[18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
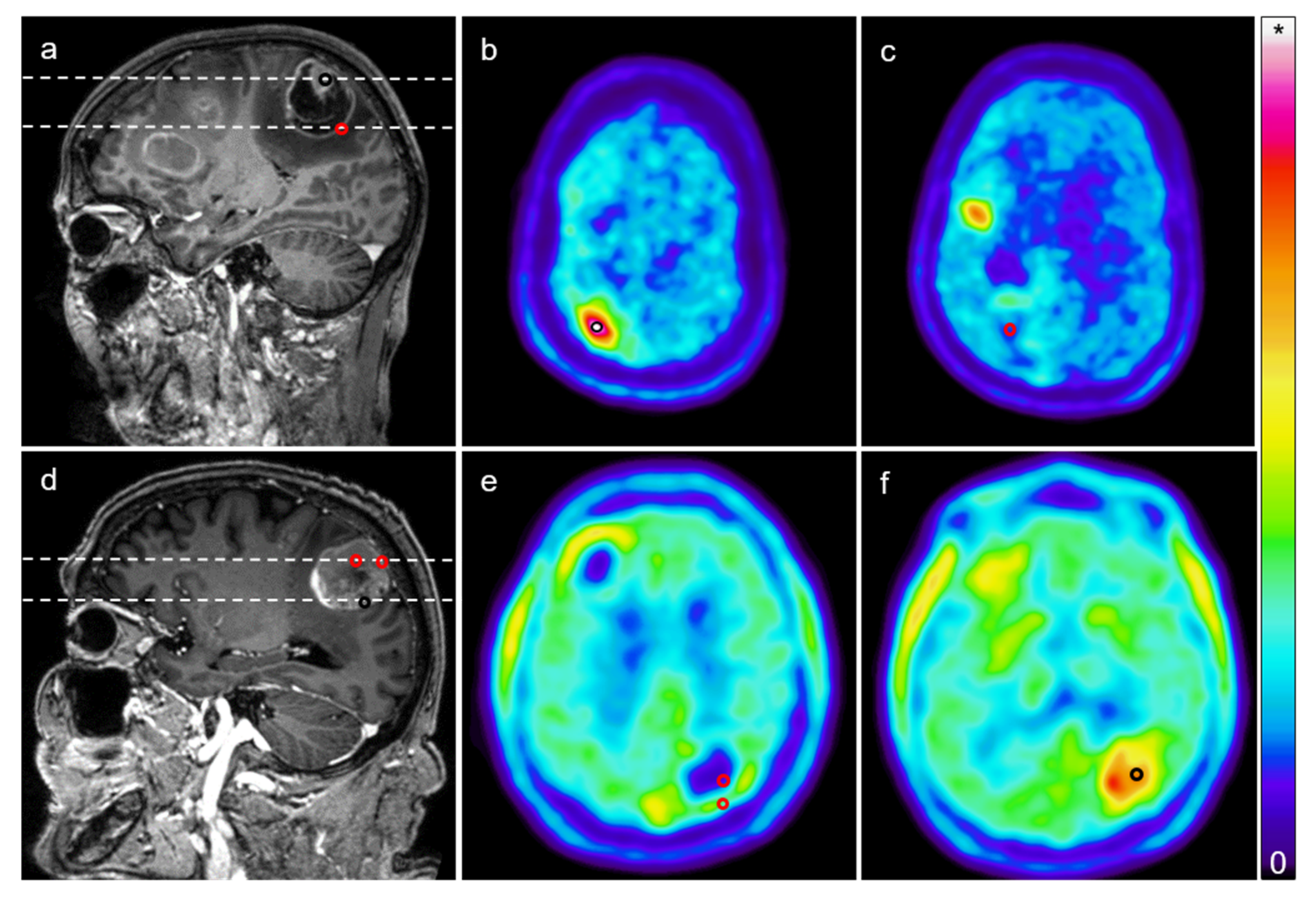
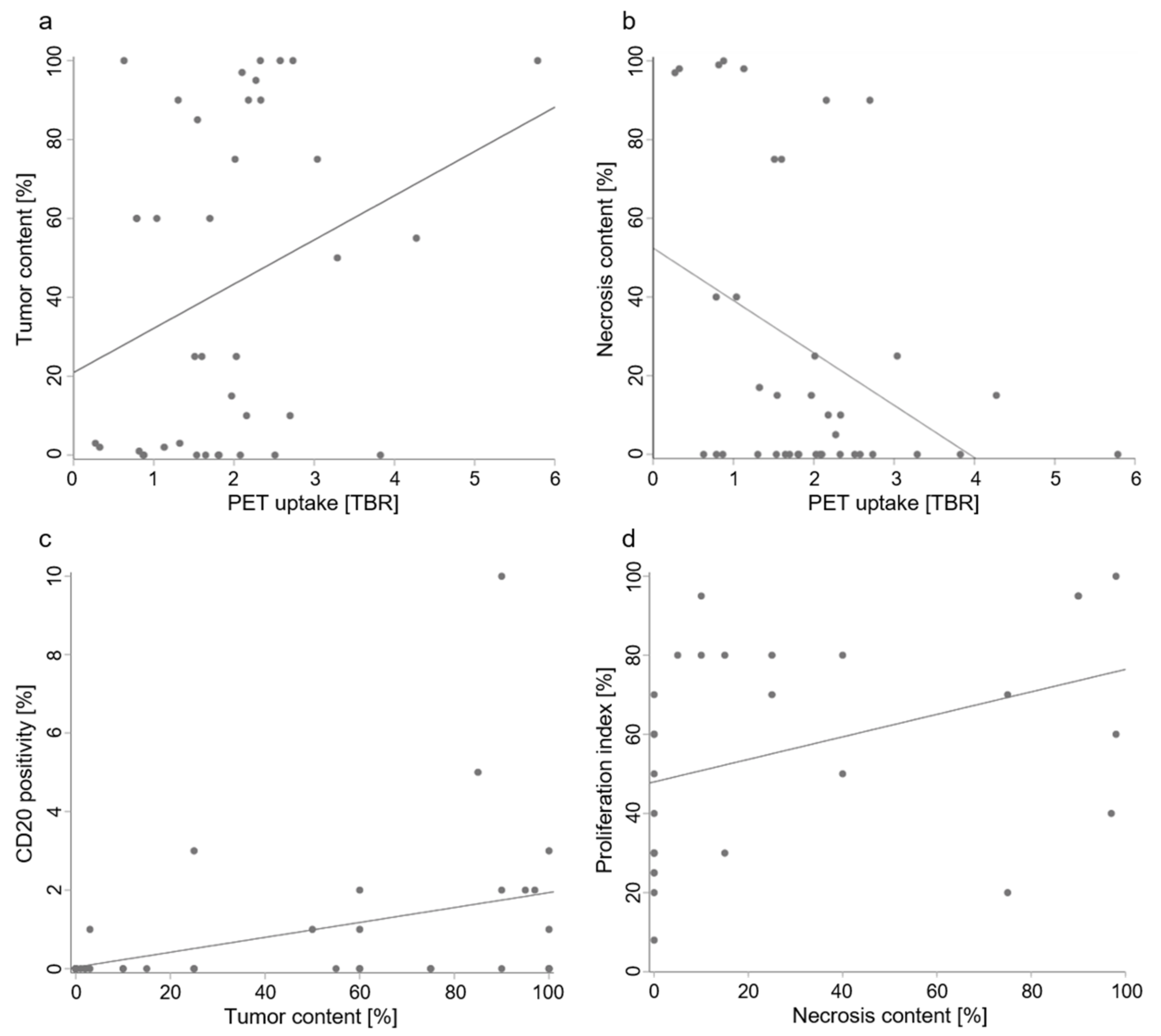
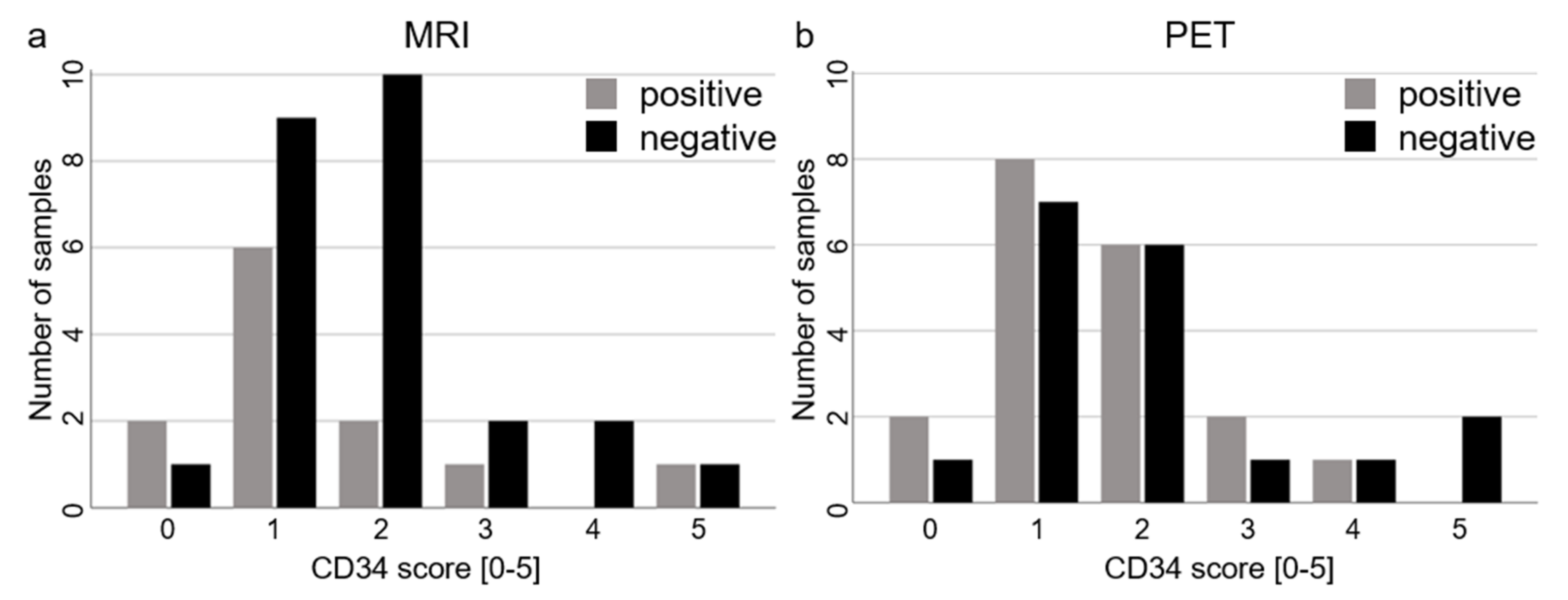
2. Results
3. Discussion
4. Materials and Methods
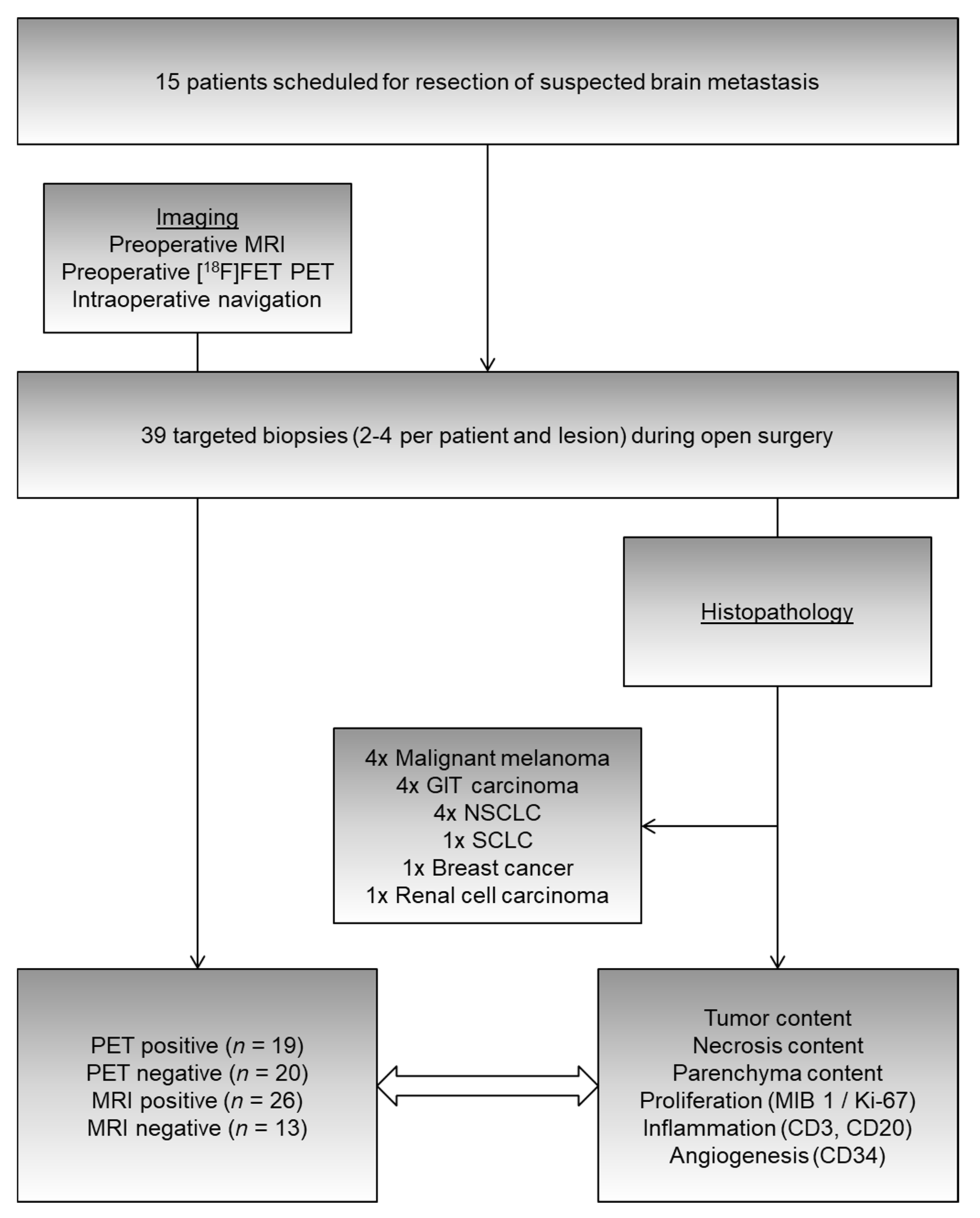
4.1. Patients
4.2. Imaging
4.3. Image Analysis
4.4. Surgery
4.5. Histopathological Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of Metastatic Brain Tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Venur, V.A.; Karivedu, V.; Ahluwalia, M.S. Systemic therapy for brain metastases. Interv. Neuroradiol. 2018, 149, 137–153. [Google Scholar] [CrossRef]
- Langen, K.-J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Pope, W. Brain metastases: Neuroimaging. Interv. Neuroradiol. 2018, 149, 89–112. [Google Scholar] [CrossRef]
- Langen, K.-J.; Galldiks, N. Update on amino acid PET of brain tumours. Curr. Opin. Neurol. 2018, 31, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wester, H.J.; Herz, M.; Weber, W.; Heiss, P.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Stöcklin, G. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J. Nucl. Med. 1999, 40, 205–212. [Google Scholar] [PubMed]
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE 2016, 11, e0157139. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; La Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Langen, K.-J.; Watts, C. Amino acid PET for brain tumours—Ready for the clinic? Nat. Rev. Neurol. 2016, 12, 375–376. [Google Scholar] [CrossRef]
