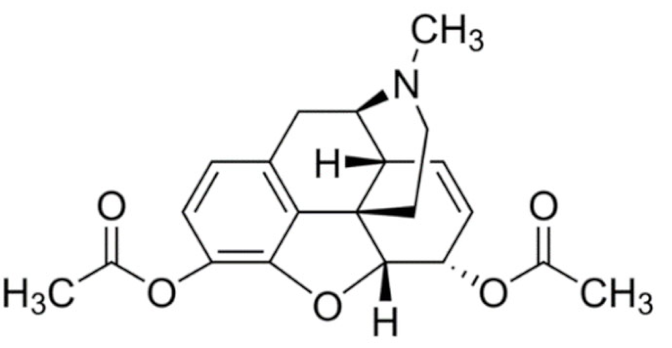 | C21H23NO5 | |||
Apomorphine  | C17H17NO2 | Parkinson’s disease | ||
Noscapine  | C22H23NO7 | Anti-tussive | ||
Trabectedin 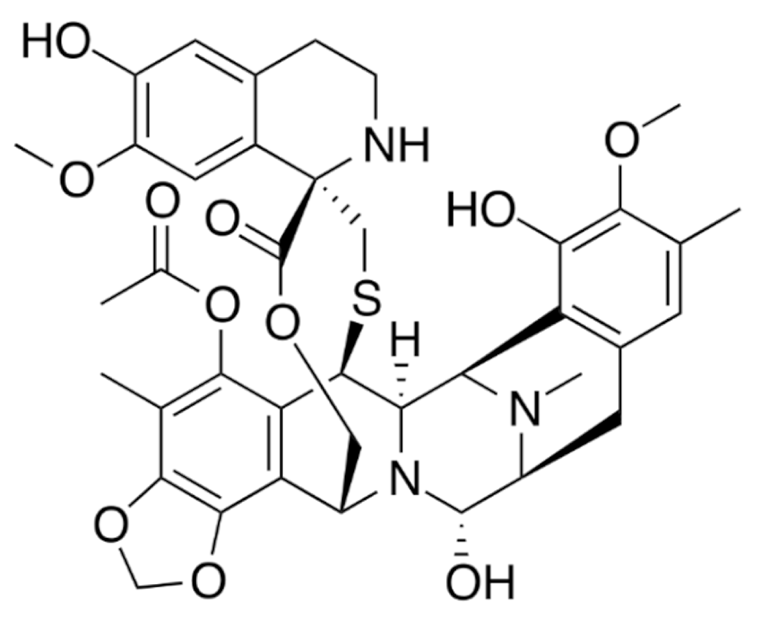 | C39H43N3O11S | Ecteinascidia turbinata | Anticancer | |
Berberine 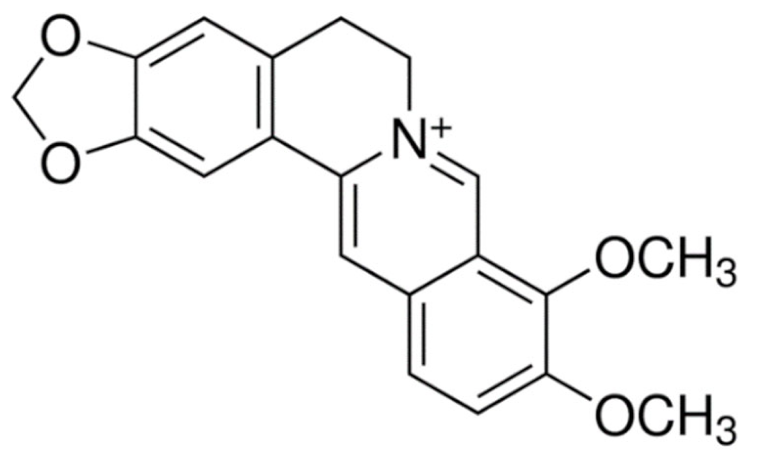 | C20H18ClNO4 | Coptis chinensis | Antimicrobial | |
Tubocurarine  | C37H41ClN2O6 | Chondrodendron tomentosum | Skeletal muscle relaxant | |
Atracurium  | C53H72N2O12 | Leontice leontopetalum | ||
Tetrandrine 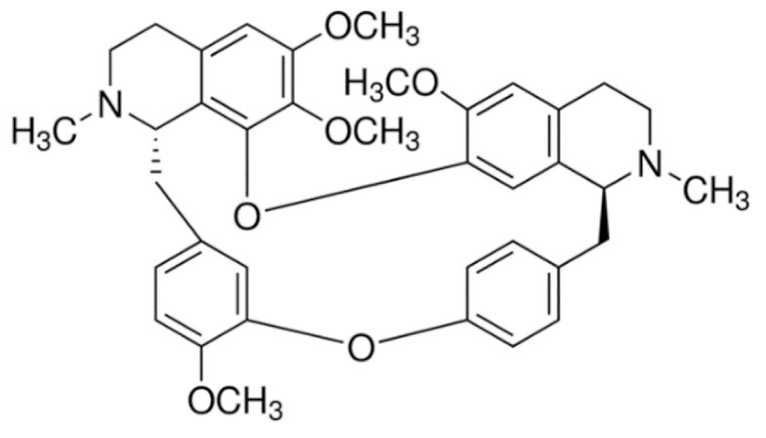 | C38H42N2O6 | Stephania tetrandra | Anti-arrhythmic | |
Galantamine 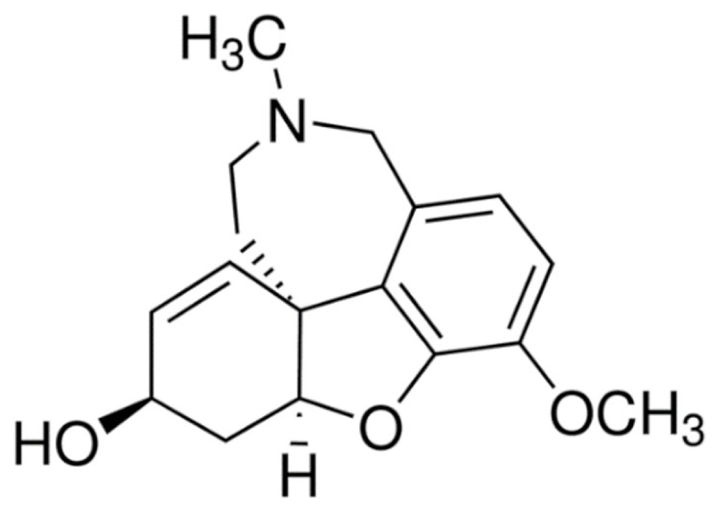 | C17H21NO3 | Galanthus nivalis | Alzheimer’s disease | |
Sanguinarine 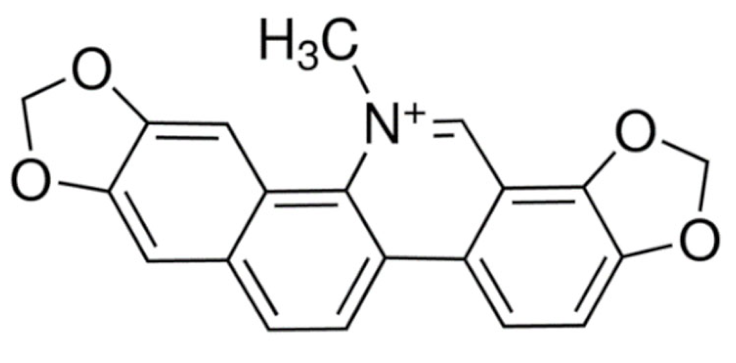 | C20H14NO4 | Sanguinaria canadenis | Antibacterial, antiplaque | |
Papaverine 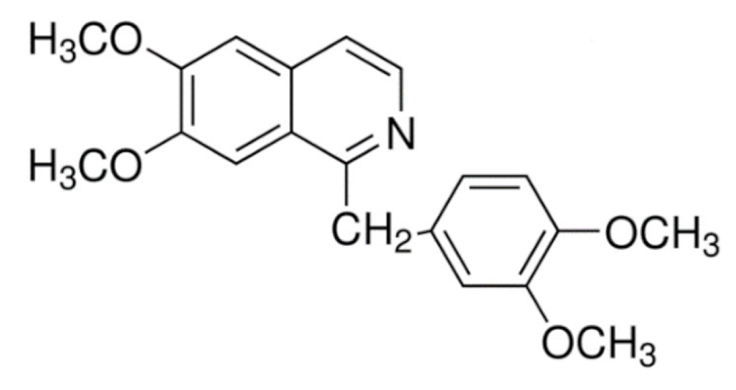 | C20H21NO4 | Papaver somniferum | Vasodilator | |
 Pyrrolidines | Hygrine  | C8H15NO | Erythroxylon coca | Laxative, diuretic |
Cuscohygrine  | C13H24N2O | |||
Stachydrine  | C7H14NO2 | Stachys tuberifera | Neuroprotectant | |
 Pyridines | Arecoline  | C8H13NO2 | Areca catechu | Muscarinic agonist |
Ricinine  | C8H8N2O2 | Ricinus communis | Insecticide | |
Trigonelline  | C7H7NO2 | Trigonella foenum-graecum | Antidiabetic | |
Nicotine  | C10H14N2 | Nicotiana tabacum | Smoking cessation | |
 Piperidine | Piperine  | C17H19NO3 | Piper nigrum | Anticancer |
Piperlongumine  | C17H19NO5 | Piper longum | ||
Pipernonaline | C21H27NO3 | Antifungal | ||
 Tropanes | Atropine  | C17H23NO3 | Atropa belladonna | Anticholinergic |
Cocaine  | C17H21NO4 | Erythroxylum coca | Local anaesthetic | |
