Simple Summary
Glioblastoma multiforme (GBM) are among the most lethal tumors. The highly invasive nature and presence of GBM stem cells, as well as the blood brain barrier (BBB) which limits chemotherapeutic drugs from entering the tumor mass, account for the high chance of treatment failure. Recent developments have found that nanoparticles can be conjugated to liposomes, dendrimers, metal irons, or polymeric micelles, which enhance the drug-loaded compounds to efficiently penetrate the BBB, thus offering new possibilities for overcoming GBM stem cell-mediated resistance to chemotherapy and radiation therapy. In addition, there have been new emerging strategies that use nanocarriers for successful GBM treatment in animal models. This review highlights the recent development of nanotechnology and nanocarrier-based drug delivery for treatment of GBMs, which may be a promising therapeutic strategy for this tumor entity.
Abstract
Glioblastoma multiforme (GBM) is the most common and malignant brain tumor with poor prognosis. The heterogeneous and aggressive nature of GBMs increases the difficulty of current standard treatment. The presence of GBM stem cells and the blood brain barrier (BBB) further contribute to the most important compromise of chemotherapy and radiation therapy. Current suggestions to optimize GBM patients’ outcomes favor controlled targeted delivery of chemotherapeutic agents to GBM cells through the BBB using nanoparticles and monoclonal antibodies. Nanotechnology and nanocarrier-based drug delivery have recently gained attention due to the characteristics of biosafety, sustained drug release, increased solubility, and enhanced drug bioactivity and BBB penetrability. In this review, we focused on recently developed nanoparticles and emerging strategies using nanocarriers for the treatment of GBMs. Current studies using nanoparticles or nanocarrier-based drug delivery system for treatment of GBMs in clinical trials, as well as the advantages and limitations, were also reviewed.
1. Introduction
1.1. Clinical Features of Glioblastoma Multiforme (GBM)
Glioblastoma multiforme (GBM) is the most common malignant brain tumor, accounting for approximately 50% of all primary malignant tumors in the central nervous system [1,2]. GBM consists of de novo (primary) GBM, arising from normal glial cells, and secondary GBM, developing from an existing low-grade diffuse or anaplastic astrocytoma [2,3,4]. The median survival after diagnosis of GBM is 12.5–18 months and the five-year survival is reported to be approximately 4–7%, despite the launch of new advanced targeted therapy and immunotherapy [5,6,7,8]. The median age at diagnosis of GBM is mid 60 s; males have a 1.6 times higher incidence than females. The incidence rate of GBM is 1.1–5.0 cases per 100,000 per year [7,8,9,10] and is highest among Caucasians compared to that among Africans and Asians [11,12]. However, recent reports found a sustained and statistically significant increase of GBM across all ages [9,10]. Clinical manifestations of GBM are initially nonspecific, including headaches, nausea, memory loss, personality changes, and seizures. Patients may progress rapidly to unconsciousness when the tumor grows to a very large size [7].
Magnetic resonance imaging (MRI) is the first-line imaging examination procedure for diagnosing GBM. It provides information on a tumor’s location, boundary, size, and characteristics. GBM imaging features are often present as ring-enhancing mass lesions, and are characterized by hypointensity on T1-weighted images and heterogeneous enhancement following contrast infusion (Figure 1) [13]. The standard T2-weighted (T2w), T2-fluid-attenuated inversion recovery (T2-FLAIR) (Figure 2), and T1-weighted contrast-enhanced sequences are typically arranged, showing the important characteristics of the mass, including vascularity, single foci, multiple foci, the necrotic region, and changes in brain structure due to tumor compression [14,15]. The typical features of GBM can be observed in other disease entities, such as metastasis, malignant lymphoma, or infective abscess [16]. For better delineation of tumors and disease monitoring of tumor progression, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is currently the most common imaging diagnostic tracer for GBM diagnosis and assessment of early therapeutic responses [13,17,18]. Patients with increased FDG uptake are found to be associated with a poor survival rate [17]. Because GBMs show variously high intratumor heterogeneity, especially in primary GBM [19,20], and it is challenging to obtain multiple regions of a tumor for analyses, the 3D quantitative image features of GBMs after radiogenomic analysis have been developed to identify GBM phenotypic subtypes and predict patient prognosis [17,18,21,22].