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.-C.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.-J.; Albert, N.L. 18 F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2016, 58, 584–589. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis—Report of the RANO/PET group. Neuro Oncol. 2019, 21, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-18F-Fluoroethyl)-L-Tyrosine PET for Differentiation of Local Recurrent Brain Metastasis from Radiation Necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.P.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.I.; Kocher, M.; et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2016, 19, 281–288. [Google Scholar] [CrossRef]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Enrici, R.M.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tomura, N.; Kokubun, M.; Saginoya, T.; Mizuno, Y.; Kikuchi, Y. Differentiation between Treatment-Induced Necrosis and Recurrent Tumors in Patients with Metastatic Brain Tumors: Comparison among11C-Methionine-PET, FDG-PET, MR Permeability Imaging, and MRI-ADC—Preliminary Results. Am. J. Neuroradiol. 2017, 38, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Kebir, S.; Rauschenbach, L.; Galldiks, N.; Schlaak, M.; Hattingen, E.; Landsberg, J.; Bundschuh, R.A.; Langen, K.-J.; Scheffler, B.; Herrlinger, U.; et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016, 18, 1462–1464. [Google Scholar] [CrossRef]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro Oncol. 2013, 15, 1058–1067. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Maurizi, E.R. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Gempt, J.; Bette, S.; Buchmann, N.; Ryang, Y.-M.; Förschler, A.; Pyka, T.; Wester, H.-J.; Förster, S.; Meyer, B.; Ringel, F. Volumetric Analysis of F-18-FET-PET Imaging for Brain Metastases. World Neurosurg. 2015, 84, 1790–1797. [Google Scholar] [CrossRef]
- Romagna, A.; Unterrainer, M.; Schmid-Tannwald, C.; Brendel, M.; Tonn, J.C.; Nachbichler, S.B.; Muacevic, A.; Bartenstein, P.; Kreth, F.-W.; Albert, N.L. Suspected recurrence of brain metastases after focused high dose radiotherapy: Can [18F]FET-PET overcome diagnostic uncertainties? Radiat. Oncol. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Terakawa, Y.; Tsuyuguchi, N.; Iwai, Y.; Yamanaka, K.; Higashiyama, S.; Takami, T.; Ohata, K. Diagnostic Accuracy of 11C-Methionine PET for Differentiation of Recurrent Brain Tumors from Radiation Necrosis After Radiotherapy. J. Nucl. Med. 2008, 49, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuguchi, N.; Sunada, I.; Iwai, Y.; Yamanaka, K.; Tanaka, K.; Takami, T.; Otsuka, Y.; Sakamoto, S.; Ohata, K.; Goto, T.; et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: Is a differential diagnosis possible? J. Neurosurg. 2003, 98, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Saginoya, T.; Kondo, C.; Tomura, N.; Ito, K.; Matsuo, Y.; Matsunaga, S.; Shuto, T.; Akabane, A.; Miyata, Y.; et al. Differentiation of Brain Tumor Recurrence from Post-Radiotherapy Necrosis with 11C-Methionine PET: Visual Assessment versus Quantitative Assessment. PLoS ONE 2015, 10, e0132515. