Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Dataset Analysis and NTCP Modeling
3. Results
3.1. RION Incidence
3.2. Univariable Correlation to RION
3.3. Elimination of Cross-Correlation and Bootstrapping
3.4. Multi-Parametric Model Selection and Cross-Validation Analysis
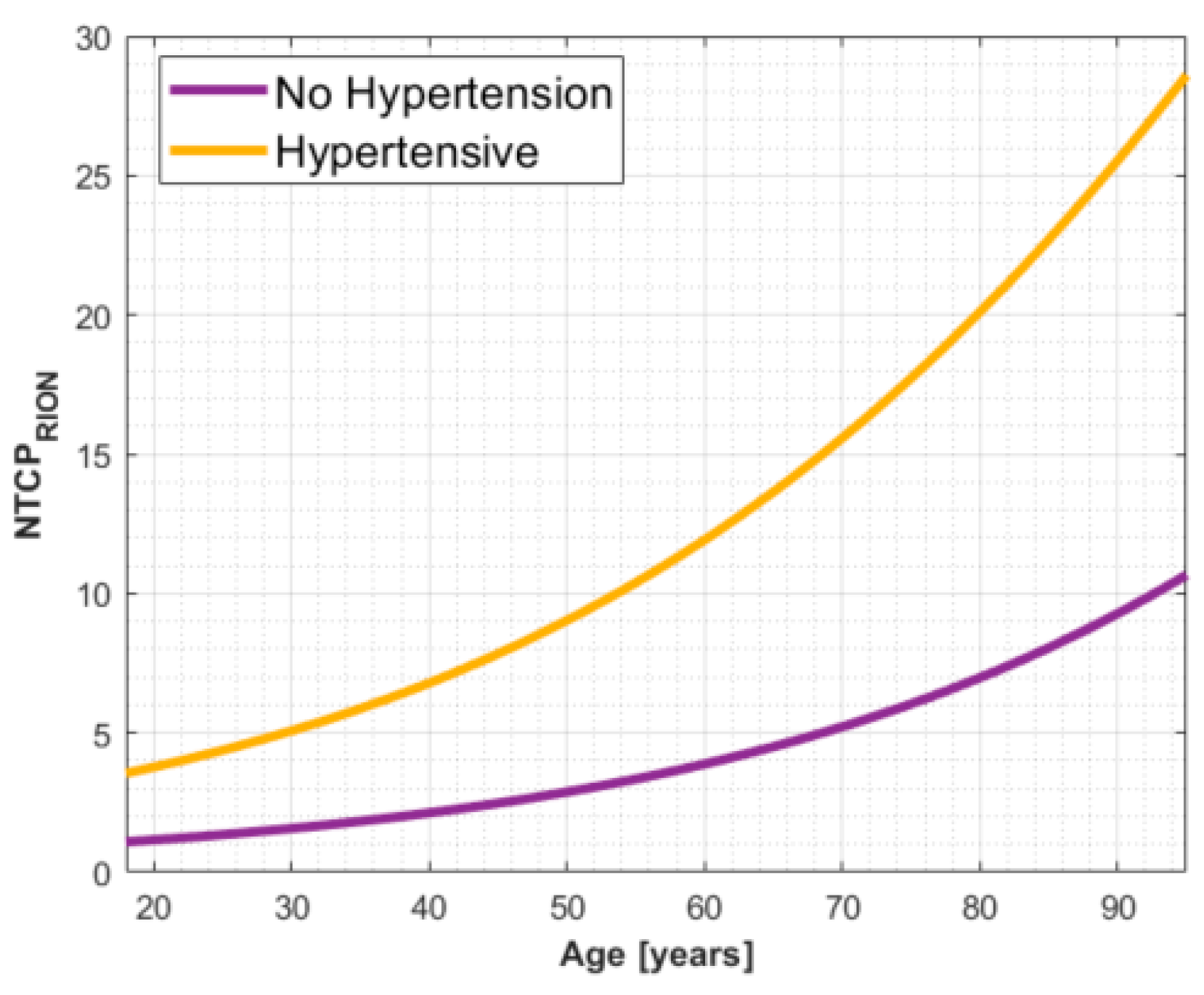
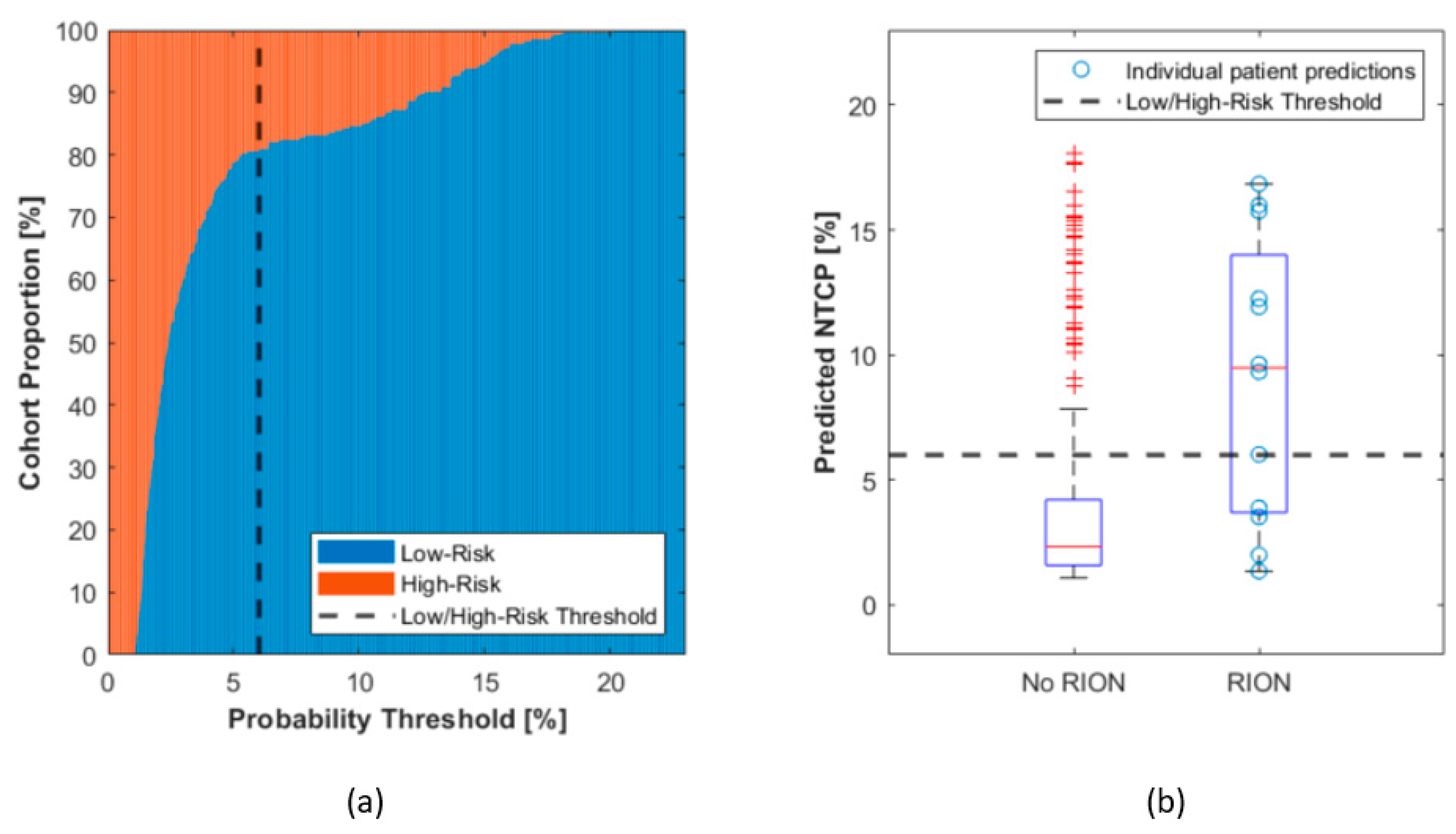
3.5. A Threshold for Patient Segmentation into Risk Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Mean over All k-Fold (LOO,5,10) with 10 Repetitions | |||||
|---|---|---|---|---|---|
| Mean AUC | Mean CE | Mean HL p-Value | AIC | BIC | |
| Age | 0.68 | 0.17 | 0.45 | 100.4 | 107.8 |
| HBP | 0.50 | 0.17 | 0.00 | 97.3 | 104.5 |
| TI | 0.09 | 0.18 | 0.00 | 106.5 | 113.8 |
| Surg | 0.08 | 0.18 | 0.00 | 106.0 | 113.4 |
| GTV | 0.08 | 0.19 | 0.00 | 112.1 | 119.4 |
| Age HBP | 0.68 | 0.17 | 0.14 | 100.5 | 111.3 |
| Age TI | 0.65 | 0.17 | 0.33 | 105.8 | 116.8 |
| Age Surg | 0.65 | 0.17 | 0.55 | 104.3 | 115.3 |
| Age GTV | 0.62 | 0.19 | 0.10 | 112.3 | 123.2 |
| HBP TI | 0.58 | 0.18 | 0.00 | 101.3 | 112.1 |
| HBP Surg | 0.53 | 0.17 | 0.00 | 101.1 | 111.9 |
| HBP GTV | 0.51 | 0.21 | 0.00 | 113.9 | 124.6 |
| TI Surg | 0.07 | 0.18 | 0.00 | 107.8 | 118.7 |
| TI GTV | 0.08 | 0.19 | 0.00 | 110.7 | 121.5 |
| Surg GTV | 0.07 | 0.19 | 0.00 | 111.9 | 122.7 |
| Age HBP TI | 0.65 | 0.18 | 0.15 | 105.7 | 120.1 |
| Age HBP Surg | 0.63 | 0.18 | 0.05 | 104.0 | 118.5 |
| Age HBP GTV | 0.56 | 0.22 | 0.03 | 120.8 | 135.0 |
| Age TI Surg | 0.61 | 0.18 | 0.47 | 108.2 | 122.8 |
| Age TI GTV | 0.61 | 0.19 | 0.13 | 114.9 | 129.4 |
| Age Surg GTV | 0.62 | 0.19 | 0.13 | 112.4 | 126.9 |
| HBP TI Surg | 0.52 | 0.18 | 0.00 | 103.1 | 117.5 |
| HBP TI GTV | 0.50 | 0.21 | 0.00 | 115.9 | 130.2 |
| HBP Surg GTV | 0.51 | 0.21 | 0.00 | 115.9 | 130.2 |
| TI Surg GTV | 0.07 | 0.19 | 0.00 | 112.4 | 126.9 |
| Age HBP TI Surg | 0.59 | 0.18 | 0.05 | 108.4 | 126.3 |
| Age HBP TI GTV | 0.55 | 0.21 | 0.02 | 120.2 | 138.0 |