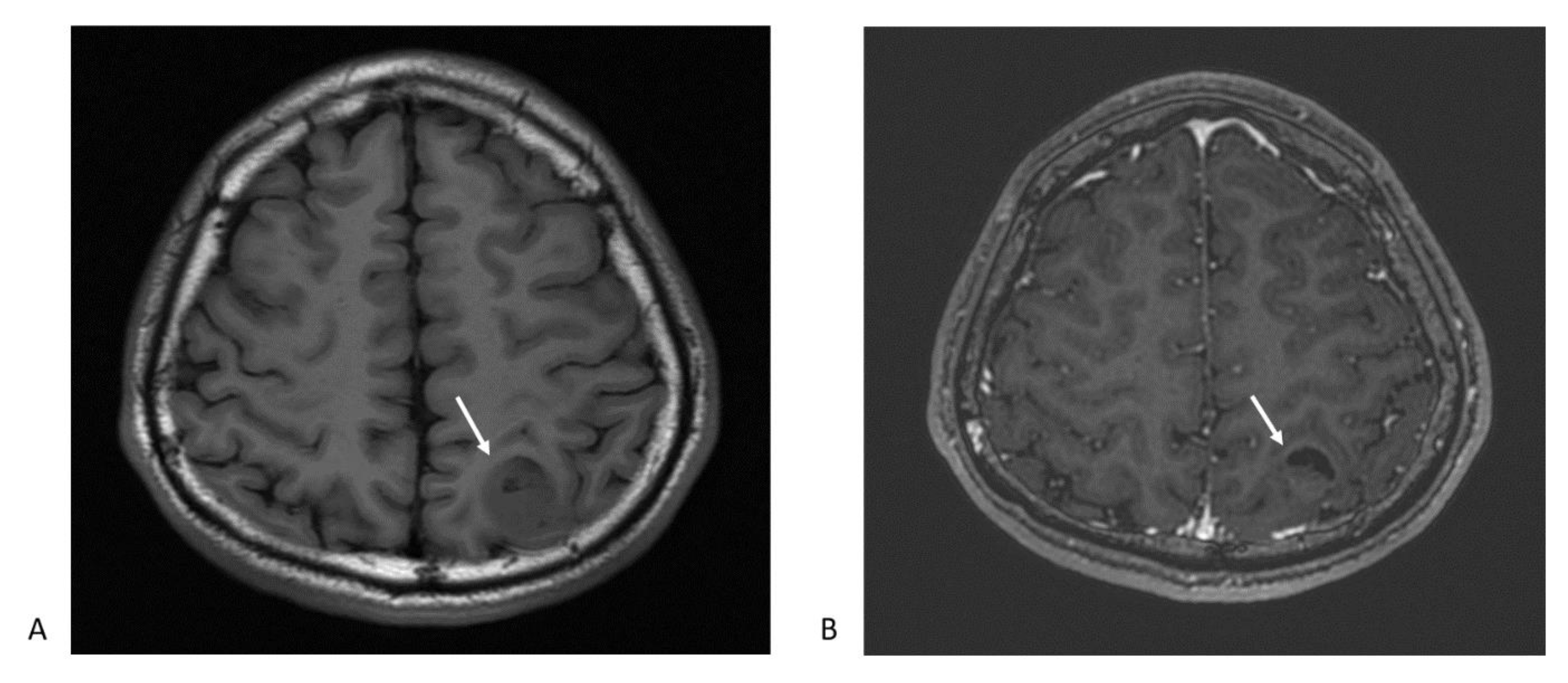
Figure 1.
Magnetic resonance imaging (MRI) typical features glioblastoma multiforme: (A) The axial T1-weighted image shows heterogeneous hypointense mass lesion at left parietal lobe (arrow); (B) postcontrast T1-weighted axial image depicts an enhancing ring lesion with central heterogeneous enhancement. The crescent-shape dark area suggests a necrotic part of the tumor (arrow).
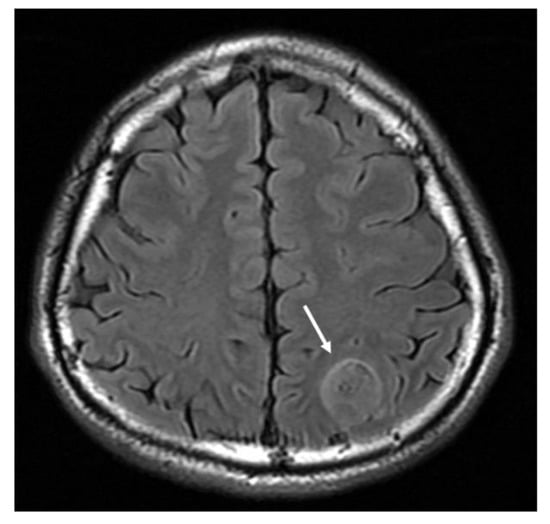
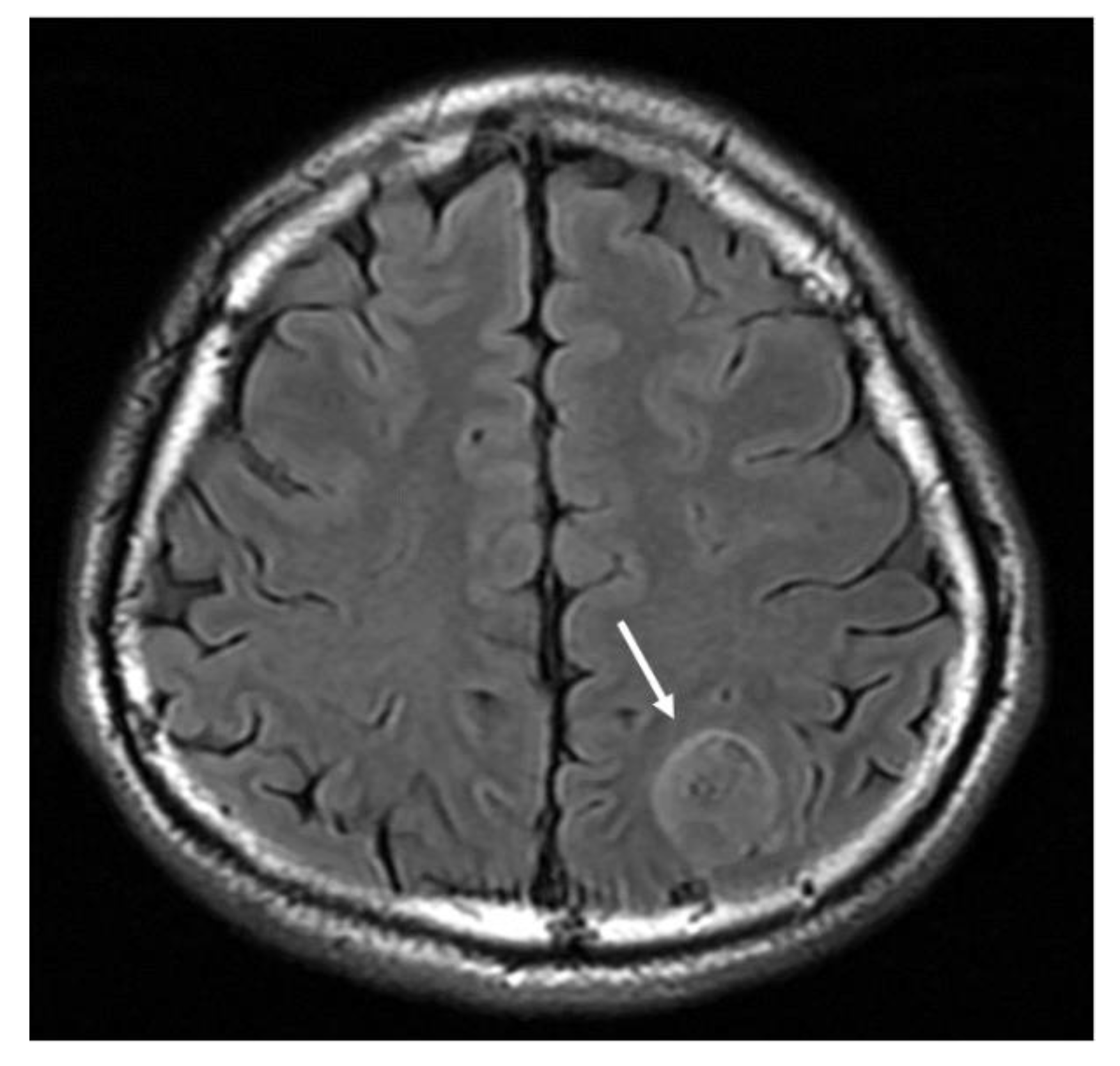
Figure 2.
MRI T2-weighted fluid attenuation inversion recovery (FLAIR) axial image demonstrates a hyperintense mass lesion (arrow).
In gross patterns, GBMs demonstrate a diversity of morphological features. Most GBMs have extensive vascularity, high endothelial proliferation, high cell density mixed with various necrosis, and some atypia [23,24]. The 2016 World Health Organization (WHO) classification of CNS tumors uses molecular parameters and histology to define brain tumors, which formulate the concept that the diagnosis of CNS tumors should be structured in the molecular era [25]. According to the 2016 WHO classification, GBM can be divided into isocitrate dehydrogenase (IDH)-wild type (90% of all cases), IDH-mutant (10%), and IDH not otherwise specified (in cases without diagnostic procedure or those IDH cannot be performed). The IDH-wide type GBM corresponds to primary or de novo glioblastoma and commonly occurs in adults over 55 years old, while the IDH-mutant GBM corresponds to secondary GBM arising from preexisting astrocytoma and is predominantly found in younger patients [25,26].
1.2. Current Treatment
GBM is one of the most lethal cancers with a very poor 5-year survival rate despite advanced therapeutic options in chemotherapy, immunotherapy, and radiation [27]. The current standard policy of GBM includes maximal surgical resection if possible, followed by a combination of chemotherapy and/or radiotherapy. Maximal surgical resection is the first step after confirmation through medical imaging. Radiographic total resection is the most prognostic and a higher extent of tumor resection (>90%) is significantly associated with better one-year survival [28,29]. In the current temozolomide (TMZ) era, maximal resection also confers a significant overall survival benefit in patients with recurrent and resectable GBM [29].
