Mastocytosis—A Review of Disease Spectrum with Imaging Correlation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathophysiology
3. Clinical Presentation and Diagnostic Criteria
4. Cutaneous Mastocytosis (CM)
5. Systemic Mastocytosis
6. Imaging Findings
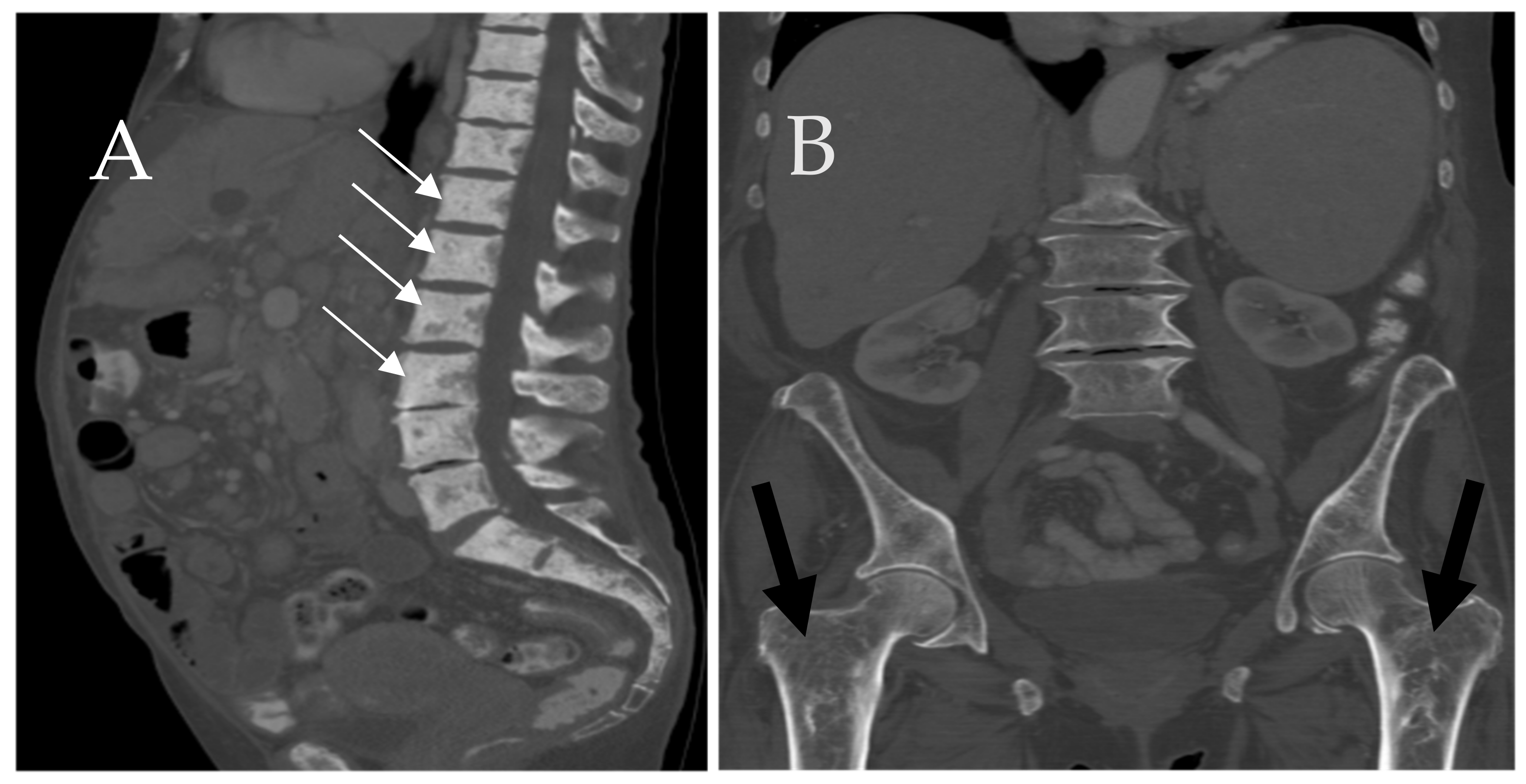
6.1. Skeletal
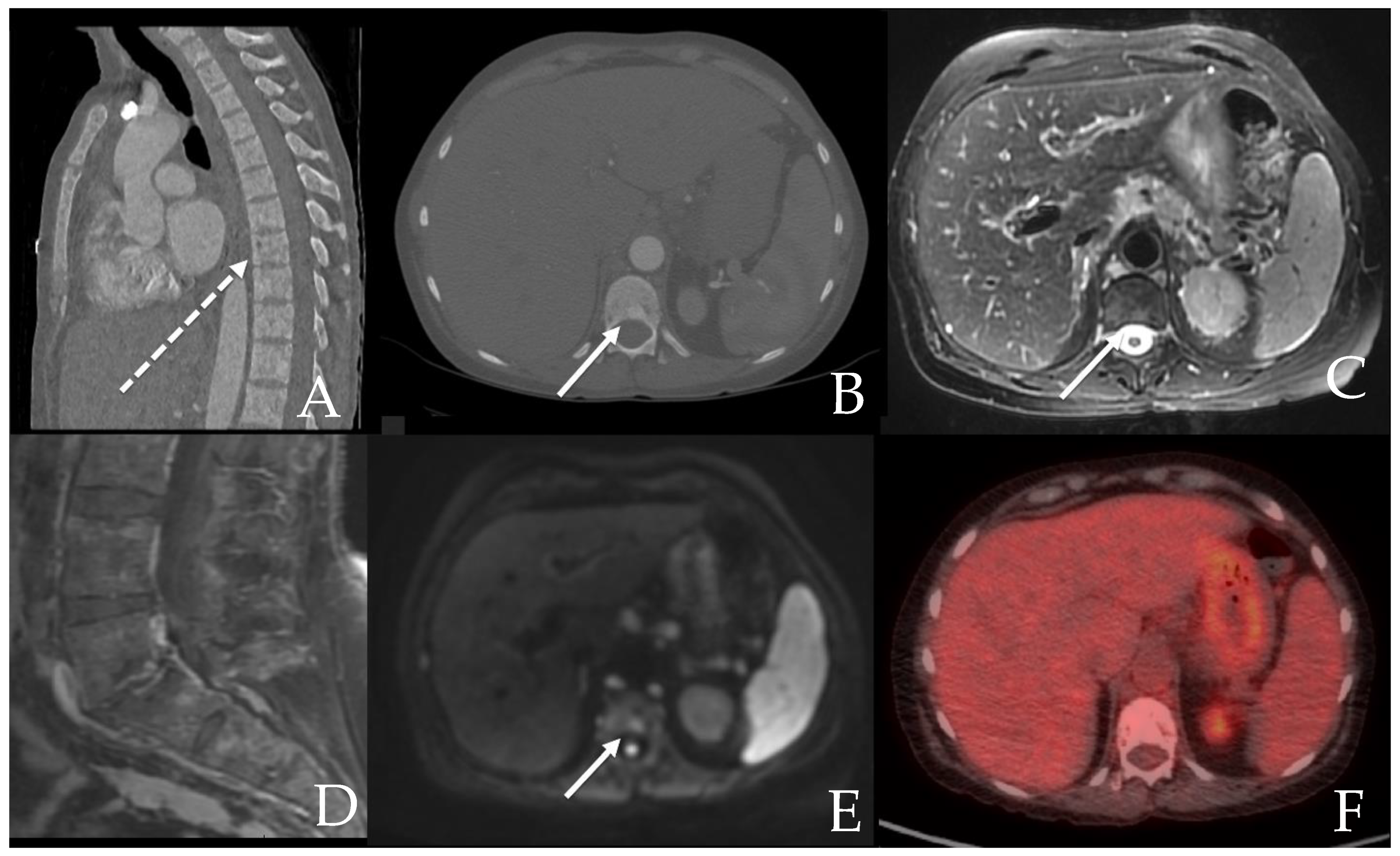
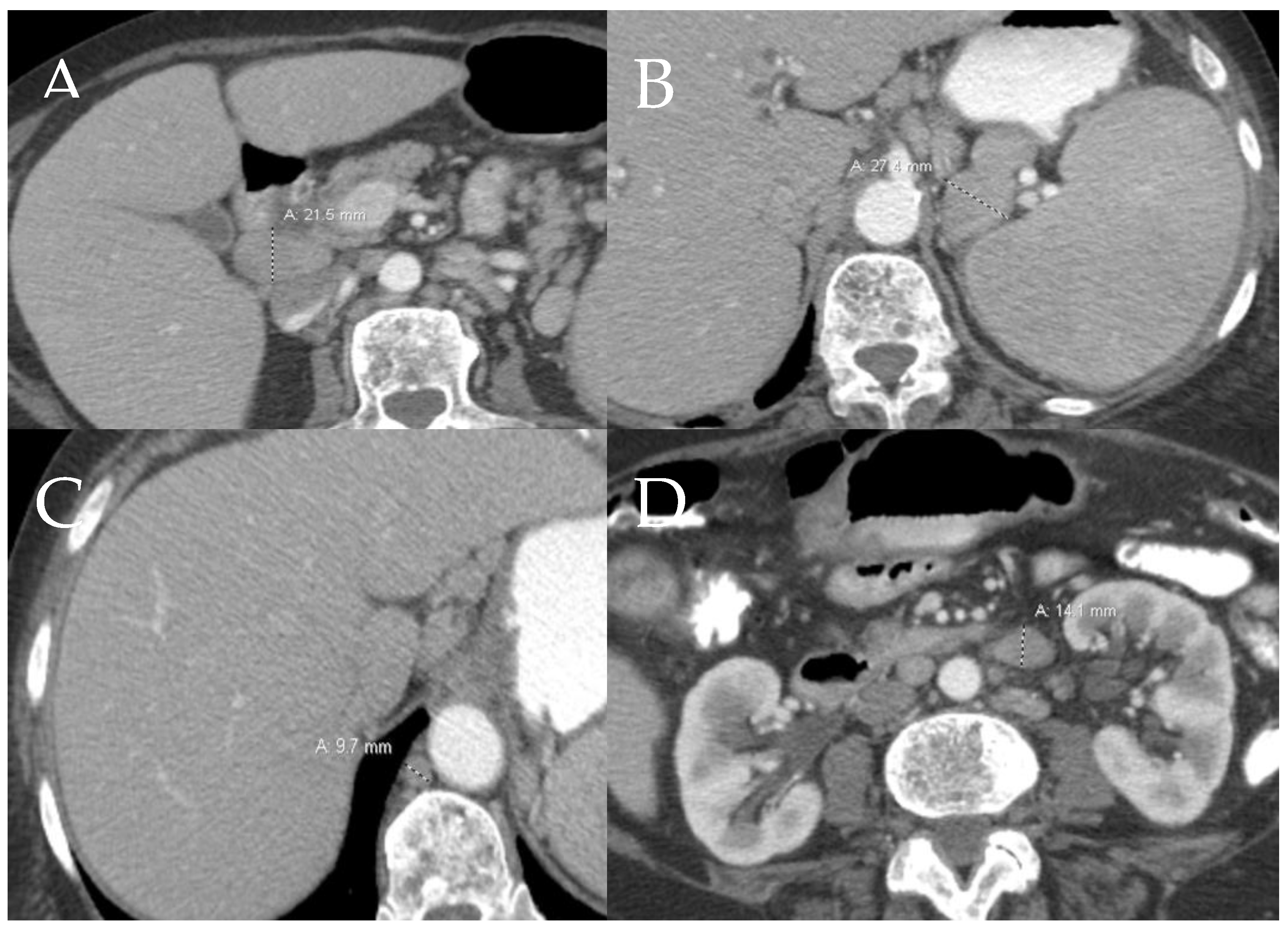
6.2. GI Tract, Liver and Spleen
6.2.1. GI Tract
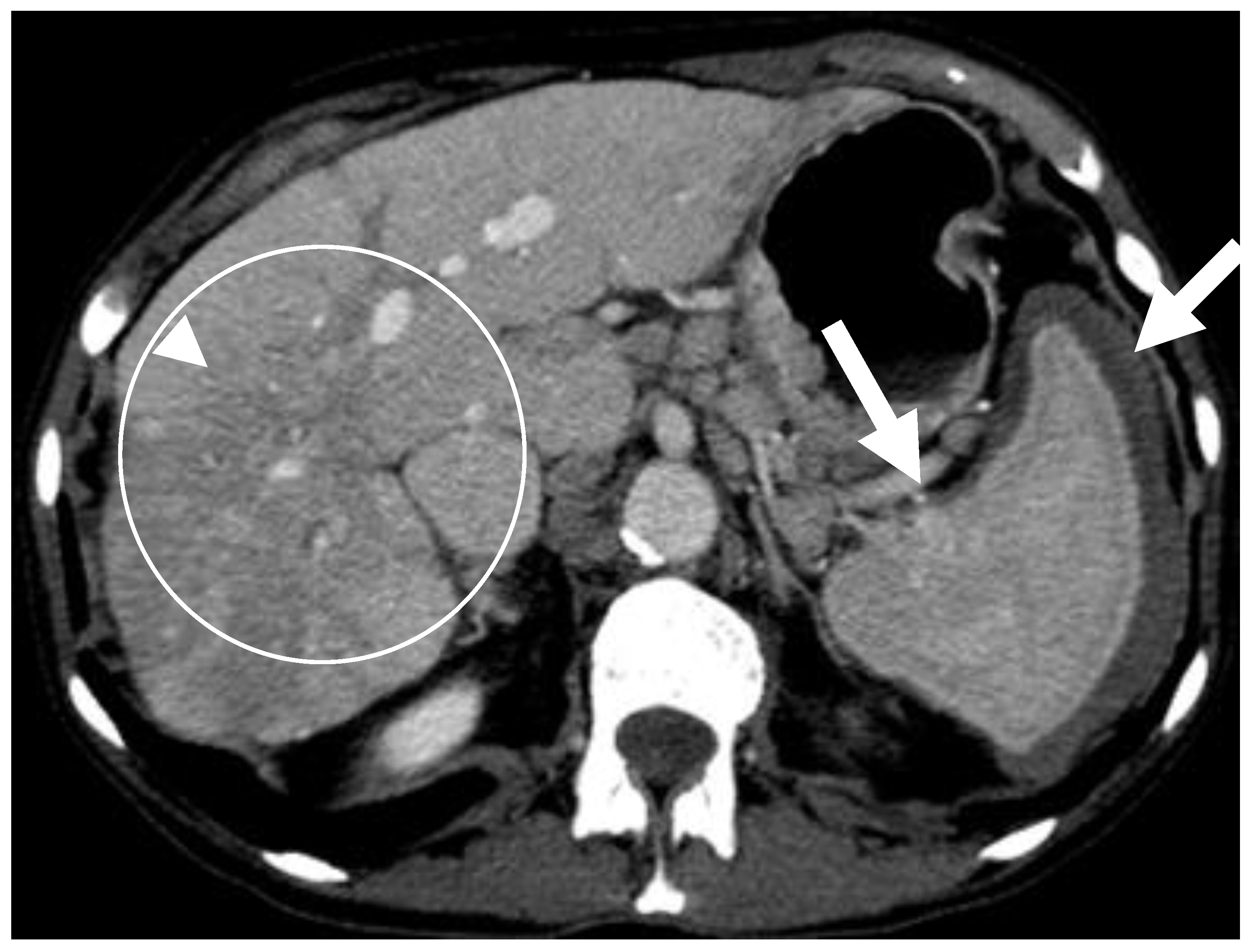
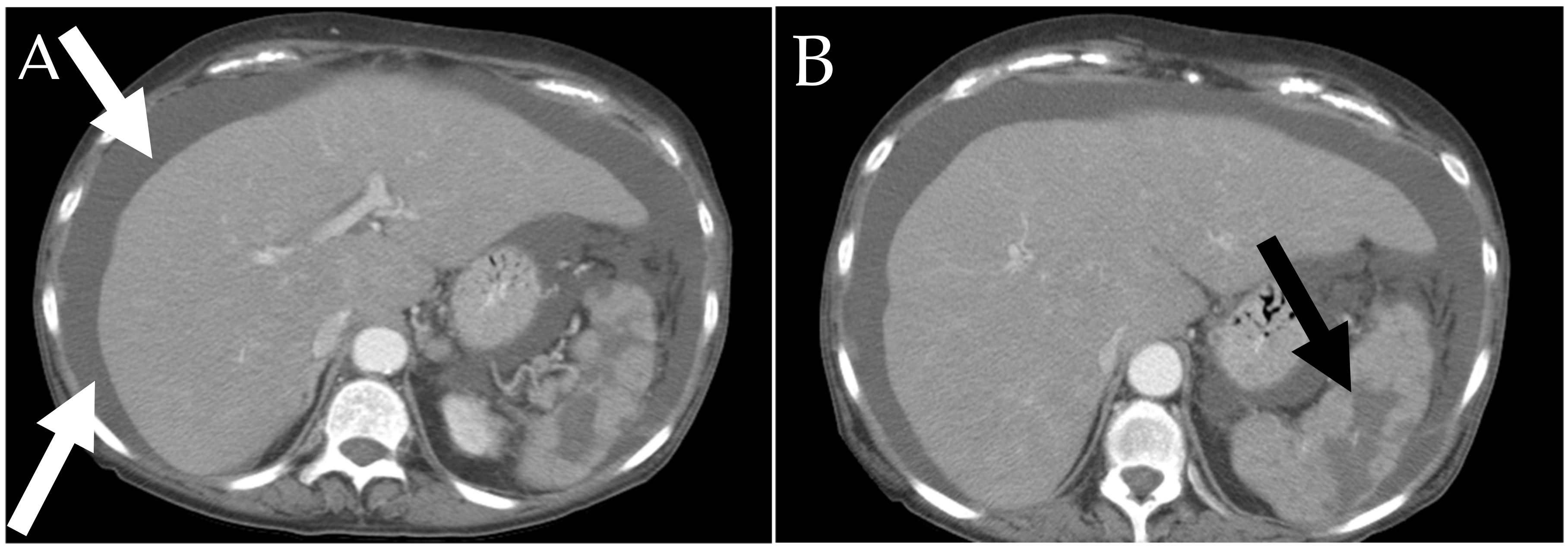
6.2.2. Liver and Spleen
6.3. Future Implications
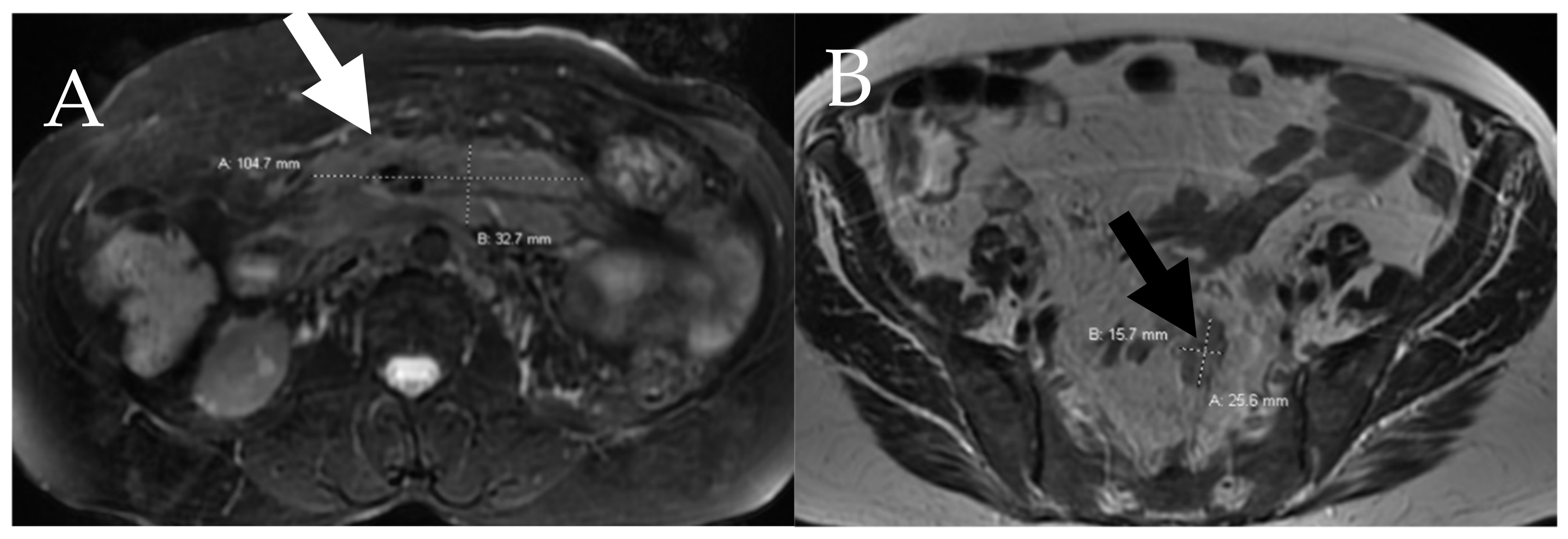
6.4. Lymphatic
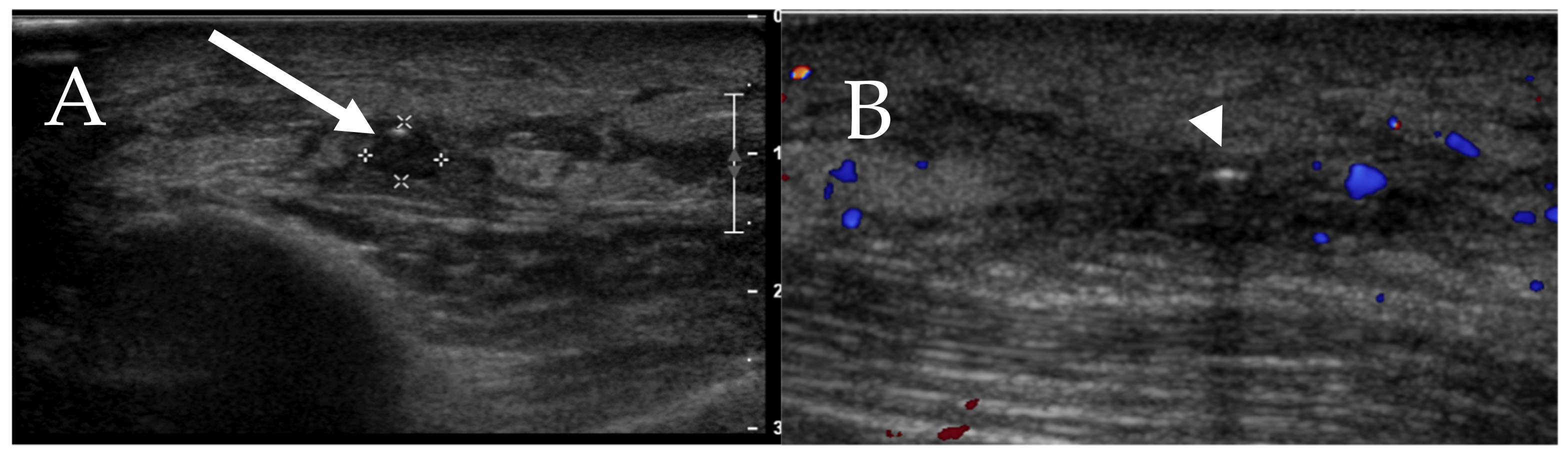
6.5. Skin
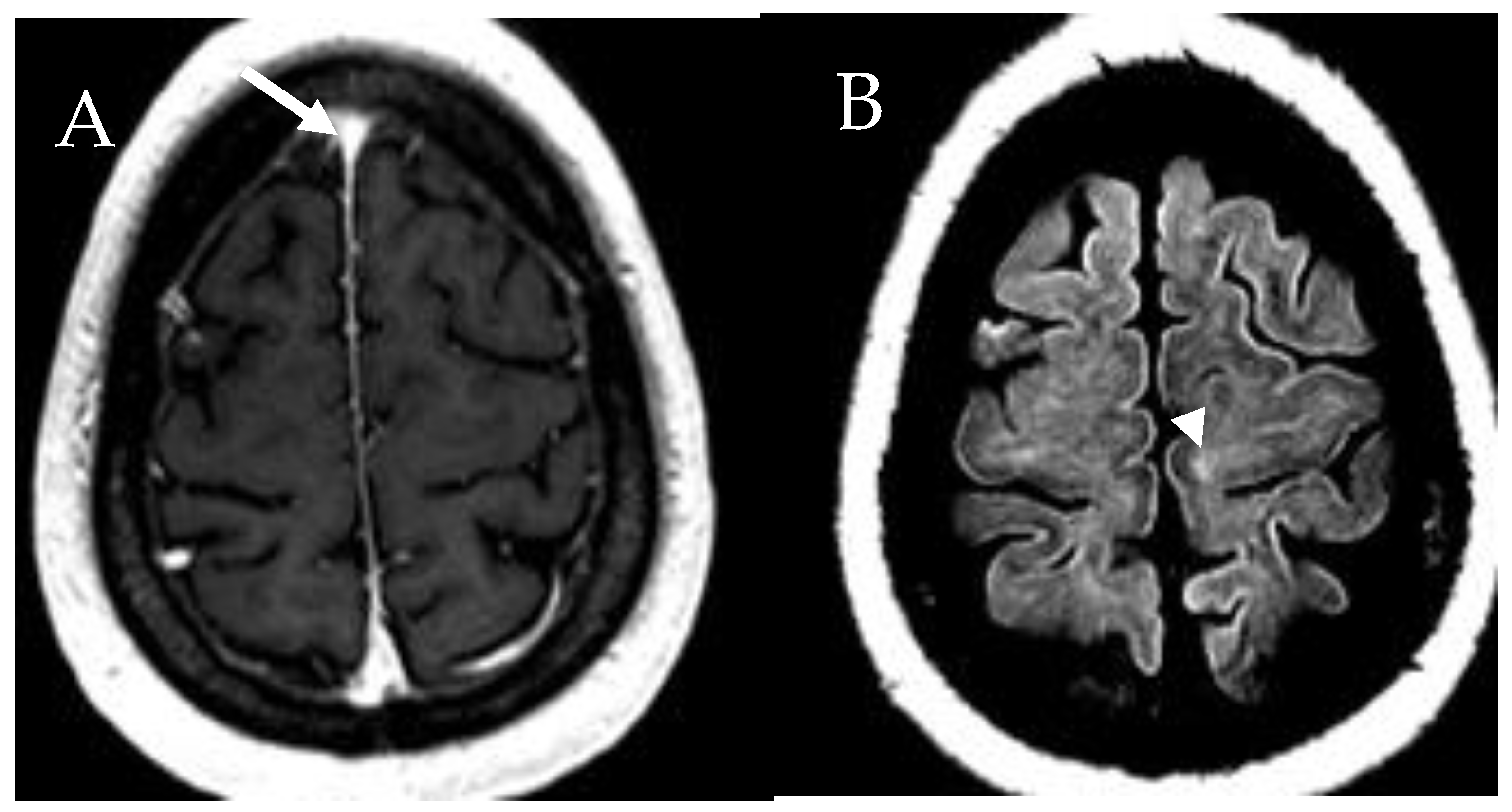
6.6. Central Nervous System
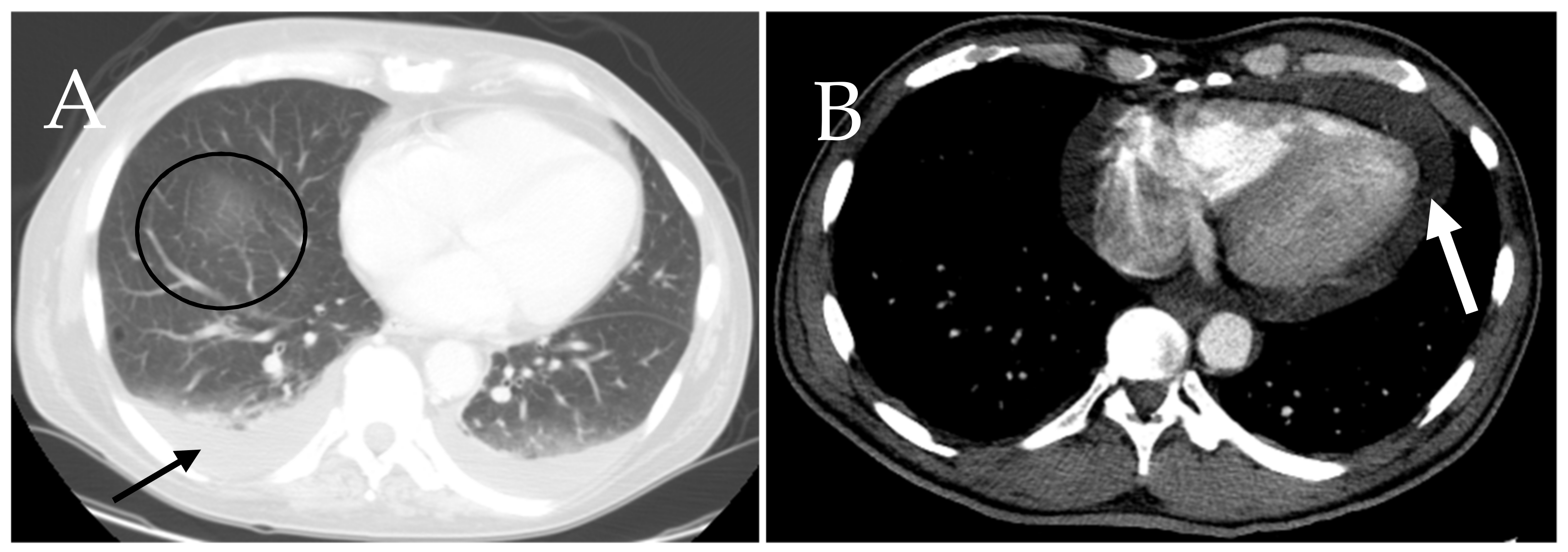
6.7. Cardiac and Respiratory
6.8. Coronavirus and Mastocytosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brockow, K. Epidemiology, Prognosis, and Risk Factors in Mastocytosis. Immunol. Allergy Clin. 2014, 34, 283–295. [Google Scholar] [CrossRef]
