Clinical Characteristics of Resected Acinar Cell Carcinoma of the Pancreas: A Korean Multi-Institutional Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Database
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
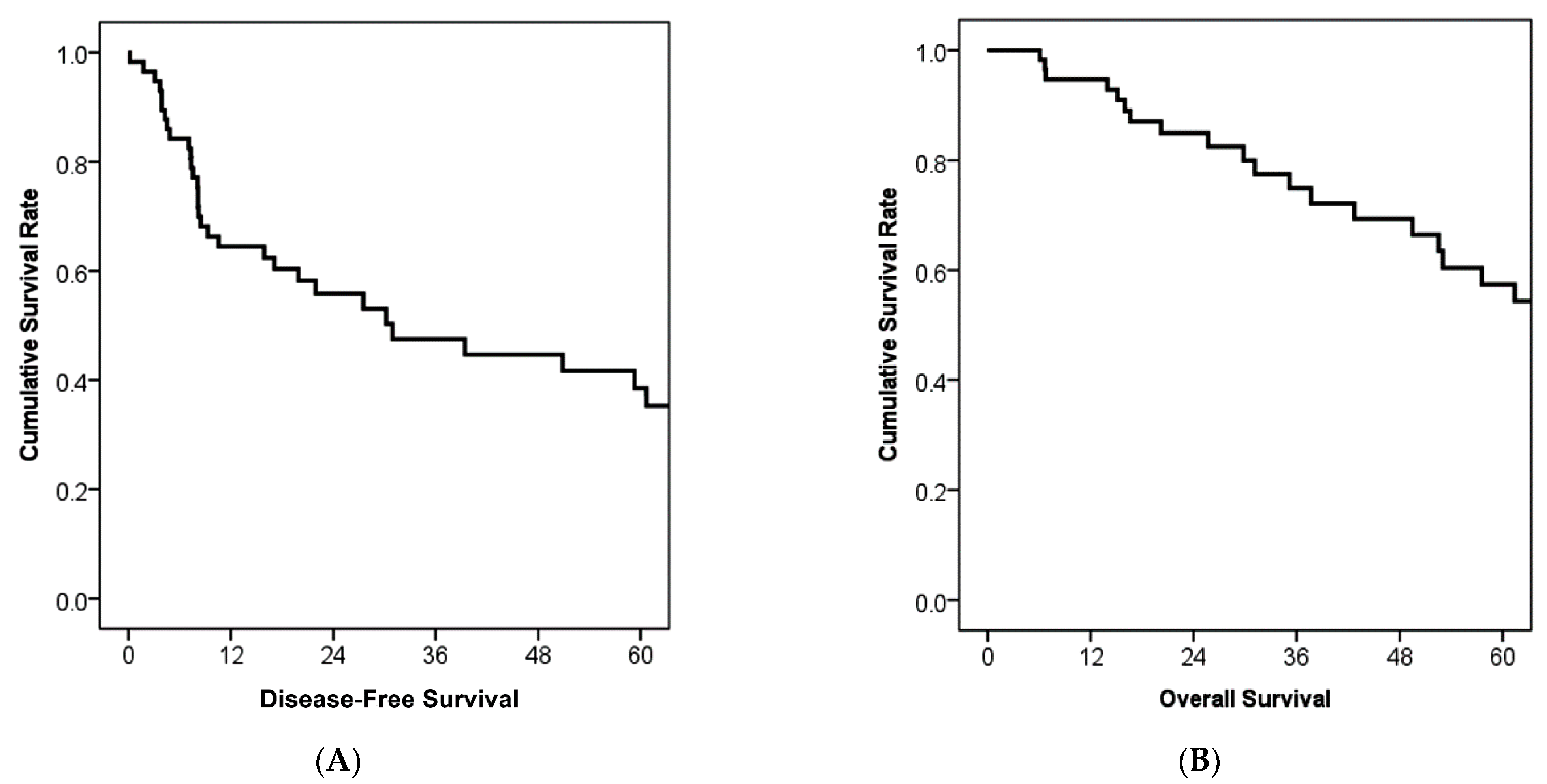
3.2. Treatment Outcomes and Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, J.A. Regulation of pancreatic acinar cell function. Curr. Opin. Gastroenterol. 2006, 22, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Baithun, S.I. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J. Pathol. 1985, 146, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Baithun, S.I.; Ramsay, M.A. Histogenesis of pancreatic carcinomas: A study based on 248 cases. J. Pathol. 1985, 146, 65–76. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Pancreatic acinar cell carcinoma. Adv. Anat. Pathol. 2001, 8, 144–159. [Google Scholar]
- He, C.; Zhang, Y.; Cai, Z.; Duan, F.; Lin, X.; Li, S. Nomogram to predict cancer-specific survival in patients with pancreatic acinar cell carcinoma: A competing risk analysis. J. Cancer 2018, 9, 4117–4127. [Google Scholar] [CrossRef]
- Holen, K.D.; Klimstra, D.S.; Hummer, A.; Gonen, M.; Conlon, K.; Brennan, M.; Saltz, L.B. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J. Clin. Oncol. 2002, 20, 4673–4678. [Google Scholar] [CrossRef]
- Matos, J.M.; Schmidt, C.M.; Turrini, O.; Agaram, N.P.; Niedergethmann, M.; Saeger, H.D.; Merchant, N.; Johnson, C.S.; Lillemoe, K.D.; Grützmann, R. Pancreatic acinar cell carcinoma: A multi-institutional study. J. Gastrointest. Surg. 2009, 13, 1495–1502. [Google Scholar] [CrossRef]
- Patel, D.J.; Lutfi, W.; Sweigert, P.; Eguia, E.; Abood, G.; Knab, L.; Kuo, P.C.; Baker, M.S. Clinically resectable acinar cell carcinoma of the pancreas: Is there a benefit to adjuvant systemic therapy? Am. J. Surg. 2020, 219, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Yoo, C.; Kim, K.P.; Ryoo, B.Y.; Chang, H.M.; Hong, S.M.; Lee, J.H.; Song, K.B.; Hwang, D.W.; Kim, K.H. Clinical outcomes of patients with resectable pancreatic acinar cell carcinoma. J. Dig. Dis. 2017, 18, 480–486. [Google Scholar] [CrossRef]
- Xing-Mao, Z.; Hong-Juan, Z.; Qing, L.; Qiang, H. Pancreatic acinar cell carcinoma—Case report and literature review. BMC Cancer 2018, 18, 1083. [Google Scholar] [CrossRef]