| Age HBP Surg GTV | 0.58 | 0.22 | 0.02 | 122.9 | 140.7 |
| Age TI Surg GTV | 0.59 | 0.19 | 0.09 | 114.0 | 132.1 |
| HBP TI Surg GTV | 0.50 | 0.21 | 0.00 | 116.8 | 134.5 |
| Age HBP TI Surg GTV | 0.56 | 0.20 | 0.03 | 118.1 | 139.4 |
References
- Fossati, P.; Vavassori, A.; Deantonio, L.; Ferrara, E.; Krengli, M.; Orecchia, R. Review of photon and proton radiotherapy for skull base tumours. Rep. Pract. Oncol. Radiother. 2016, 21, 336–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh-Meyer, H.V. Radiation-induced optic neuropathy. J. Clin. Neurosci. 2008, 15, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kountouri, M.; Pica, A.; Walser, M.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Bachtiary, B.; Combescure, C.; Lomax, A.J.; Schneider, R.; et al. Radiation induced optic neuropathy after pencil beam scanning proton therapy for skull-base and head and neck tumours. Br. J. Radiol. 2019, 93, 20190028. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Murakami, M.; Miyawaki, D.; Niwa, Y.; Akagi, T.; Sasaki, R.; Terashima, K.; Suga, D.; Kamae, I.; Hishikawa, Y. Analysis of Vision Loss Caused by Radiation-Induced Optic Neuropathy After Particle Therapy for Head-and-Neck and Skull-Base Tumors Adjacent to Optic Nerves. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Liebsch, N.J.; Niemierko, A.; Giantsoudi, D.; Lessell, S.; Fullerton, B.C.; Adams, J.; Shih, H.A. Radiation tolerance of the optic pathway in patients treated with proton and photon radiotherapy. Radiother. Oncol. 2019, 131, 112–119. [Google Scholar] [CrossRef]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation Dose–Volume Effects of Optic Nerves and Chiasm. Int. J. Radiat. Oncol. 2010, 76, S28–S35. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Soltys, S.G.; Yorke, E.; Moiseenko, V.; Tomé, W.A.; Sahgal, A.; Xue, J.; Ma, L.; Solberg, T.D.; et al. Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways. Int. J. Radiat. Oncol. 2021, 110, 87–99. [Google Scholar] [CrossRef]