Hyoscyamine 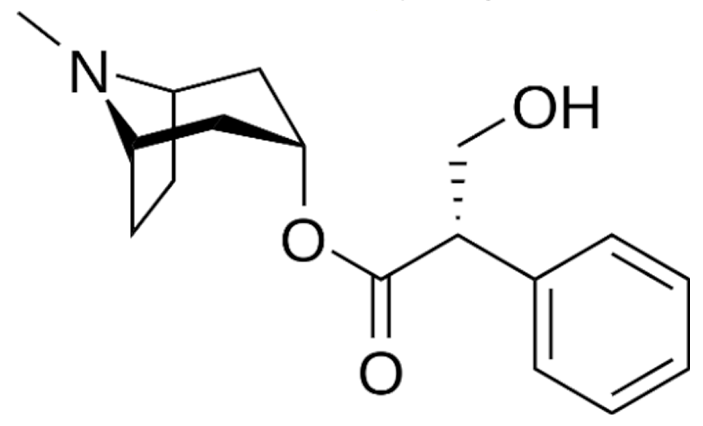 | C17H23NO3 | Atropa belladonna, Hyoscyamus niger | Anticholinergic | |
Hyoscine 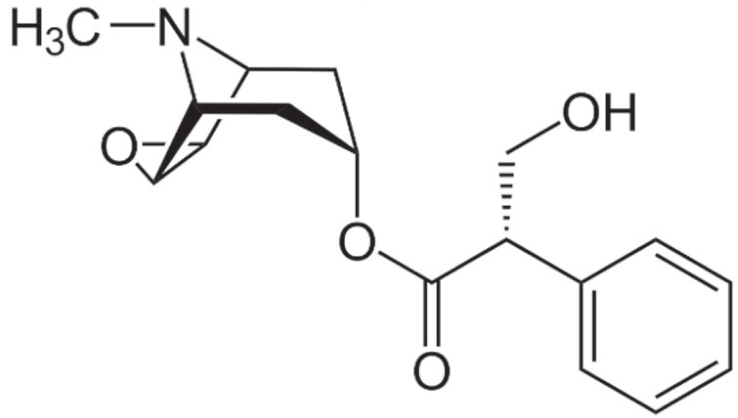 | C17H21NO4 | Atropa belladonna | Motion sickness | |
 Indolizidine | Swainsonine  | C8H15NO3 | Swainsona canescens | Anticancer |
Castanospermine 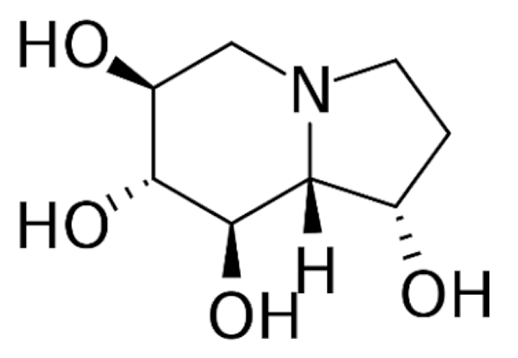 | C8H15NO4 | Castanospermum australe | Antiviral | |
Securinine 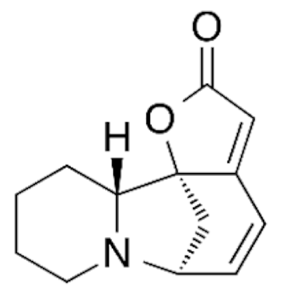 | C13H15NO2 | Securinega suffruticosa | Neuroprotection | |
Tylophorine 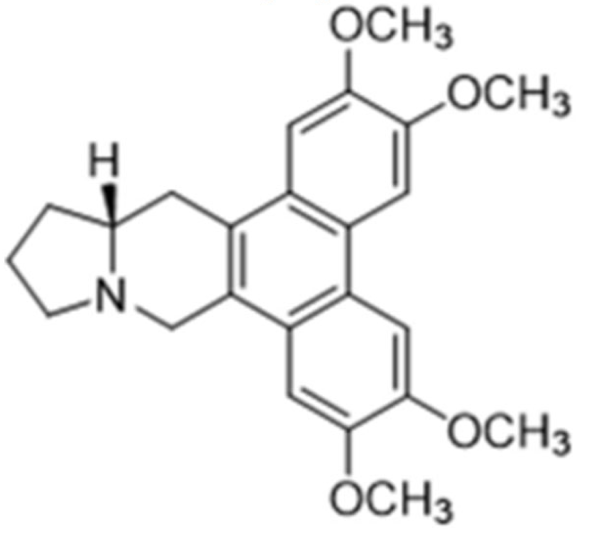 | C24H27NO4 | Tylophora indica | Anticancer | |
Lycorine 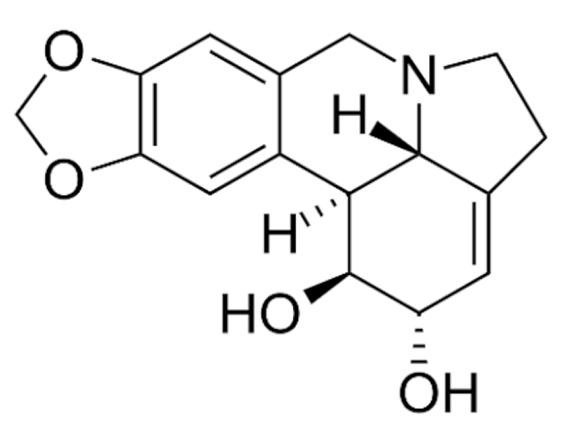 | C16H17NO4 | Clivia miniata | ||
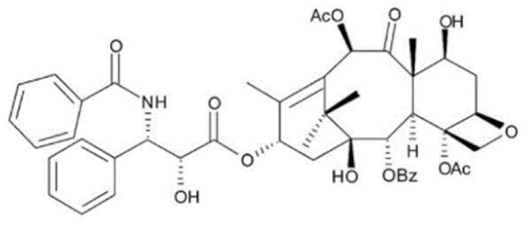 Terpenoids | Paclitaxel 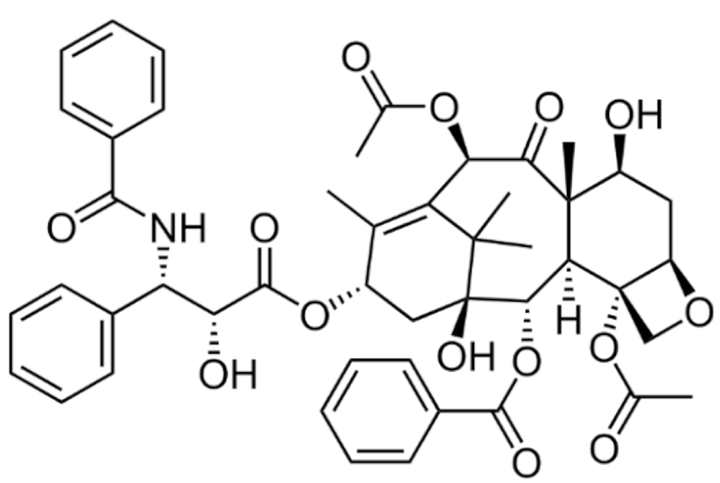 | C47H51NO14 | Taxus brevifolia | |
Docetaxel 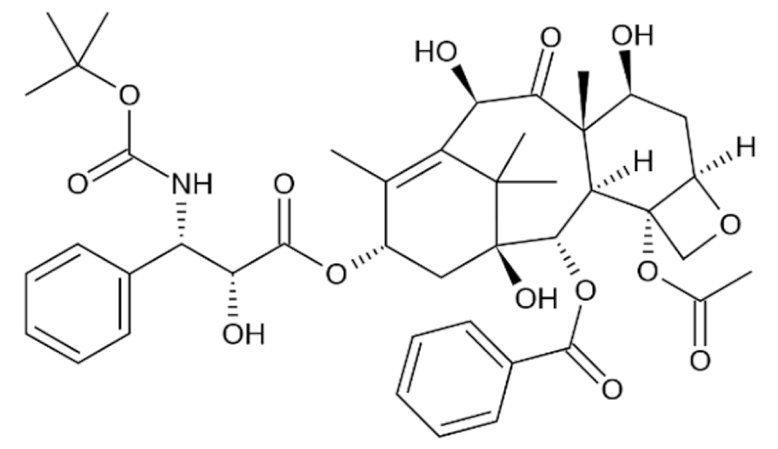 | C43H53NO14 | Taxus baccata | ||
 Purine | Caffeine 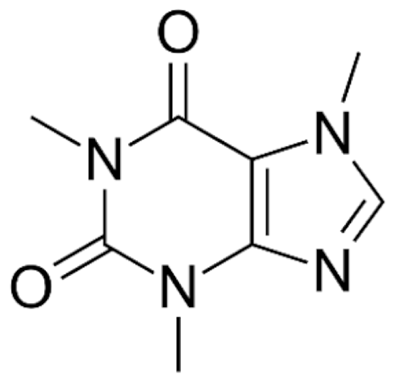 | C8H10N4O2 | Coffee arabica | CNS stimulant |
Theobromine 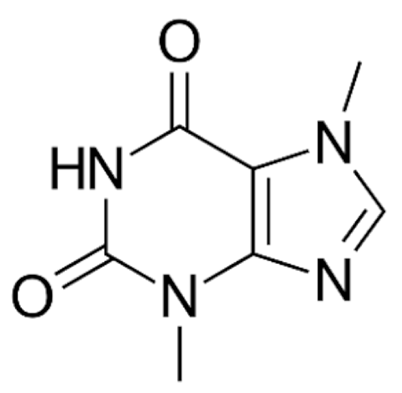 | C7H8N4O2 | Theobroma cacao | Cardioprotectant | |
Theophylline 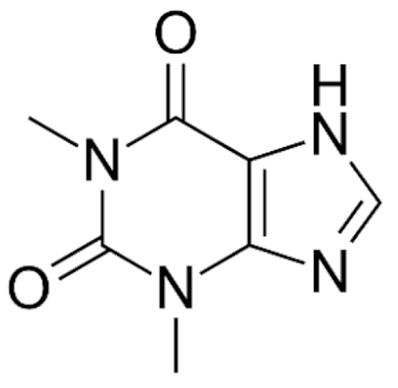 | C7H8N4O2 | COPD and asthma | ||
 Imidazole | Pilocarpine  | C11H16N2O2 | Pilocarpus microphyllus | Glaucoma |
Epiisopiloturine  | C₁₆H₁₈N₂O₃ | Anthelmintic | ||
 Steroidal | Pancuronium  | C35H60N2O4 | Malouetia bequaertiana | Skeletal muscle relaxant |
Conessine 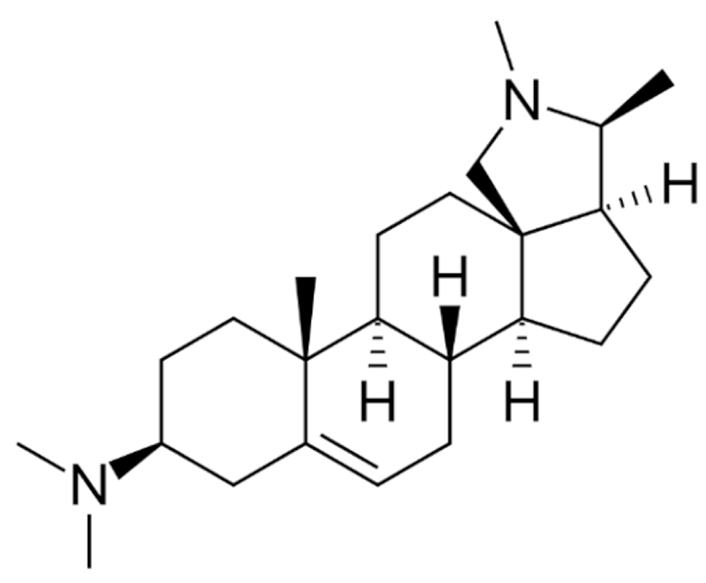 | C24H40N2 | Holarrhena antidysenterica | Antidysenteric | |
Solanidine 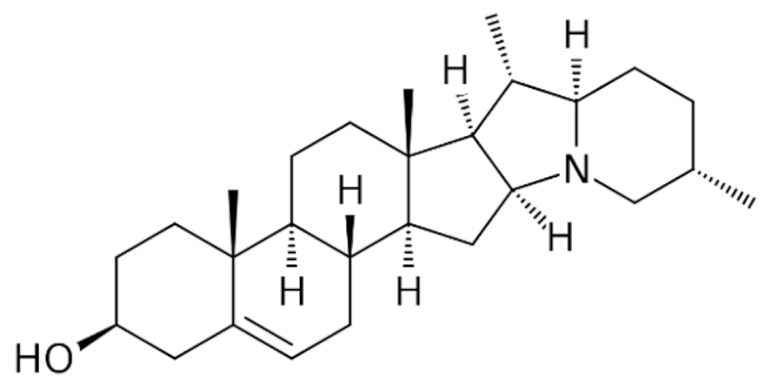 | C27H43NO | Solanum tuberosum | Anticancer | |
