Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Exposures Definition and Data Availability
2.3. Outcomes Definition and Data Availability
2.4. Selection of Genetic Instruments
2.5. Statistical Power
2.6. Statistical Analyses
2.6.1. Univariable MR
2.6.2. Multivariable MR
3. Results
3.1. Instrument Strength and Statistical Power
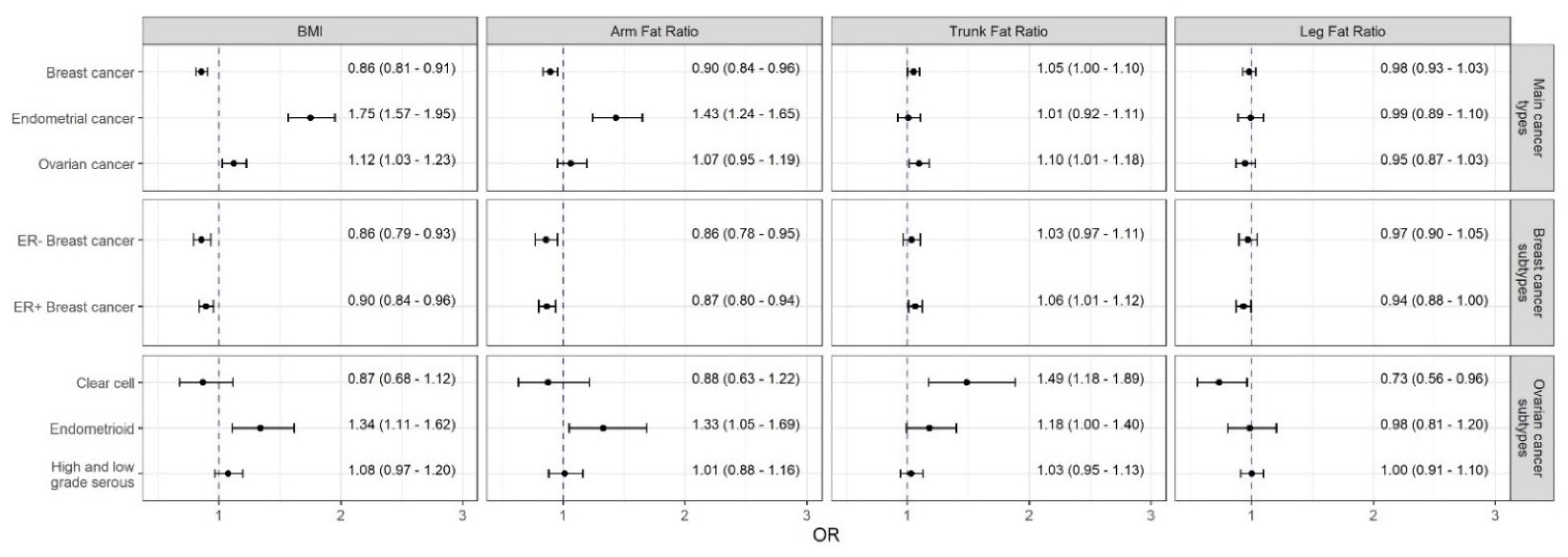
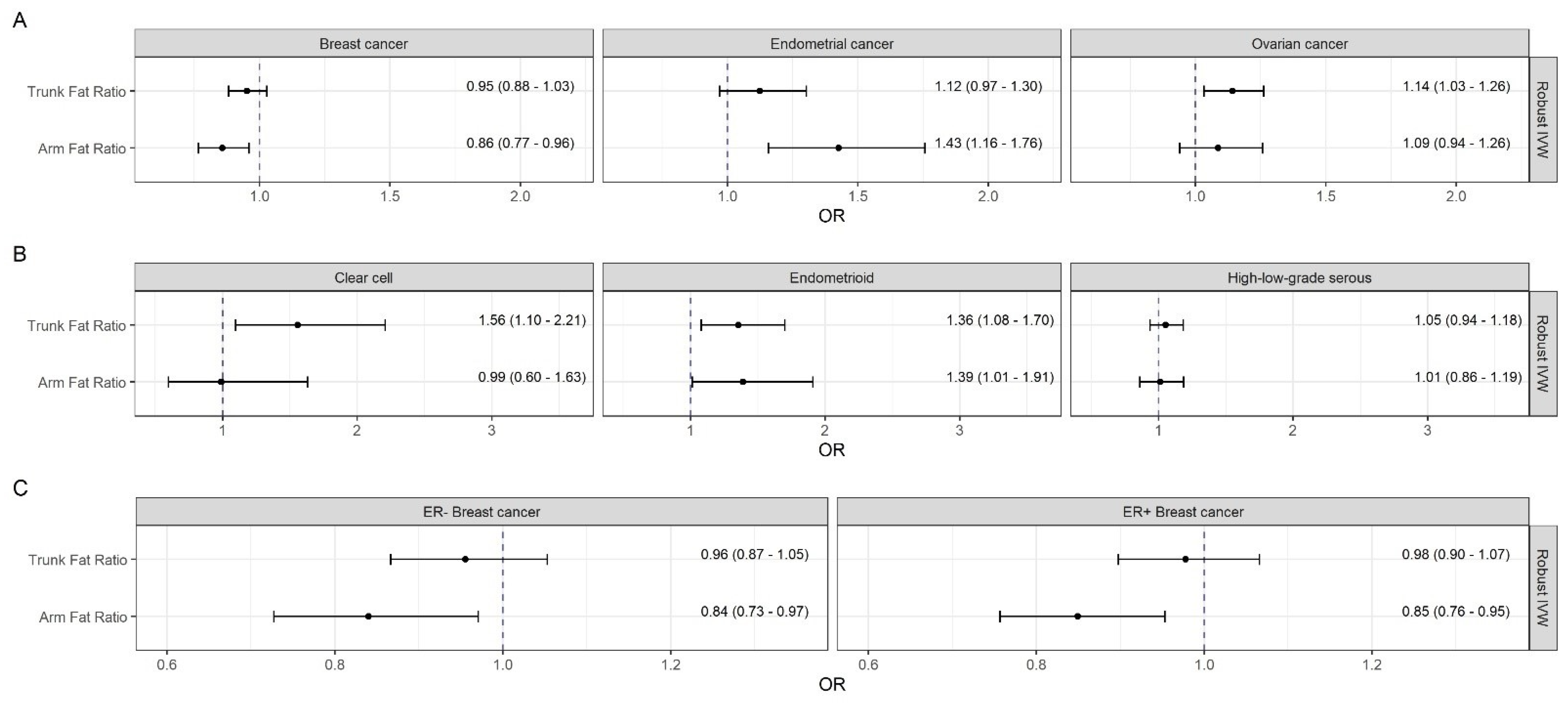
3.2. Effects of Body Fat on Breast Cancer and Its Subtypes
3.3. Effects of Body Fat on Endometrial Cancer
3.4. Effects of Body Fat on Ovarian Cancer and Its Histotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Mitra, A.; Terzidou, V.; Bennett, P.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Obesity and Gynaecological and Obstetric Conditions: Umbrella Review of the Literature. BMJ 2017, 359, j4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and Cancer at Major Anatomical Sites: Umbrella Review of the Literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk Factors for Endometrial Cancer: An Umbrella Review of the Literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, C.L.; Ross, R.K.; Paganini-Hill, A.; Bernstein, L. Effect of Family History, Obesity and Exercise on Breast Cancer Risk among Postmenopausal Women. Int. J. Cancer 2003, 106, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-Mass Index and Risk of 22 Specific Cancers: A Population-Based Cohort Study of 5.24 Million Uk Adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Risch, H.; Irwin, M.L.; Mayne, S.T.; Cartmel, B.; Schwartz, P.; Rutherford, T.; Yu, H. Long-Term Overweight and Weight Gain in Early Adulthood in Association with Risk of Endometrial Cancer. Int. J. Cancer 2011, 129, 1237–1243. [Google Scholar] [CrossRef] [Green Version]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Aune, D.; Rosenblatt, D.A.N.; Chan, D.S.; Vingeliene, S.; Abar, L.; Vieira, A.R.; Greenwood, D.C.; Bandera, E.V.; Norat, T. Anthropometric Factors and Endometrial Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Ann. Oncol. 2015, 26, 1635–1648. [Google Scholar] [CrossRef]
- Wentzensen, N.; Poole, E.M.; Trabert, B.; White, E.; Arslan, A.A.; Patel, A.V.; Setiawan, V.W.; Visvanathan, K.; Weiderpass, E.; Adami, H.O.; et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis from the Ovarian Cancer Cohort Consortium. J. Clin. Oncol. 2016, 34, 2888–2898. [Google Scholar] [CrossRef]