Radiotherapy has been used in patients with residual tumors for more than several decades [30]. Radiation therapy causes tumor cell apoptosis through DNA double-strand breaks [31]. However, approximately 50% of GBMs express amplification of the epidermal growth factor receptor (EGFR) gene, whose truncated variant III, EGFRvIII, which is expressed in nearly one-fourth of all GBMs, confers resistance of GBM to radiation by promoting the rapid repair of DNA double-strand breaks [32]. Furthermore, radiotherapy of brain tumors could promote tumor recurrence or trigger secondary gliomas [33,34]. A recent study demonstrated that radiation-induced DNA double-strand breaks combined with preexisting tumor suppressor losses could contribute to the development of high-grade gliomas in both in vivo and in vitro models [33]. Currently, it is potentially possible to significantly improve GBM therapy by combining ionizing radiation and bioactive DNA repair inhibitors [35]. While radiosurgery techniques have been developed in recent years, stereotactic radiosurgery can confine treatment to the targeted tumor site. Therefore, the role of stereotactic radiosurgery in recurrent GBM has been documented to be significantly associated with longer overall survival and/or progression-free survival [36].
The current standard chemotherapy for GBM is TMZ. Radiotherapy plus concomitant and adjuvant TMZ for GBM was first described in 2005 [37]. It was found to provide better survival outcomes than radiotherapy alone among patients who underwent surgical resection [37,38] and those who only received biopsy [39]. TMZ is an alkylating agent that induces tumor cell apoptosis by methylating the purines of DNA. The ineffectiveness of TMZ comes from O6-methylguanine-DNA methyltransferase (MGMT) expression. MGMT is a DNA repair protein that can reverse the TMZ-induced alkylation process and has emerged as a predictor of responsiveness to alkylating agents [40]. Furthermore, TMZ-induced DNA damage in healthy cells causes significant concern. Given the presences of disadvantages and concerns arising from the current standard care of GBM, namely concomitant chemotherapy after maximal resection, novel therapeutic options are urgently needed to improve the treatment efficacy and target the GBM tumor cells.
2. Obstacles of GBM Treatment and the Resolution
2.1. GBM Stem Cells
The most common mechanism of GBM resistances is the presence of stem-like glioblastoma stem cells (GSCs) and poor permeability restricted by the blood brain barrier (BBB) for most chemotherapeutic agents. GSCs are functionally defined and distinguished from their differentiated glioblastoma cell progeny by the properties of tumor-initiating capacity following serial transplantation, self-renewal, and the ability to recapitulate tumor heterogeneity [41]. The origin of GSCs remains controversial, but it is believed that these progenitor cells arise from neural stem cells or are transformed astrocytes that gain access to stem-specific transcriptional programs [41,42]. The majority of therapeutic modalities to target GSCs have failed during clinical trials, because GSCs have various epigenetic and posttranscriptional regulations that can drive GSCs differentiation, invasive growth, and support GSC maintenance [41,42,43]. GSCs also have high metabolic power to support the rapid proliferation and adapt to harsh microenvironments [44].
The heterogeneity of GBMs further increases the difficulty of treatment. Recent advances in sequencing techniques found the complete genomic landscape of GBMs and revealed profound heterogeneity of individual tumors even at the single cell level [45]. For example, the EGFR genes have been found to have amplifications and mutations in more than half of GMBs, which frequently result in the ineffectiveness of anti-EGFR therapies [46]. Current researchers have tried to find specific biomarkers for GSC populations to distinguish them from non-GSC population in order to target GSCs and sensitize tumors to conventional treatment [47,48]. Cell membrane surface antigens are ideal biomarkers to which antitumor agents can easily bind, leading to increased therapeutic efficacy [48]. However, the optimal markers for GSCs have not yet been identified. Potential biomarkers for GSCs include CD133, CD15/SSEA-1, CD44, integrin-α, and A2B5. Some of these biomarkers can also be used as an indicator of therapeutic response and a prognostic index of GBMs [49].
2.2. Transport across the Blood-Brain Barrier
Another obstacle comes from the low permeability of the BBB which makes the delivery of drugs to the intracranial tumors very difficult [50]. The presence of tight junction complexes in the BBB, which lines the endothelial cells of brain capillaries, results in the absence of pinocytosis and fenestrations and reduces permeability to anticancer agents [51]. Furthermore, active efflux transporters (AETs) will vehicle drugs back to the blood and the presence of metabolizing enzymes further makes drugs inactive before they can be released to the tumor site [51]. To overcome the clearance effects of AETs and promote the transport of anticancer agents to across the BBB, the receptor-mediated transport process has to be active through binding of the drugs to a cell-surface receptor.
GBMs compromise the integrity of the BBB and result in a highly heterogeneous vasculature with distinct features of nonuniform permeability and active efflux of molecules. This phenomenon is known as the blood-tumor barrier (BTB) [52]. Both the BBB and BTB limit the access of potentially effective chemotherapeutic agents to metastatic lesions. Recently, numerous strategies have been investigated to overcome these barriers, including new small molecules capable of penetrating the BBB, novel formulations of anticancer agents, and various disruptive techniques [52,53,54]. A drug-loaded nanocarrier has been designed to overcome the BBB and BTB through the increased affinity for an endocytic receptor expressed on the endothelial cell surface, leading to the efficient release to tumor sites [55,56].