Tomatidine 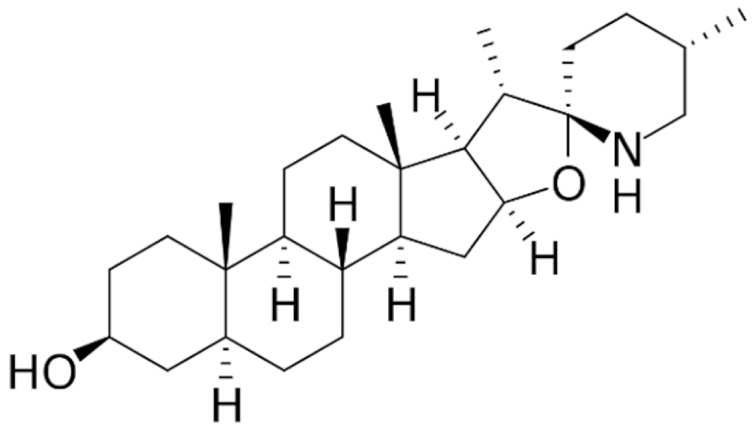 | C27H45NO2 | Lycopersicon esculentum |
| Class | Compound | Type of Lipid Carrier | Type of Cancer | In Vitro/In Vivo | Cell Type/Animal Model | Ref. |
|---|---|---|---|---|---|---|
| Terpenoids | Docetaxel 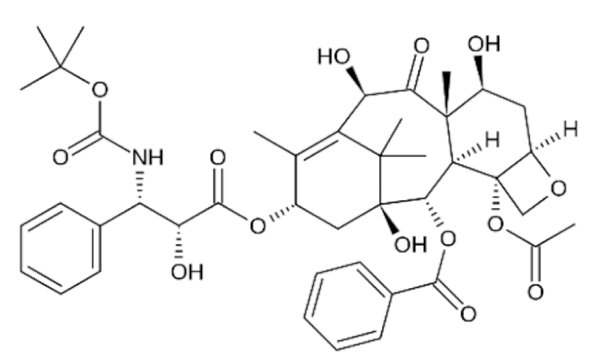 | Liposome | Breast | in vitro; in vivo | MCF-7/4T1 xenograft mice | [243] |
| in vivo | MDA-MB-435/LCC6 xenograft mice | [244] | ||||
| in vitro | MCF-7 | [245] | ||||
| Lung | in vivo | A549 xenograft rat | [246] | |||
| in vitro | A549 | [247] | ||||
| in vitro | A549 | [245] | ||||
| Liver | in vitro | HepG2 | [245] | |||
| Melanoma | in vitro; in vivo | B16F10/B16-F10 xenograft mice | [248] | |||
| Micelle | Breast | in vitro | MCF-7 | [249] | ||
| in vitro | MCF-7 | [250] | ||||
| Lung | in vitro | A549 | [250] | |||
| Niosome | Breast | in vitro; in vivo | MDA-MB-s31 and MCF-7 | [251] | ||
| NLC | Liver, Ovarian, Lung, Melanoma | in vitro; in vivo | HepG2, SKOV3, A549, B16 cells | [252] | ||
Paclitaxel 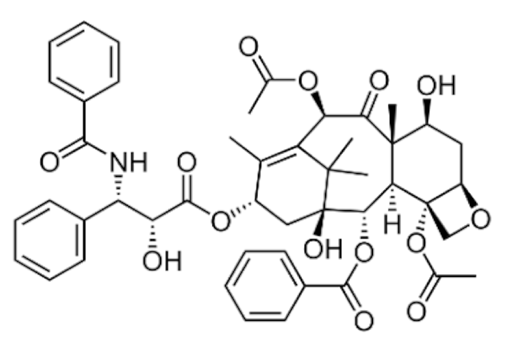 | Liposome | Breast | in vitro; in vivo | 4T1 xenograft mice | [253] | |
| in vivo | ICR male mice | [254] | ||||
| Lung | in vivo | A549 xenograft mice | [255] | |||
| in vitro; in vivo | A549 | [69] | ||||
| NLC | Breast, Ovarian | in vitro | MCF-7, SKOV3 | [256] | ||
| Ethosome | Squamous Cell Carcinoma | in vitro | DJM-1 | [257] | ||
| Micelle | Glioma | in vitro; in vivo | C6/C6 xenograft rat | [258] | ||
| Indole | Vincristine 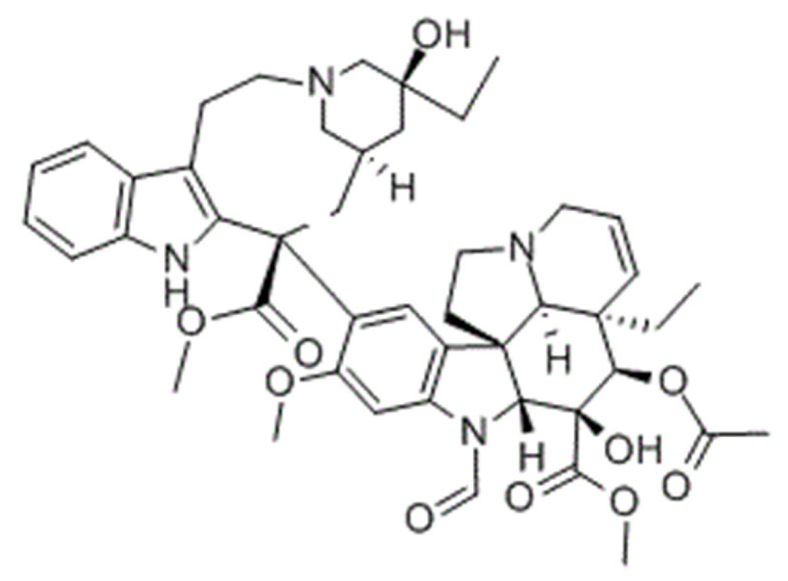 | Liposome | Acute Lymphoblastic Leukemia | in vivo | Namwala xenograft mice | [190] |
| Brain Glioma | in vitro, in vivo | Glioma bearing mice | [259] | |||