- Moiseenko, V.; Song, W.Y.; Mell, L.K.; Bhandare, N. A comparison of dose-response characteristics of four NTCP models using outcomes of radiation-induced optic neuropathy and retinopathy. Radiat. Oncol. 2011, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Mathis, T.; Bensadoun, R.-J.J.; Thariat, J. Radiation Induced Optic Neuropathy: Does Treatment Modality Influence the Risk? Bull. Cancer 2019, 106, 1160–1176. [Google Scholar] [CrossRef]
- Köthe, A.; van Luijk, P.; Safai, S.; Kountouri, M.; Lomax, A.J.; Weber, D.C.; Fattori, G. Combining Clinical and Dosimetric Features in a PBS Proton Therapy Cohort to Develop a NTCP Model for Radiation-Induced Optic Neuropathy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 587–595. [Google Scholar] [CrossRef]
- Lomax, A.J.; Böhringer, T.; Bolsi, A.; Coray, D.; Emert, F.; Goitein, G.; Jermann, M.; Lin, S.; Pedroni, E.; Rutz, H.; et al. Treatment planning and verification of proton therapy using spot scanning: Initial experiences. Med. Phys. 2004, 31, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72. [Google Scholar] [CrossRef]
- El Naqa, I.; Bradley, J.; Blanco, A.I.; Lindsay, P.E.; Vicic, M.; Hope, A.; Deasy, J.O. Multivariable modeling of radiotherapy outcomes, including dose–volume and clinical factors. Int. J. Radiat. Oncol. 2006, 64, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Weber, D.C.; Chan, A.W.; Lessell, S.; McIntyre, J.F.; Goldberg, S.I.; Bussiere, M.R.; Fitzek, M.M.; Thornton, A.F.; DeLaney, T.F. Visual outcome of accelerated fractionated radiation for advanced sinonasal malignancies employing photons/protons. Radiother. Oncol. 2006, 81, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Urie, M.M.; Fullerton, B.; Tatsuzaki, H.; Birnbaum, S.; Suit, H.D.; Convery, K.; Skates, S.; Goitein, M. A dose response analysis of injury to cranial nerves and/or nuclei following proton beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 27–39. [Google Scholar] [CrossRef]