2.3. Applicable Strategies for Drug Delivery to GBMs
There are various strategies to enhance most drugs to cross the BBB, including chemical modification of anticancer drugs, strategies to increase the BBB permeability, and efflux transporter inhibitors [55,57]. For example, increased solubility and lipophilicity of methotrexate can be achieved by adding the translocator protein to form the TSPO-MTX conjugates, which will enhance their delivery through the BBB [58]. A targeted ultrasonic wave can be used to transiently alter the permeability of the BBB through an interaction between administered microbubbles and the capillary bed [59]. MRI-guided focused ultrasound has been demonstrated to open the BBB in the targeted region without compromising its histological and functional integrity [59,60]. Recently, a low dose of systemically injected recombinant human vascular endothelial growth factor was shown to help induce a short period of increased BBB permeability [61,62].
Furthermore, the efflux transporter inhibitors have been used in a mouse model to investigate the effects of improving drug delivery across the BBB [63]. Both P-glycoprotein (Pgp, also known as ABCB1 or MDR) and breast cancer resistance protein (BCRP, also known as ABCG2) are well known efflux transporter proteins found on the endothelial cells of the brain that present an additional functional barrier by pumping drugs back into the blood circulation [64]. Pgp is also highly expressed on the BBB and GSCs and limits the therapeutic efficacy of several chemotherapeutic drugs targeting GSCs. Therefore, safe inhibitors of Pgp, including thiosemicarbazone derivatives and tetrahydroisoquinoline derivatives, can bypass Pgp-mediated drug efflux in primary human BBB and GSC cells [65]. The limitations of efflux transporter inhibitors include poor bioavailability and varied permeability depending on the drug or molecules measured and the heterogeneity of GSCs [66]. For example, statins can reduce the efflux activity of Pgp and BCRP by increasing NO synthesis, which have been documented in statins plus doxorubicin-loaded nanoparticles to be efficient vehicle to cross the BBB [67].
Another strategy to overcome BBB-associated drug delivery is continuous local drug delivery using a convection-enhanced delivery (CED) to achieve great distribution within the brain [68]. In cases of diffuse tumor infiltration and inability of curative surgical resection, CED is also applicable by facilitating concentrated therapeutic drug delivery regardless of molecular size and charge [68,69]. The implantable reservoir-catheter system is the basic CED that uses a pump to provide continuous positive pressure for local drug delivery. In addition, a CED has the advantages of delivering a diverse range of chemotherapeutic agents, monitoring the volume of distribution, and inducing almost no systemic toxicity [69]. However, optimal drugs for CED require specific considerations, including limited toxicity to the normal brain and tumor cell specific cytotoxicity, and a long therapeutic half-life [70]. In addition, CED causes white matter edema, active tumor/BBB disruption, backflow through the catheter, and air bubbles, all of which need technical improvements to facilitate the application of CED to treat GBMs [70].
3. Nanocarriers for Delivery of Anticancer Agents
3.1. Basic Concept and Characteristics of Nanocarriers
Because only a small number of molecules can cross the BBB, novel technologies and delivery systems are therefore necessary to efficiently transport the drugs into the brain matrix. Nanocarriers and nanotechnology-based delivery of drugs are colloidal-based particulate systems that can overcome the BBB due to their characteristics of biosafety, sustained drug release, increased solubility, enhanced drug bioactivity, BBB penetrability, and self-assembly [55,56].
Chemotherapeutic agents are entrapped inside the matrix or attached to the surface of nanoparticles, which are capable of penetrating small capillaries because of their small size. After extravasation and receptor-mediated transcytosis, nanoparticle-drug complexes are absorbed by cells; then, the drug is released into their cytoplasm or compartment (Figure 3). After penetrating the BBB, the accumulation of chemotherapeutic agents-loaded nanoparticles in tumor sites is influenced by the interaction of nanoparticle with tumor cells and intra-tumoral diffusion, which is significantly affected by the particle size, morphology, and surface properties of the nanoparticle [71,72]. Nanoparticles from biodegradable materials have the most important advantage of sustained drug release at the targeted site in a tunable manner [73,74]. Through appropriately engineering with proper ligands on the surface, the drug-loaded nanoparticles can be nontoxic, nonimmunogenic, and stable inside the blood circulation [75]. The presence of ligands on the surface of nanoparticles can deliver the carrier system to the target sites with specific receptors [76,77]. Good candidates to be the ligands that enable drug-nanoparticle complex to efficiently pass through the BBB via receptor-mediated endocytosis include transferrin, apolipoprotein (Apo) E, B, A and some antibodies on the surface of nanoparticles [76,77,78,79]. In addition, nanotechnology can improve the bioavailability of short half-life chemotherapeutic agents and reduce the adverse side effects through the combination [80].