| Nasopharyngeal cancer | in vitro, in vivo | KB/KBv200 xenograft mice | [260] | |||
| SLN | Breast | in vitro; in vivo | MDA-MB-231 | [261] | ||
| NLC | Breast | in vitro | MCF-7 | [262] | ||
Vinblastine 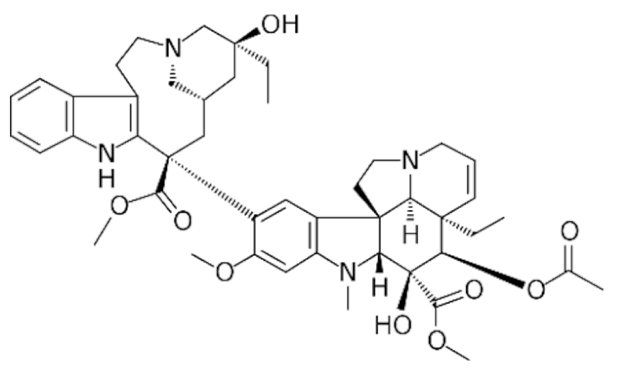 | Liposome | Non-Small Cell Lung | in vitro; in vivo | LLT/LLT xenograft mice | [263] | |
| Niosome | Lung | in vitro; in vivo | TC-1/TC-1 xenograft mice | [264] | ||
Vinorelbine 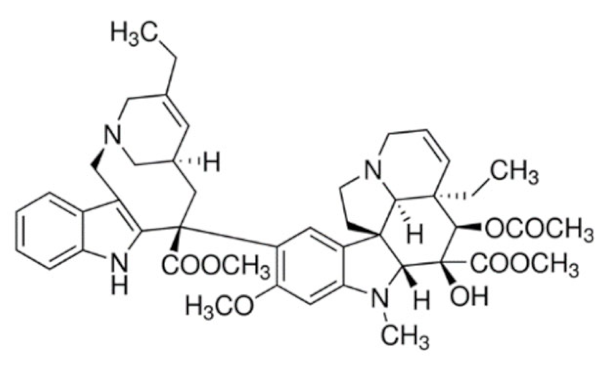 | Micelle | Breast | in vitro | MCF-7 | [265] | |
| Quinoline | Topotecan 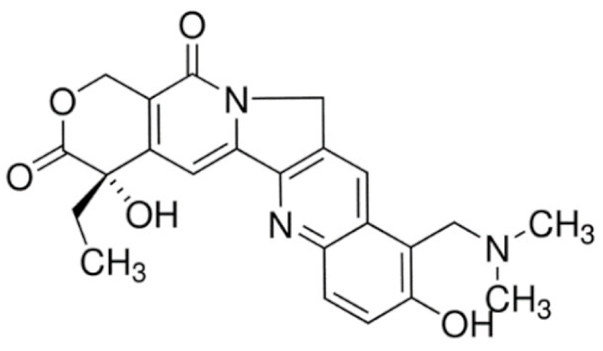 | Liposome | Lung and Adenocarcinoma | in vitro | LLC | [266] |
| Breast | in vitro; in vivo | MCF-7/MCF-7 xenograft mice | [267] | |||
| SLN/NLC | Leukemia | in vitro | K-562 | [268] | ||
Irinotecan 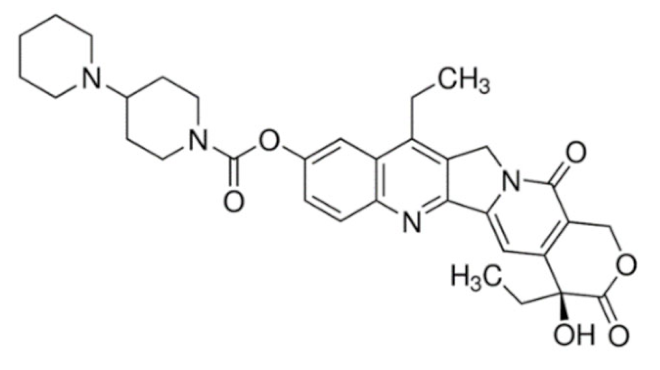 | Liposome | Colon | in vivo | Colo 320DM and Colon 26 xenograft mice | [269] | |
| SLN | Rectal, Colon | in vivo | SCC7 xenograft mice | [270] | ||
| Isoquinoline | Berberine  | Liposome | Hepatic | in vitro; in vivo | HepG2/HepG2 xenograft mice | [271] |
| Breast | in vitro; in vivo | MCF-7/MCF-7 CSC xenograft mice | [272] | |||
| SLN | Breast, Hepatic, Lung | in vitro | MCF-7, HepG2, A549 | [273] |
| Type of Lipid Carrier | Alkaloids | Type of Cancer | Cell Line | IC50 | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Exposure Time (h) | Free Drug (μg/mL) | Drug-Carrier (μg/mL) | Reduction in IC50 (%) | |||||
| Liposome | Vincristine | Oral Epidermoid | KB | 72 | 3.47 | 0.0021 | 99.94 | [260] |
| MDR Oral Epidermoid | KBv200 | 72 | 1.31 | 0.33 | 74.88 | |||
| Topotecan | Breast | BT20 | 24 | 1.88 | 0.33 | 82.45 | [266] | |
| MDR Breast | MCF-7/ADR | 48 | 2.47 | 0.71 | 71.43 | [267] | ||