- Kitagami, H.; Kondo, S.; Hirano, S.; Kawakami, H.; Egawa, S.; Tanaka, M. Acinar cell carcinoma of the pancreas: Clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2007, 35, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Tafur, A.; Smithedajkul, P.; Corsini, M.; Quevedo, F.; Miller, R. Mayo Clinic experience with very rare exocrine pancreatic neoplasms. Pancreas 2010, 39, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Wisnoski, N.C.; Townsend, C.M., Jr.; Nealon, W.H.; Freeman, J.L.; Riall, T.S. 672 patients with acinar cell carcinoma of the pancreas: A population-based comparison to pancreatic adenocarcinoma. Surgery 2008, 144, 141–148. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017; p. 3319406175. [Google Scholar]
- Seth, A.K.; Argani, P.; Campbell, K.A.; Cameron, J.L.; Pawlik, T.M.; Schulick, R.D.; Choti, M.A.; Wolfgang, C.L. Acinar cell carcinoma of the pancreas: An institutional series of resected patients and review of the current literature. J. Gastrointest. Surg. 2008, 12, 1061–1067. [Google Scholar] [CrossRef]
- Glazer, E.S.; Neill, K.G.; Frakes, J.M.; Coppola, D.; Hodul, P.J.; Hoffe, S.E.; Pimiento, J.M.; Springett, G.M.; Malafa, M.P. Systematic review and case series report of acinar cell carcinoma of the pancreas. Cancer Control 2016, 23, 446–454. [Google Scholar] [CrossRef]
- Verbeke, C.; Leitch, D.; Menon, K.; McMahon, M.; Guillou, P.; Anthoney, A. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Li, H.; Wu, Y.; Zhang, H.; Chen, W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 11683–11691. [Google Scholar] [PubMed]
- Wong, D.; Ko, A.H.; Hwang, J.; Venook, A.P.; Bergsland, E.K.; Tempero, M.A. Serum CA19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas 2008, 37, 269–274. [Google Scholar] [CrossRef]
- Hammad, N.; Heilbrun, L.K.; Philip, P.A.; Shields, A.F.; Zalupski, M.M.; Venkatramanamoorthy, R.; El-Rayes, B.F. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia Pac. J. Clin. Oncol. 2010, 6, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Girgis, M.D.; Olafsen, T.; Kenanova, V.; McCabe, K.E.; Wu, A.M.; Tomlinson, J.S. CA19-9 as a potential target for radiolabeled antibody-based positron emission tomography of pancreas cancer. Int. J. Mol. Imaging 2011, 2011, 834515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Gao, J.; Du, Y.; Li, Z.; Ren, Y.; Gu, J.; Wang, X.; Gong, Y.; Wang, W.; Kong, X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int. J. Cancer 2012, 131, 683–691. [Google Scholar] [CrossRef]