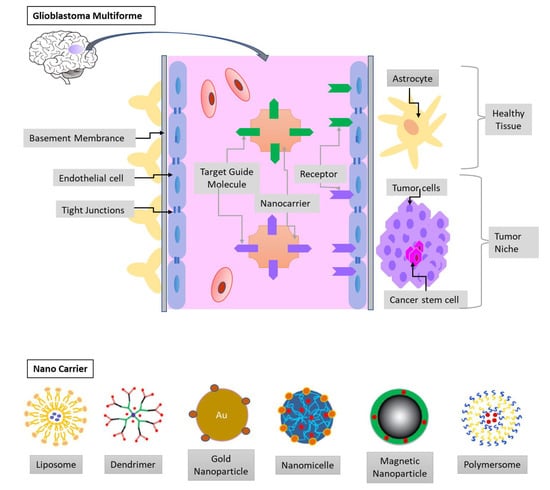
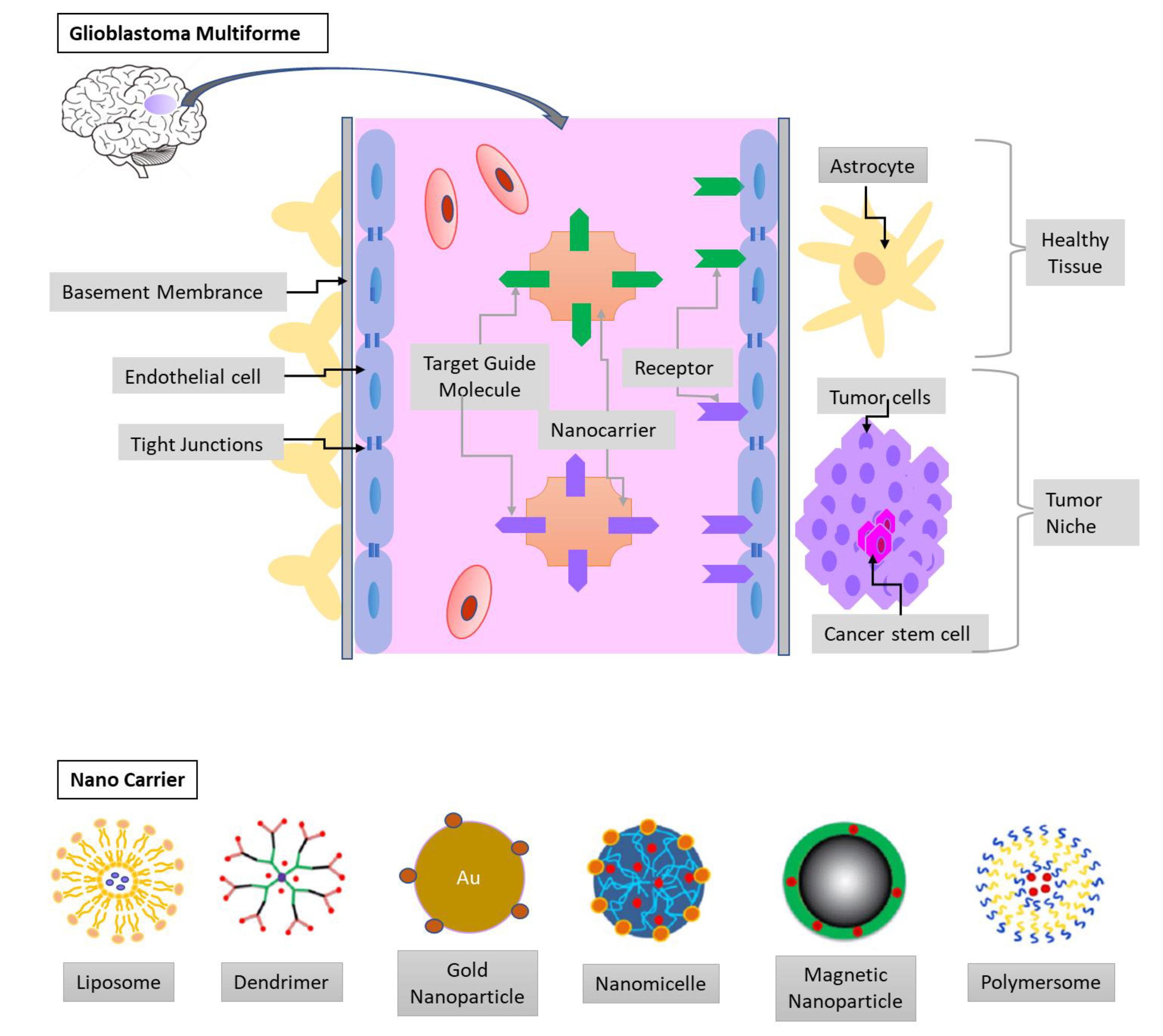
Figure 3.
The blood-brain barrier (BBB) and the glioblastoma multiforme (GBM) niche. The endothelia cells, tight junctions, and basement membrane limit the drug delivery to the tumor niche. Therefore, nanocarrier conjugated with target guide molecules and loaded with chemotherapeutic agents can efficiently cross the BBB. The composites of nanocarrier can be liposomes, micelles, dendrimers, metal, and polymeric nanoparticles.