- Scherber, R.M.; Borate, U. How we diagnose and treat systemic mastocytosis in adults. Br. J. Haematol. 2018, 180, 11–23. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Kloc, M.; Uosef, A.; Kubiak, J.Z.; Ghobrial, R.M. Macrophage proinflammatory responses to microorganisms and transplanted organs. Int. J. Mol. Sci. 2020, 21, 9669. [Google Scholar] [CrossRef]
- Xu, J.M.; Shi, G.P. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr. Rev. 2012, 33, 71–108. [Google Scholar] [CrossRef] [Green Version]
- Kloc, M.; Uosef, A.; Leśniak, M.; Kubiak, J.Z.; Ghobrial, R.M. Reciprocal interactions between mesenchymal stem cells and macrophages. Int. J. Dev. Biol. 2020, 64, 465–469. [Google Scholar] [CrossRef]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef] [Green Version]
- Bibi, S.; Langenfeld, F.; Jeanningros, S.; Brenet, F.; Soucie, E.; Hermine, O.; Damaj, G.; Dubreuil, P.; Arock, M. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol. Allergy Clin. 2014, 34, 239–262. [Google Scholar] [CrossRef]
- Cruse, G.; Metcalfe, D.D.; Olivera, A. Functional deregulation of KIT: Link to mast cell proliferative diseases and other neoplasms. Immunol. Allergy Clin. 2014, 34, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef]