- Painter, J.N.; O’Mara, T.A.; Marquart, L.; Webb, P.M.; Attia, J.; Medland, S.E.; Cheng, T.; Dennis, J.; Holliday, E.G.; McEvoy, M.; et al. Genetic Risk Score Mendelian Randomization Shows That Obesity Measured as Body Mass Index, but Not Waist:Hip Ratio, Is Causal for Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1503–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mara, T.A.; Glubb, D.M.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Bolla, M.K.; et al. Identification of Nine New Susceptibility Loci for Endometrial Cancer. Nat. Commun. 2018, 9, 3166. [Google Scholar] [CrossRef]
- Guo, Y.; Andersen, S.W.; Shu, X.O.; Michailidou, K.; Bolla, M.K.; Wang, Q.; Garcia-Closas, M.; Milne, R.L.; Schmidt, M.K.; Chang-Claude, J. Borresen-Dale Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent. PLoS Med. 2016, 13, e1002105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Patel, C.J.; Michailidou, K.; Peters, U.; Gong, J.; Schildkraut, J.; Schumacher, F.R.; Zheng, W.; Boffetta, P.; Stucker, I.; et al. Mendelian Randomization Study of Adiposity-Related Traits and Risk of Breast, Ovarian, Prostate, Lung and Colorectal Cancer. Int. J. Epidemiol. 2016, 45, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wu, L.; Khankari, N.K.; Shu, X.O.; Wang, T.J.; Michailidou, K.; Bolla, M.K.; Wang, Q.; Dennis, J.; Milne, R.L.; et al. Associations of Obesity and Circulating Insulin and Glucose with Breast Cancer Risk: A Mendelian Randomization Analysis. Int. J. Epidemiol. 2019, 48, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.C.; Nagle, C.M.; Thrift, A.P.; Pharoah, P.D.; Pearce, C.L.; Zheng, W.; Painter, J.N.; Group, A.; Cancer, S.A.; Chenevix-Trench, G.; et al. Adult Body Mass Index and Risk of Ovarian Cancer by Subtype: A Mendelian Randomization Study. Int. J. Epidemiol. 2016, 45, 884–895. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.L.; Shaw, J.E.; Anstey, K.J.; Adams, R.; Balkau, B.; Brennan-Olsen, S.L.; Briffa, T.; Davis, T.M.; Davis, W.A.; Dobson, A.; et al. Comparison of Anthropometric Measures as Predictors of Cancer Incidence: A Pooled Collaborative Analysis of 11 Australian Cohorts. Int. J. Cancer 2015, 137, 1699–1708. [Google Scholar] [CrossRef]
- Freisling, H.; Arnold, M.; Soerjomataram, I.; O’Doherty, M.G.; Ordonez-Mena, J.M.; Bamia, C.; Kampman, E.; Leitzmann, M.; Romieu, I.; Kee, F.; et al. Comparison of General Obesity and Measures of Body Fat Distribution in Older Adults in Relation to Cancer Risk: Meta-Analysis of Individual Participant Data of Seven Prospective Cohorts in Europe. Br. J. Cancer 2017, 116, 1486–1497. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and Cancer: An Update of the Global Impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef]