The size and surface charge of nanoparticles contribute significantly to their ability of escape from the reticuloendothelial system (RES) [81]. Nanoparticles with a size between 5 nm and 500 nm and a positive charge is very important for better cellular uptake. Particles < 200 nm are especially preferred and suitable for systemic administration [81,82]. Particles cannot be less than 5 nm because they are easily excreted by the kidney. The reason for preferred positive charge nanoparticles is that the better interaction with negatively charged cell membranes, target biological area, or some proteins enhance the in vivo stability in the circulation [82]. The small size of nanoparticles is also the double-sided blade because nanoparticles can enter the cytoplasm of normal cells after crossing the tissue junctions and cellular membranes. Inside the cells, nanoparticles can induce mitochondria structural damage, cause damage to DNA and RNA, and lead to cell death [83]. To make the nanoparticles applicable in clinical medicine, surface coatings and other modifications to increase the safety of nanoparticles in the body are mandatory.
To minimize unwanted interactions between nanomaterials and normal tissues, surface modification of nanoparticles with different molecules has been investigated for more than a decade [83]. Initially, polyethylene glycol (PEG) was used as a surface coating because of its hydrophilic external surface and inner hydrophobic polymeric matrix, which helps nanoparticles escape RES recognition and increases the half-life and persistence in the circulation [84]. In order to increase the affinity and specificity of nanoparticles for the targeted tissue, chitosan PEGylated albumin coated nanoparticles coupled with some antibodies were later developed for brain drug targeting through receptor-mediated transporter endocytosis [85,86]. Although there have been various drug delivery systems to the CNS [59,60,61,62,63,64,65,66,67,68,69,70], a recently developed nanoparticles from poly (ethylene glycol)-poly(ῳ-pentadecalactone-co- p-dioxanone) can have a longer period of sustained release and no requirement of repeated infusions, which enhances safety and translatability [87].
3.2. Applicable Strategies of Nanocarriers to Improve Delivery of Anti-GBM Drugs
The major obstacles of GBM treatment are the presence of the BBB, the capture and clearance of anticancer agents by the RES, and the lack of a specific targeting mechanism by which the drugs can bind specifically to GSCs. Special designs and administration route of nanocarrier-based delivery systems are desperately needed to overcome these obstacles. Through CED and an intratumor administration route, nano-formulated drugs can be maintained in or around the tumor site for a longer period, which cannot be achieved in non-nano-formulated drugs [88]. The technique of CED has additional advantages of allowing nano-formulated chemotherapeutic agents to be released toward GBM cells at a precisely controlled infusion rate [68,89], which further enhances anti-tumor efficacy [89].
A new synthesized nanoparticle from magnetotactic bacteria was recently intratumor injected in mice bearing intracranial glioma and followed by alternating magnetic field or magnetic hyperthermia, which showed enhanced anti-tumor efficacy with almost full tumor disappearance [90,91]. This approach indicates an available and alternative strategy for the treatment of infiltrating tumors such as glioma. The whole tumor coverage by nanoparticles is difficult to be achieved.
Another method is to inject anticancer drugs via the intranasal route. For example, the potential of the nose-to-brain direct transport, which bypasses the BBB, has been investigated in GBM mice using theranostic polyfunctional gold-iron oxide nanoparticles surface loaded with therapeutic miRNAs [92]. This nanoformulation also allows GBM cells to be systemically delivered to TMZ [92]. The intranasal route of nose-to-brain drug delivery can potentially present several advantages over the traditional IV route. However, this delivery system is mostly in a preclinical phase of development, and intranasal administration also has limitations [93]. Lower bioavailability of peptides and high clearance from the nasal cavity and some restrictions from the anatomy of the nasal cavity are currently obstacles that need to be overcome [93].
It is possible to weaken or open the major barrier by MRI-guided focused ultrasounds or administering bradykinin, which enable the chemotherapeutic agents to diffuse through the BBB more efficiently [94,95]. Cisplatin-loaded nanoparticles coated with PEG, which prevent capture by macrophage, can have brain-penetrating ability to cross the BBB and BTB after MR image-guided focused ultrasound [60,94]. The successful combination in animal models may offer a new powerful approach for treatment of refractory GBM and control of recurrence [60].
AET targeted and tight junction targeted strategies are important methods to achieve the goal of circumventing and modulating the BBB and BTB [96]. Previously, inhibitors against multidrug resistance efflux transporters have failed in most studies. However, Pgp inhibitors encapsulated into surfactant-based nanoparticles have been developed to reverse multidrug resistance efflux transporters, which can be used to improve the therapeutic effect of the drug [97].