- Martelli, M.; Monaldi, C.; De Santis, S.; Bruno, S.; Mancini, M.; Cavo, M.; Soverini, S. Recent advances in the molecular biology of systemic mastocytosis: Implications for diagnosis, prognosis, and therapy. Int. J. Mol. Sci. 2020, 21, 3987. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Mekori, Y.A. Pathogenesis and Pathology of Mastocytosis. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am. J. Hematol. 2019, 94, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost. Agents 2021, 35, 65–70. [Google Scholar] [PubMed]
- Compton, R.A.; Lonergan, A.R.; Tsillioni, I.; Conti, P.; Ronconi, G.; Lauritano, D.; Rebeiz, E.E.; Theoharides, T.C. Neurohormonal markers in chronic rhinosinusitis. J. Biol. Regul. Homeost. Agents 2021, 35, 901–908. [Google Scholar]
- Di Raimondo, C.; Del Duca, E.; Silvaggio, D.; Di Prete, M.; Lombardo, P.; Mazzeo, M.; Spallone, G.; Campione, E.; Botti, E.; Bianchi, L. Cutaneous mastocytosis: A dermatological perspective. Australas. J. Dermatol. 2021, 62, e1–e7. [Google Scholar] [CrossRef]
- Hosking, A.M.; Makdisi, J.; Ortenzio, F.; de Feraudy, S.; Smith, J.; Linden, K. Diffuse cutaneous mastocytosis: Case report and literature review. Pediatr. Dermatol. 2018, 35, e348–e352. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Review Article Mastocytosis: 2016 updated WHO classi fi cation and novel emerging treatment concepts. Blood 2017, 129, 1420–1428. [Google Scholar] [CrossRef]
- Valent, P.; Horny, H.P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Leone, A.; Criscuolo, M.; Gullì, C.; Petrosino, A.; Carlo Bianco, N.; Colosimo, C. Systemic mastocytosis revisited with an emphasis on skeletal manifestations. Radiol. Med. 2021, 126, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am. J. Hematol. 2021, 96, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riffel, P.; Schwaab, J.; Lutz, C.; Naumann, N.; Metzgeroth, G.; Fabarius, A.; Schoenberg, S.O.; Hofmann, W.K.; Valent, P.; Reiter, A.; et al. An increased bone mineral density is an adverse prognostic factor in patients with systemic mastocytosis. J. Cancer Res. Clin. Oncol. 2020, 146, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.; Fishman, E.K.; Carrino, J.A.; Horger, M.S. Advanced imaging of skeletal manifestations of systemic mastocytosis. Skelet. Radiol. 