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, K.J.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.A.F.; Yong, W.H.; Phelps, M.E.; Chen, W. 18F-FDOPA PET for Differentiating Recurrent or Progressive Brain Metastatic Tumors from Late or Delayed Radiation Injury After Radiation Treatment. J. Nucl. Med. 2013, 55, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yomo, S.; Oguchi, K. Prospective study of 11C–methionine PET for distinguishing between recurrent brain metastases and radiation necrosis: Limitations of diagnostic accuracy and long-term results of salvage treatment. BMC Cancer 2017, 17, 713. [Google Scholar] [CrossRef]
- Smits, A.J.J.; Kummer, J.A.; De Bruin, P.C.; Bol, M.; Tweel, J.G.V.D.; Seldenrijk, K.A.; Willems, S.M.; Offerhaus, G.J.A.; De Weger, R.A.; Van Diest, P.J.; et al. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Mod. Pathol. 2014, 27, 168–174. [Google Scholar] [CrossRef]
- Kanai, Y.; Segawa, H.; Miyamoto, K.-I.; Uchino, H.; Takeda, E.; Endou, H. Expression Cloning and Characterization of a Transporter for Large Neutral Amino Acids Activated by the Heavy Chain of 4F2 Antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.-I.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro Oncol. 2013, 15, 1664–1672. [Google Scholar] [CrossRef]