| Non-Small Cell Lung | LLC | 24 | 2.49 | 0.78 | 68.67 | [266] | ||
| Breast | MCF-7 | 48 | 2.07 | 2.07 | -0.22 | [267] | ||
| Docetaxel | Breast | MCF-7 | 72 | 14.40 | 1.90 | 86.81 | [243] | |
| Non-Small Cell Lung | A549 | 24 | 9.39 | 2.42 | 74.23 | [245] | ||
| Hepatocellular | HepG2 | 24 | 7.91 | 3.12 | 60.56 | [245] | ||
| Breast | MCF-7 | 24 | 8.40 | 3.77 | 55.12 | [245] | ||
| Melanoma | B16F10 | 48 | 12.46 | 6.60 | 47.03 | [248] | ||
| Breast | 4TI | 72 | 13.00 | 8.70 | 33.08 | [243] | ||
| Breast | TUBO | 72 | 4.70 | 4.10 | 12.77 | [243] | ||
| Berberine | Hepatocellular | HepG2 | 72 | 4.23 | 1.67 | 60.52 | [271] | |
| Paclitaxel | Non-Small Cell Lung | A549 | 72 | 35.42 | 28.25 | 20.24 | [69] | |
| NLC | Vincristine | Breast | MCF-7 | 24 | 0.011 | 0.0031 | 71.97 | [262] |
| Diffuse large B-cell lymphoma | LY1 | 48 | 9.82 | 3.31 | 66.29 | [277] | ||
| Docetaxel | Non-Small Cell Lung | A549 | 96 | 0.60 | 0.12 | 79.73 | [252] | |
| Ovarian | SKOV3 | 96 | 0.065 | 0.016 | 75.00 | |||
| Hepatocellular | HepG2 | 96 | 0.78 | 0.38 | 51.04 | |||
| Murine melanoma | B16 | 96 | 0.58 | 0.36 | 38.89 | |||
| Paclitaxel | MDR Breast | MCF-7/ADR | 48 | 8.61 | 0.065 | 99.25 | [256] | |
| MDR Ovarian | SKOV3-TR30 | 48 | 9.35 | 0.10 | 98.93 | |||
| Breast | MCF-7 | 48 | 0.29 | 0.075 | 74.14 | |||
| Ovarian | SKOV3 | 48 | 0.16 | 0.053 | 66.88 | |||
| SLN | Berberine | Non-Small Cell Lung | A549 | 24 | 13.45 | 5.11 | 62.00 | [273] |
| Hepatocellular | HepG2 | 24 | 3.46 | 1.61 | 53.40 | |||
| Breast | MCF-7 | 24 | 13.45 | 6.90 | 48.75 | |||
| Vincristine | Breast | MDA-MB-231 | 72 | 0.09 | 0.078 | 11.05 | [261] | |
| Micelle | Docetaxel | Breast | MCF-7 | 72 | 9.00 | 0.090 | 99.00 | [250] |