2012, 41, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Barete, S.; Assous, N.; De Gennes, C.; Grandpeix, C.; Feger, F.; Palmerini, F.; Dubreuil, P.; Arock, M.; Roux, C.; Launay, J.M.; et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann. Rheum. Dis. 2010, 69, 1838–1841. [Google Scholar] [CrossRef]
- Roca, M.; Mota, J.; Giraldo, P.; García Erce, J.A. Systemic mastocytosis: MRI of bone marrow involvement. Eur. Radiol. 1999, 9, 1094–1097. [Google Scholar] [CrossRef]
- Ozturk, K.; Cayci, Z.; Gotlib, J.; Akin, C.; George, T.I.; Ustun, C. Non-hematologic diagnosis of systemic mastocytosis: Collaboration of radiology and pathology. Blood Rev. 2021, 45, 100693. [Google Scholar] [CrossRef]
- Sokol, H.; Georgin-Lavialle, S.; Grandpeix-Guyodo, C.; Canioni, D.; Barete, S.; Dubreuil, P.; Lortholary, O.; Beaugerie, L.; Hermine, O. Gastrointestinal involvement and manifestations in systemic mastocytosis. Inflamm. Bowel Dis. 2010, 16, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Scolapio, J.S.; Wolfe, J.; Malavet, P.; Woodward, T.A. Endoscopic findings in systemic mastocytosis. Gastrointest. Endosc. 1996, 44, 608–610. [Google Scholar] [CrossRef]