- Martin, L.K.; Wei, L.; Trolli, E.; Bekaii-Saab, T. Elevated baseline CA19-9 levels correlate with adverse prognosis in patients with early-or advanced-stage pancreas cancer. Med. Oncol. 2012, 29, 3101–3107. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.C.; Song, K.-B.; Hwang, D.W.; Lee, J.H.; Park, K.-M.; Lee, Y.-J. Chronologic changes in clinical and survival features of pancreatic ductal adenocarcinoma since 2000: A single-center experience with 2029 patients. Surgery 2018, 164, 432–442. [Google Scholar] [CrossRef]
- Oba, A.; Croce, C.; Hosokawa, P.; Meguid, C.; Torphy, R.J.; Al-Musawi, M.H.; Ahrendt, S.; Gleisner, A.; Schulick, R.D.; Del Chiaro, M. Prognosis based definition of resectability in pancreatic cancer: A road map to new guidelines. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Kim, N.; Han, I.W.; Ryu, Y.; Hwang, D.W.; Heo, J.S.; Choi, D.W.; Shin, S.H. Predictive Nomogram for Early Recurrence after Pancreatectomy in Resectable Pancreatic Cancer: Risk Classification Using Preoperative Clinicopathologic Factors. Cancers 2020, 12, 137. [Google Scholar] [CrossRef]
- Neoptolemos, J.; Dunn, J.; Stocken, D.; Almond, J.; Link, K.; Beger, H.; Bassi, C.; Falconi, M.; Pederzoli, P.; Dervenis, C. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001, 358, 1576–1585. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Matos, J.M.; Bentrem, D.J.; Talamonti, M.S.; Lillemoe, K.D.; Bilimoria, K.Y. Acinar cell carcinoma of the pancreas in the United States: Prognostic factors and comparison to ductal adenocarcinoma. J. Gastrointest. Surg. 2008, 12, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Zhou, X.; Zhou, H.; Cui, Y.; Li, Q.; Zhang, L. Acinar cell carcinoma: A report of 19 cases with a brief review of the literature. World J. Surg. Oncol. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | n (%) or Mean (±SD) | Characteristics | n (%) or Mean (±SD) |
|---|---|---|---|
| Age, year | 59.2 (±11.6) | T stage, AJCC 8th edition | - |
| BMI, kg/m2 | 23.3 (±3.3) | T1 | 8 (13.6) |
| Sex | - | T2 | 26 (44.1) |
| Male | 49 (83.1) | T3 | 25 (42.4) |
| Female | 10 (16.9) | N stage, AJCC 8th edition | - |
| CEA | - | N0 | 44 (74.6) |
| Normal | 55 (93.2) | N1 | 11 (18.6) |
| Elevated | 2 (3.4) | N2 | 4 (6.8) |
| NA | 2 (3.4) | Staging, AJCC 8th edition | - |
| CA19-9 | - | Stage IA | 8 (13.6) |
| Normal | 47 (79.7) | Stage IB | 16 (27.1) |
| Elevated | 7 (11.9) | Stage IIA | 20 (33.9) |