- Huang, Z.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Manson, J.E.; Rosner, B.; Speizer, F.E.; Hankinson, S.E. Waist Circumference, Waist:Hip Ratio, and Risk of Breast Cancer in the Nurses’ Health Study. Am. J. Epidemiol. 1999, 150, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- White, A.J.; Nichols, H.B.; Bradshaw, P.T.; Sandler, D.P. Overall and Central Adiposity and Breast Cancer Risk in the Sister Study. Cancer 2015, 121, 3700–3708. [Google Scholar] [CrossRef] [Green Version]
- Recalde, M.; Davila-Batista, V.; Diaz, Y.; Leitzmann, M.; Romieu, I.; Freisling, H.; Duarte-Salles, T. Body Mass Index and Waist Circumference in Relation to the Risk of 26 Types of Cancer: A Prospective Cohort Study of 3.5 Million Adults in Spain. BMC Med. 2021, 19, 10. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Smith, G.D. Reading Mendelian Randomisation Studies: A Guide, Glossary, and Checklist for Clinicians. BMJ 2018, 362. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian Randomization: The Use of Pleiotropic Genetic Variants to Estimate Causal Effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask-Andersen, M.; Karlsson, T.; Ek, W.E.; Johansson, A. Genome-Wide Association Study of Body Fat Distribution Identifies Adiposity Loci and Sex-Specific Genetic Effects. Nat. Commun. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-Analysis of Genome-Wide Association Studies for Body Fat Distribution in 694 649 Individuals of European Ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association Analysis Identifies 65 New Breast Cancer Risk Loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Phelan, C.M.; Kuchenbaecker, K.B.; Tyrer, J.P.; Kar, S.P.; Lawrenson, K.; Winham, S.J.; Dennis, J.; Pirie, A.; Riggan, M.J.; Chornokur, G.; et al. Identification of 12 New Susceptibility Loci for Different Histotypes of Epithelial Ovarian Cancer. Nat. Genet. 2017, 49, 680–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brion, M.-J.A.; Shakhbazov, K.; Visscher, P.M. Calculating Statistical Power in Mendelian Randomization Studies. Int. J. Epidemiol. 2012, 42, 1497–1501. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; Group International Agency for Research on Cancer Handbook Working. Body Fatness and Cancer—Viewpoint of the Iarc Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Body Fatness and Weight Gain and the Risk of Cancer. Available online: https://www.dietandcancerreport.org (accessed on 7 May 2021).