- Avila, N.A.; Ling, A.; Worobec, A.S.; Mican, J.A.M.; Metcalfe, D.D. Systemic mastocytosis: CT and US features of abdominal manifestations. Radiology 1997, 202, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.N.; Liu Yin, J.A.; Azzawi, S.; Warnes, T.W.; Turck, W.P.G. Portal hypertension and ascites in systemic mastocytosis. Postgrad. Med. J. 1989, 65, 394–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Lucey, B.C.; Stuhlfaut, J.W.; Soto, J.A. Nodes Seen at Imaging: Causes and significance. Radiographics 2005, 25, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Karande, G.Y.; Hedgire, S.S.; Sánchez, Y.; Baliyan, V.; Mishra, V.; Ganguli, S.; Prabhakar, A.M. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Boddaert, N.; Salvador, A.; Chandesris, M.O.; Lemaître, H.; Grévent, D.; Gauthier, C.; Naggara, O.; Georgin-Lavialle, S.; Moura, D.S.; Munsch, F.; et al. Neuroimaging evidence of brain abnormalities in mastocytosis. Transl. Psychiatry 2017, 7, e1197. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Dercken, C.; Loke, O.; Reimann, S.; Diederich, S.; Blasius, S.; Heidenreich, S. Pulmonary manifestation of systemic mast cell disease. Eur. Respir. J. 2000, 15, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Indhirajanti, S.; van Daele, P.L.A.; Bos, S.; Mulder, M.T.; Bot, I.; Roeters van Lennep, J.E. Systemic mastocytosis associates with cardiovascular events despite lower plasma lipid levels. Atherosclerosis 2018, 268, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Pillai, R.; Maehara, D.; Chitkara, N. Systemic Mastocytosis With Pulmonary Involvement. Chest 2016, 150, 1136A. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. COVID-19 and Multisystem Inflammatory Syndrome, or is it Mast Cell Activation Syndrome? J. Biol. Regul. Homeost. Agents. 2020, 34, 1633–1636. [Google Scholar] [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]


| B Findings | C Findings |
|---|---|
|
|
| Major Criteria | Minor Criteria |
|---|---|
| Mast cells infiltrate the bone marrow in different foci (i.e., >15 mast cell collected in one location in bone marrow biopsy and/or other extra-cutaneous organs). |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaiey, A.; Mahmoud, H.S.; Jensen, C.T.; Klimkowski, S.; Taher, A.; Chaudhry, H.; Morani, A.C.; Wong, V.K.; Salem, U.I.; Palmquist, S.M.; et