| NA | 5 (8.5) | Stage IIB | 11 (18.6) |
| Surgery | - | Stage III | 4 (6.8) |
| PD/PPPD | 30 (50.8) | R status | - |
| DP | 21 (35.6) | R0 | 55 (93.2) |
| TP | 3 (5.1) | R1 | 4 (6.8) |
| CP | 1 (1.7) | Adjuvant therapy | - |
| Enucleation | 4 (6.8) | No | 28 (47.5) |
| Tumor location | - | Chemotherapy | 22 (37.3) |
| Head | 33 (55.9) | Chemo-Radiation therapy | 9 (15.3) |
| Body | 3 (5.1) | Chemotherapy regimen | - |
| Tail | 22 (37.3) | No | 28 (47.5) |
| Diffuse | 1 (1.7) | 5-fluorouracil based | 18 (30.5) |
| Size, cm | 4.6 (3.0) | Gemcitabine based | 13 (22.0) |
| Pathology | - | - | - |
| Acinar cell carcinoma | 43 (72.9) | - | - |
| Ductal differentiation | 5 (8.5) | - | - |
| Neuroendocrine mixed | 7 (11.9) | - | - |
| Intraductal and papillary variant | 4 (6.8) | - | - |
| Factors | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Median Survival (Months) | p | p | Hazard Ratio | 95% CI | |
| Elevated CA 19-9 | 52.5 | 0.045 | 0.01 | 24.078 | 2137–271,319 |
| Intraductal and papillary variant | Not reached | 0.023 | 0.014 | 0.018 | 0.001–0.445 |
| N2 stage | 20.2 | 0.036 | 0.027 | 13.882 | 1339–143,931 |
| 5-fluorouracil-based | 53.0 | 0.042 | 0.048 | 5.733 | 1015–32,379 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.H.; Hwang, H.K.; Jang, J.-Y.; Kim, H.; Park, S.J.; Han, S.-S.; Han, I.W.; Hwang, D.W.; Heo, J.S. Clinical Characteristics of Resected Acinar Cell Carcinoma of the Pancreas: A Korean Multi-Institutional Study. Cancers 2021, 13, 5095. https://doi.org/10.3390/cancers13205095
Shin SH, Hwang HK, Jang J-Y, Kim H, Park SJ, Han S-S, Han IW, Hwang DW, Heo JS. Clinical Characteristics of Resected Acinar Cell Carcinoma of the Pancreas: A Korean Multi-Institutional Study. Cancers. 2021; 13(20):5095. https://doi.org/10.3390/cancers13205095
Chicago/Turabian StyleShin, Sang Hyun, Ho Kyoung Hwang, Jin-Young Jang, Hongbeom Kim, Sang Jae Park, Sung-Sik Han, In Woong Han, Dae Wook Hwang, and Jin Seok Heo. 2021. "Clinical Characteristics of Resected Acinar Cell Carcinoma of the Pancreas: A Korean Multi-Institutional Study" Cancers 13, no. 20: 5095. https://doi.org/10.3390/cancers13205095
APA StyleShin, S. H., Hwang, H. K., Jang, J.-Y., Kim, H., Park, S. J., Han, S.-S., Han, I. W., Hwang, D. W., & Heo, J. S. (2021). Clinical Characteristics of Resected Acinar Cell Carcinoma of the Pancreas: A Korean Multi-Institutional Study. Cancers, 13(20), 5095. https://doi.org/10.3390/cancers13205095