| Non-Small Cell Lung | A549 | 72 | 12.80 | 0.56 | 95.63 | |||
| Breast | MCF-7 | 48 | 1.28 | 0.25 | 80.39 | [249] | ||
| Vinorelbine | Breast | MCF-7 | 72 | 8.13 | 1.20 | 85.24 | [265] | |
| Paclitaxel | Glioma | C6 | 48 | 0.36 | 0.24 | 32.92 | [258] | |
| Niosome | Docetaxel | Breast | MDA-MB-231 | 24 | 12.34 | 5.47 | 55.67 | [251] |
| MCF-7 | 24 | 0.72 | 0.51 | 28.95 | ||||
| Vinblastine | Lung | TC-1 | 72 | 7.40 | 13.30 | -79.73 | [264] | |
| Type of Lipid Carrier | Alkaloids | Type of Cancer | Animal Model | Cancer Cell | Route of Administration | Reduction in Tumor Volume (%) | Weight Changes (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Vs. Negative Control | Vs. Free Drug | Free Drug | Lipid-Carrier | |||||||
| Liposome | Irinotecan | Colorectal Adenocarcinoma | Female ddY mice | Colo 320DM | IV | 97 | 92.5 | −18.1 | −5.2 | [269] |
| Murine Colon | Female ddY mice | Colon 26 | IV | 36 | 14.7 | +7.8 | +3.9 | |||
| Paclitaxel | Breast | BALB/c mice | 4T1 | IV | 96.6 | 89.8 | +8.6 | +19 | [253] | |
| Non-Small Cell Lung | Male BALB/c mice | A549 | IV | 55.6 | 23.8 | +7.9 | +6.4 | [255] | ||
| Breast | ICR male mice | - | IV | 38.2 | 10.5 | 0 | +30 | [254] | ||
| Docetaxel | Breast | Female BALB/c mice | TUBO | IV | 85.7 | 83.3 | +14 | +22 | [243] | |
| 4T1 | IV | 78.9 | 71.4 | +18 | +36 | |||||
| Non-Small Cell Lung | Sprague- Dawley rats | A549 | IV | 96.3 | 50 | −13 | +5.6 | [246] | ||
| Breast | Female RAG2-M mice | MDA-MB-435/LCC6 | IV | 34.8 | +25 | NR | NR | [183] | ||
| Melanoma | C57BL/6 mice | B16-F10 | IV | 12 | 1.8 | −14.3 | +14.3 | [248] | ||
| Topotecan | Breast | NCR nu/nu athymic female mice | BT474 | IV | 83.3 | 63.6 | NR | NR | [284] | |