- Premenopausal Breast Cancer Collaborative Group; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O’Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.S.; Costantino, J.P.; Cauley, J.A.; Cronin, W.M.; Wickerham, D.L.; Land, S.R.; Weissfeld, J.L.; Wolmark, N. Body Mass Index and the Risk for Developing Invasive Breast Cancer among High-Risk Women in Nsabp P-1 and Star Breast Cancer Prevention Trials. Cancer Prev. Res. 2012, 5, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, T.G.; Sanderson, E.; Elsworth, B.; Tilling, K.; Smith, G.D. Use of Genetic Variation to Separate the Effects of Early and Later Life Adiposity on Disease Risk: Mendelian Randomisation Study. BMJ 2020, 369, m1203. [Google Scholar] [CrossRef] [PubMed]
- Baer, H.J.; Tworoger, S.S.; Hankinson, S.E.; Willett, W.C. Body Fatness at Young Ages and Risk of Breast Cancer throughout Life. Am. J. Epidemiol. 2010, 171, 1183–1194. [Google Scholar] [CrossRef]
- Baer, H.J.; Colditz, G.A.; Rosner, B.; Michels, K.B.; Rich-Edwards, J.W.; Hunter, D.J.; Willett, W.C. Body Fatness During Childhood and Adolescence and Incidence of Breast Cancer in Premenopausal Women: A Prospective Cohort Study. Breast Cancer Res. 2005, 7, R314. [Google Scholar] [CrossRef] [Green Version]
- Sandholt, C.H.; Allin, K.H.; Toft, U.; Borglykke, A.; Ribel-Madsen, R.; Sparso, T.; Justesen, J.M.; Harder, M.N.; Jorgensen, T.; Hansen, T.; et al. The Effect of Gwas Identified Bmi Loci on Changes in Body Weight among Middle-Aged Danes during a Five-Year Period. Obesity 2014, 22, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Keum, N.; Greenwood, D.C.; Lee, D.H.; Kim, R.; Aune, D.; Ju, W.; Hu, F.B.; Giovannucci, E.L. Adult Weight Gain and Adiposity-Related Cancers: A Dose-Response Meta-Analysis of Prospective Observational Studies. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Nead, K.T.; Sharp, S.J.; Thompson, D.J.; Painter, J.N.; Savage, D.B.; Semple, R.K.; Barker, A.; Study, G.A.N.E.C.; Perry, J.R.; Attia, J.; et al. Evidence of a Causal Association between Insulinemia and Endometrial Cancer: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Available online: https://www.wcrf.org/dietandcancer (accessed on 7 May 2021).