- Scoccianti, S.; Detti, B.; Gadda, D.; Greto, D.; Furfaro, I.; Meacci, F.; Simontacchi, G.; Di Brina, L.; Bonomo, P.; Giacomelli, I.; et al. Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practice. Radiother. Oncol. 2015, 114, 230–238. [Google Scholar] [CrossRef]
- Doroslovački, P.; Tamhankar, M.A.; Liu, G.T.; Shindler, K.S.; Ying, G.S.; Alonso-Basanta, M. Factors Associated with Occurrence of Radiation-induced Optic Neuropathy at “Safe” Radiation Dosage. Semin. Ophthalmol. 2018, 33, 581–588. [Google Scholar] [CrossRef]
- Seibel, I.; Cordini, D.; Hager, A.; Tillner, J.; Riechardt, A.I.; Heufelder, J.; Davids, A.M.; Rehak, M.; Joussen, A.M. Predictive risk factors for radiation retinopathy and optic neuropathy after proton beam therapy for uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1787–1792. [Google Scholar] [CrossRef]
- Mackley, H.B.; Reddy, C.A.; Lee, S.Y.; Harnisch, G.A.; Mayberg, M.R.; Hamrahian, A.H.; Suh, J.H. Intensity-modulated radiotherapy for pituitary adenomas: The preliminary report of the Cleveland Clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 232–239. [Google Scholar] [CrossRef] [PubMed]


| Property | Total | PSI | CPO | p-Value CPO vs. PSI |
|---|---|---|---|---|
| Number of patients | 289 (100%) | 143 (49%) | 146 (51%) | - |
| Age (years) | 43 (18–80) | 45 (18–77) | 44 (18–80) | 0.17 |
| Tumor Type | Chordoma (101, 35.0%), Chondrosarcoma (188, 65.0%) | Chordoma (101, 70.6%), Chondrosarcoma (42, 29.4%) | Chondrosarcoma (146, 100%) | <0.001 |
| Minimum dose to optic apparatus (GyRBE) | 2 (0, 50.5) | 0.9 (0.1,34.4) | 2.7 (0, 50.5) | 0.88 |
| Maximum dose to optic apparatus (GyRBE) | 59.6 (45,76) | 60.0 (45.6,76) | 59.3 (45,72.4) | 0.03 |
| GTV Volume (cc) | 27.2 (0.2–130) | 34.9 (2.3–130) | 19.6 (0.2–109) | <0.001 |
| Treatment doses (GyRBE) | 71.2 (67–76) | 72.7 (68–76) | 70.2 (70–70.2) | <0.001 |
| Follow-Up (months) | 73.8 (5–197) | 75.1 (11.83–183.6) | 72.5 (5–197) | 0.11 |