| MDR Breast | Female BALB/c nude mice | MCF-7/ADR | IV | 75.3 | 58.2 | NR | NR | [267] | ||
| Vinblastine | Non-Small Cell Lung | C57BL/6 mice | LLT | IV | 54.3 | 30.4 | NR | NR | [263] | |
| Berberine | Breast | Female BALB/c nude mice | MCF-7 CSC | IV | 47.4 | 23.1 | NR | NR | [272] | |
| Vincristine | MDR Oral Epidermoid | Male nude mice | KBv200 | IV | 44.3 | 2.9 | NR | NR | [260] | |
| NLC | Docetaxel | Murine melanoma | Female Kunming Mice | B16 | IV | 62 | 32 | +20 | +33.3 | [252] |
| Vincristine | Diffuse Large B-Cell Lymphoma | BALB/c mice | LY-1 | IV | 44.2 | 21.6 | −14.8 | +19.1 | [277] | |
| SLN | Irinotecan | Squamous Cell | Female athymic nude mice | SCC7 | Rectal | 70 | 58.3 | −6.9 | +0.8 | [270] |
| Niosome | Docetaxel | Breast | Female nude mice | MCF-7 | PO | 53.1 | 21.1 | +26.3 | +27 | [251] |
| Vinblastine | Murine Lung | Female C57BL/6 mice | TC-1 | IV | 33.3 | 18.5 | NR | NR | [264] | |
| Alkaloids | Product Name | Type of Lipid-Based Nanoparticles | Indication | Status |
|---|---|---|---|---|
| Paclitaxel | Lipusu | Liposome | Squamous Non-small-cell Lung Cancer | Approved by State FDA of China (2006); Phase IV ongoing (NCT02996214) |
| LEP-ETU | Liposome | Metastatic breast cancer | Phase II completed (NCT01190982) | |
| EndoTAG-1 | Cationic Liposome | Locally advanced and/or metastatic adenocarcinoma of the pancreas | Phase III ongoing (NCT03126435) | |
| Docetaxel | CPC634(CriPec docetaxel) | Micelle | Ovarian cancer | Phase II ongoing (NCT03742713) |
| Irinotecan | Onivyde | Liposome | Metastatic pancreatic cancer | Approved by FDA (2015) |
| Small cell lung cancer | Phase III ongoing (NCT03088813) | |||
| LY01610 | Liposome | Small cell lung cancer | Phase II ongoing (NCT04381910) | |