- Gempt, J.; Soehngen, E.; Förster, S.; Ryang, Y.-M.; Schlegel, J.; Zimmer, C.; Meyer, B.; Ringel, F.; Grams, A.E.; Förschler, A. Multimodal imaging in cerebral gliomas and its neuropathological correlation. Eur. J. Radiol. 2014, 83, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Heinzel, A.; Galldiks, N.; Stoffels, G.; Felsberg, J.; Ewelt, C.; Sabel, M.; Steiger, H.J.; Reifenberger, G.; Beez, T.; et al. Diagnostic Performance of 18F-FET PET in Newly Diagnosed Cerebral Lesions Suggestive of Glioma. J. Nucl. Med. 2012, 54, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Vettermann, F.; Brendel, M.; Holzgreve, A.; Lifschitz, M.; Zähringer, M.; Suchorska, B.; Wenter, V.; Illigens, B.M.; Bartenstein, P.; et al. Towards standardization of 18F-FET PET imaging: Do we need a consistent method of background activity assessment? EJNMMI Res. 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
| Imaging Property | Tumor in Specimen (n = 30) | No tumor in Specimen (n = 9) |
|---|---|---|
| PET//MRI Positive | 16//22 (combined: 28) | 3//4 (combined: 5) |
| PET//MRI Negative | 14//8 (combined: 2) | 6//5 (combined: 4) |
| Histological Property | PET-Positive | PET-Negative | MRI-Positive | MRI-Negative |
|---|---|---|---|---|
| Tumor content (%) | 75 (10–97) | 3 (0–60) | 60 (3–90) | 10 (0–50) |
| Necrosis content (%) | 0 (0–15) | 16 (0–81) | 13 (0–36) | 0 (0–75) |
| Proliferation index (%) | 60 (30–80) | 60 (30–70) | 65 (30–80) | 50 (25–65) |
| CD20 expression (%) | 0 (0–2) | 0 (0–0.5) | 0 (0–1) | 0 (0–2) |
| CD3 expression (%) | 1 (1–4) | 1 (0–3) | 1 (0–2) | 3 (1–5) |