- Aune, D.; Rosenblatt, D.A.N.; Chan, D.S.; Abar, L.; Vingeliene, S.; Vieira, A.R.; Greenwood, D.C.; Norat, T. Anthropometric Factors and Ovarian Cancer Risk: A Systematic Review and Nonlinear Dose-Response Meta-Analysis of Prospective Studies. Int. J. Cancer 2015, 136, 1888–1898. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.K.; Park, H.B.; Lee, K.H.; Park, J.H.; Eisenhut, M.; van der Vliet, H.J.; Kim, G.; Shin, J.I. Body Mass Index and 20 Specific Cancers: Re-Analyses of Dose-Response Meta-Analyses of Observational Studies. Ann. Oncol. 2018, 29, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vithayathil, M.; Carter, P.; Kar, S.; Mason, A.M.; Burgess, S.; Larsson, S.C. Body Size and Composition and Site-Specific Cancers in Uk Biobank: A Mendelian Randomisation Study. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G.; on behalf of EPIC-InterAct Consortium. Instrumental Variable Analysis with a Nonlinear Exposure-Outcome Relationship. Epidemiology 2014, 25, 877–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | BMI | AFR | TFR | LFR |
|---|---|---|---|---|
| Sample size | 434,794 | 195,043 | 195,043 | 195,043 |
| Consortium | GIANT, UK Biobank | UK Biobank | UK Biobank | UK Biobank |
| Number of genetic instruments a | 297 | 116 | 202 | 166 |
| Explained variance by instruments | 4.63% | 3.13% | 6.77% | 5.10% |
| F-statistic, mean (min; max) | 60.66 (29.07; 941.37) | 51.37 (29.73; 380.64) | 63.9 (29.84; 392.04) | 58.51 (29.73; 340.77) |
| Reference | Pulit et al., 2018 [26] | Rask-Andersen et al., 2019 [25] | Rask-Andersen et al., 2019 [25] | Rask-Andersen et al., 2019 [25] |
| Characteristics | Breast Cancer | Endometrial Cancer | Ovarian Cancer | |||
|---|---|---|---|---|---|---|
| Sample size | 228,951 | 58,396 | 66,450 | |||
| Controls | 105,974 | 46,126 | 40,941 | |||
| Cases | 122,977 | 12,270 | 25,509 | |||
| Subtypes | ER− | ER+ | Clear cell | Endometrioid | Low or high grade serous | |
| Cases | 21,468 | 69,501 | 1366 | 2810 | 14,049 | |
| Consortium | BCAC | ECAC, E2C2 a | OCAC | |||
| Reference | Michailidou et al., 2018 [27] | O’Mara et al., 2019 [12] | Phelan et al., 2017 [28] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freuer, D.; Linseisen, J.; O’Mara, T.A.; Leitzmann, M.; Baurecht, H.; Baumeister, S.-E.; Meisinger, C. Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study. Cancers 2021, 13, 5053. https://doi.org/10.3390/cancers13205053
Freuer D, Linseisen J, O’Mara TA, Leitzmann M, Baurecht H, Baumeister S-E, Meisinger C. Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study. Cancers. 2021; 13(20):5053. https://doi.org/10.3390/cancers13205053
Chicago/Turabian StyleFreuer, Dennis, Jakob Linseisen, Tracy A. O’Mara, Michael Leitzmann, Hansjörg Baurecht, Sebastian-Edgar Baumeister, and Christa Meisinger. 2021. "Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study" Cancers 13, no. 20: 5053. https://doi.org/10.3390/cancers13205053
APA StyleFreuer, D., Linseisen, J., O’Mara, T. A., Leitzmann, M., Baurecht, H., Baumeister, S.-E., & Meisinger, C. (2021). Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study. Cancers, 13(20), 5053. https://doi.org/10.3390/cancers13205053