| Advanced solid tumors | Phase I ongoing (NCT04088604) | |||
| Topotecan | FF-10850 | Liposome | Advanced solid tumors | Phase I ongoing (NCT04047251) |
| TLI | Liposome | Advanced solid tumors | Phase I ongoing (NCT00765973) | |
| Lurtotecan | OSI-211 | Liposome | Small cell lung cancer | Phase II completed (NCT00046787) |
| NX-211 | Liposome | Metastatic or locally recurrent head and neck cancer | Phase II completed (NCT00022594) | |
| Vincristine | Marqibo (Onco TCS) | Liposome | Acute lymphoblastic leukemia | Approved by FDA (2012) |
| Vinorelbine | TLC178 | Liposome | Advanced malignancies | Phase I/II ongoing (NCT02925000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loh, J.S.; Tan, L.K.S.; Lee, W.L.; Ming, L.C.; How, C.W.; Foo, J.B.; Kifli, N.; Goh, B.H.; Ong, Y.S. Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids? Cancers 2021, 13, 5346. https://doi.org/10.3390/cancers13215346
Loh JS, Tan LKS, Lee WL, Ming LC, How CW, Foo JB, Kifli N, Goh BH, Ong YS. Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids? Cancers. 2021; 13(21):5346. https://doi.org/10.3390/cancers13215346
Chicago/Turabian StyleLoh, Jian Sheng, Li Kar Stella Tan, Wai Leng Lee, Long Chiau Ming, Chee Wun How, Jhi Biau Foo, Nurolaini Kifli, Bey Hing Goh, and Yong Sze Ong. 2021. "Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids?" Cancers 13, no. 21: 5346. https://doi.org/10.3390/cancers13215346
APA StyleLoh, J. S., Tan, L. K. S., Lee, W. L., Ming, L. C., How, C. W., Foo, J. B., Kifli, N., Goh, B. H., & Ong, Y. S. (2021). Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids? Cancers, 13(21), 5346. https://doi.org/10.3390/cancers13215346