| CD34 score | 2 (1–2) | 1 (1–2) | 2 (1–2) | 1 (1–2) |
| Histological Property | PET-Positive | PET-Negative | MRI-Positive | MRI-Negative |
|---|---|---|---|---|
| Tumor content (%) | 60 ± 40 | 27 ± 35 | 50 ± 41 | 28 ± 36 |
| Necrosis content (%) | 14 ± 28 | 39 ± 42 | 26 ± 35 | 28 ± 44 |
| Proliferation index (%) | 58 ± 28 | 54 ± 27 | 58 ± 28 | 51 ± 28 |
| CD20 expression (%) | 1 ± 2 | 1 ± 1 | 1 ± 2 | 1 ± 1 |
| CD3 expression (%) | 3 ± 5 | 3 ± 4 | 3 ± 5 | 3 ± 3 |
| Tumor content (%) | 60 ± 40 | 27 ± 35 | 50 ± 41 | 28 ± 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, H.S.; Liesche-Starnecker, F.; Mustafa, M.; Yakushev, I.; Wiestler, B.; Meyer, B.; Gempt, J. [18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis. Cancers 2021, 13, 355. https://doi.org/10.3390/cancers13020355
Meyer HS, Liesche-Starnecker F, Mustafa M, Yakushev I, Wiestler B, Meyer B, Gempt J. [18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis. Cancers. 2021; 13(2):355. https://doi.org/10.3390/cancers13020355
Chicago/Turabian StyleMeyer, Hanno S., Friederike Liesche-Starnecker, Mona Mustafa, Igor Yakushev, Benedikt Wiestler, Bernhard Meyer, and Jens Gempt. 2021. "[18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis" Cancers 13, no. 2: 355. https://doi.org/10.3390/cancers13020355
APA StyleMeyer, H. S., Liesche-Starnecker, F., Mustafa, M., Yakushev, I., Wiestler, B., Meyer, B., & Gempt, J. (2021). [18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis. Cancers, 13(2), 355. https://doi.org/10.3390/cancers13020355