al. Mastocytosis—A Review of Disease Spectrum with Imaging Correlation. Cancers 2021, 13, 5102. https://doi.org/10.3390/cancers13205102
Elsaiey A, Mahmoud HS, Jensen CT, Klimkowski S, Taher A, Chaudhry H, Morani AC, Wong VK, Salem UI, Palmquist SM, et al. Mastocytosis—A Review of Disease Spectrum with Imaging Correlation. Cancers. 2021; 13(20):5102. https://doi.org/10.3390/cancers13205102
Chicago/Turabian StyleElsaiey, Ahmed, Hagar S. Mahmoud, Corey T. Jensen, Sergio Klimkowski, Ahmed Taher, Humaira Chaudhry, Ajaykumar C. Morani, Vincenzo K. Wong, Usama I. Salem, Sarah M. Palmquist, and et al. 2021. "Mastocytosis—A Review of Disease Spectrum with Imaging Correlation" Cancers 13, no. 20: 5102. https://doi.org/10.3390/cancers13205102
APA StyleElsaiey, A., Mahmoud, H. S., Jensen, C. T., Klimkowski, S., Taher, A., Chaudhry, H., Morani, A. C., Wong, V. K., Salem, U. I., Palmquist, S. M., & Elsayes, K. M. (2021). Mastocytosis—A Review of Disease Spectrum with Imaging Correlation. Cancers, 13(20), 5102. https://doi.org/10.3390/cancers13205102