| Treatment Year | 1996–2019 | 1999–2013 | 1996–2019 | - |
| Number of Surgeries | 1.6 (1–8) | 1.9 (1–8) | 1.3 (1–4) | <0.001 |
| RION cases (grade ≥ 1) | 12 (4.2%) | 7 (4.9%) | 5 (3.4%) | 0.57 |
| Sex | 152F/137M | 67F/76M | 85F/61M | 0.06 |
| Hypertension (yes/no/no data) | 52/221/16 | 30/98/15 | 22/123/1 | 0.09 |
| Tumor Involvement in the Optic Apparatus (yes/no/no data) | 112/174/3 | 42/101/0 | 70/73/3 | 0.001 |
| Protons Only | 195 (67%) | 143 (100%) | 52 (35.6%) | <0.001 |
| Pencil Beam Scanning | 143 (49%) | 143 (100%) | 0 (0%) | <0.001 |
| Parameter | p-Value |
|---|---|
| Age | 0.008 |
| Tumor (Chordoma/Chondrosarcoma) | 0.264 |
| Hypertension | <0.001 |
| Tumor Involvement | 0.432 |
| Sex | 0.684 |
| Number of surgeries | 0.468 |
| Number of surgeries > 1 | 0.932 |
| Number of surgeries > 2 | 0.273 |
| Number of surgeries > 3 | 0.038 |
| Number of surgeries > 4 | 0.577 |
| GTV Volume | 0.731 |
| Prescription Dose | 0.167 |
| Photon Dose | 0.419 |
| Proton Dose | 0.321 |
| Dmean | 0.773 |
| Dmax | 0.131 |
| Dmin | 0.594 |
| D1 | 0.150 |
| D99 | 0.663 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köthe, A.; Feuvret, L.; Weber, D.C.; Safai, S.; Lomax, A.J.; Fattori, G. Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy. Cancers 2021, 13, 5327. https://doi.org/10.3390/cancers13215327
Köthe A, Feuvret L, Weber DC, Safai S, Lomax AJ, Fattori G. Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy. Cancers. 2021; 13(21):5327. https://doi.org/10.3390/cancers13215327
Chicago/Turabian StyleKöthe, Andreas, Loïc Feuvret, Damien Charles Weber, Sairos Safai, Antony John Lomax, and Giovanni Fattori. 2021. "Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy" Cancers 13, no. 21: 5327. https://doi.org/10.3390/cancers13215327
APA StyleKöthe, A., Feuvret, L., Weber, D. C., Safai, S., Lomax, A. J., & Fattori, G. (2021). Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy. Cancers, 13(21), 5327. https://doi.org/10.3390/cancers13215327