A magnetic field has been applied to trigger the diffusion of magnetic anti-GBM drugs towards the GBM cells [90,91]. Previous difficulties came from the unavailability of equipment to generate a sufficient and precise magnetic field and concerns of unwanted influences on normal tissue [98,99]. However, direct intratumoral administration of magnetic nanoparticles (MNPs) is now applicable for GBM treatment because MNPs can be highly accumulated to the tumor site after the development of a magnetic platform drivable through an external magnetic field [100]. Another example is the new design of hybrid magnetic nanovectors, which are angiopep-2-functionalized lipid-based and promote GBM cell death through a combined effect of lysosomal membrane permeabilization and chemotherapy [101].
3.3. Targeting the GBM Cells and Glioblastoma Stem Cells
The current strategy of active targeting for GBM uses substances attached to the surface of nanoparticles that can specifically target the receptors or antigen on GBM cells or GSCs [89,102,103]. GBM cells express several receptors or proteins, such as metalloproteinase-2, IL-13 receptor, Integrinα5β3, CD33, and CD133, which can be the candidate for nanoparticle targeting.
Since the presence of GSCs account for an important cause of GBM recurrence, the important targeting of GSCs has been investigated in recent years [104]. GSCs express several specific receptors or markers that can be the target of nanocarrier-based drug delivery system. Based on the locations, GSCs have cell surface markers (e.g., CD15, CD133), transcription factors (e.g., OCT4), post-transcriptional factors, and cytoskeletal proteins (e.g., nestin) [105]. The majority of treatments to target GSCs have failed in clinical trials, despite a number of treatment options for targeting GSCs being theoretically available [106].
Recent examples of GSC targeting using nanotechnology includes the preparation of mixing calf thymus DNA with gold nanoparticles, which sensitizes GSCs to radiotherapy [107]. The neurofilament-derived NFL-TBS.40-63 peptide and LinTT1 peptide with enhanced binding targets GSCs [108,109]. Nestin positive GSCs can also be specifically recognized by gold nanorods functionalized with an engineered peptide, which has been proven as a promising tool to develop an efficient nanomedicine for treatment of recurrent GBM [110]. For cell surface marker CD133 in GSCs, the targeting peptide CBP4-coated gold nanoparticles has been developed as a drug carrier for therapeutic approaches [111].
4. Current Nanocarriers and Nanocarrier-Associated Strategies for the Treatment of GBM
The new development of nanocarrier-based combination therapy for GBMs has additional advantages, including facilitation of sequential drug exposure, well confirmation of the synergistic drug ratio, and improved localization of anticancer agents into the tumor site [55,57]. Nanocarriers can be classified into nanocapsules, nanoparticles, and nanospheres depending on their preparation methods. Among them, nanoparticles are the most widely used to treat GBMs and can be classified based on the type of colloidal drug carriers from which they are made of, including liposomes, polymeric nanoparticles, solid lipid nanoparticles, polymeric micelles, silica, and dendrimers.
4.1. Liposomes
The structure of liposomes is similar to that of cell membrane, as they are composed of a water soluble core surrounded by an outer phospholipid membrane. This characteristic increases lipophilicity and enables lipophilic macromolecules to cross the BBB. Liposomal nanoparticles have a lot of advantages, including easy preparation, easy encapsulation of a wide range of anticancer drugs, favorable biocompatibility, efficiency, non-immunogenicity, improved solubility of anticancer agents, and commercial availability [112,113]. Liposomes were initially designed to encapsulate radiosensitizers and chemotherapeutic agents such as doxorubicin for the treatment of various refractory cancers for more than two decades ago [112]. During the last decade, various methods of liposomal formulations for the treatment of GBMs, novel conjugated agents, and receptor-mediated transcytosis have been investigated to facilitate their transport across the BBB [113,114,115]. For example, conjugation of polyethylene glycol (PEG) to the surface of a liposome phospholipid bilayer can extend the half-life of liposomes in the circulation because PEG can help the nanoparticles escape from the capture of RES [84].
Some unique receptors or antigens overexpressed on GBM cells are the potential tumor targets for the development of novel nanotechnology. For example, interleukin (IL)-13-conjugated liposomes and IL-4 receptor-targeted liposomal doxorubicin have been investigated in mouse models, which showed evidence of significant tumor size reduction when compared with unconjugated liposomes [114,115]. This approach does not increase toxicity in animals receiving receptor-conjugated liposomes [114], indicating it as a potential application of nanotechnology. Furthermore, an antibody can be used to label liposomes to target tumors. Anti-EGFR immunoliposomes were developed more than ten years ago to target GBM cells with overexpression of EGFR in an animal model and demonstrated that they can significantly enhance the efficacy of multiple anticancer drugs [116].
Despite the common application of liposomal nanoparticles in GBM treatment, there are some disadvantages we need to overcome. Non-uniform effects across all brain regains are noted in liposomal nanoparticles, and its permeability across the BBB varies depending on the loaded drug or surface molecules [114,115].
4.2. Polymeric Micelles
Polymeric micelles are composed of a hydrophobic polymer core and hydrophilic shell architecture. This architecture is formed through the self-assembly of block copolymers, and the design can modulate the incorporation efficiency and controlled release rate of chemotherapeutic agents [117]. The characteristic core-shell structures and narrow size distribution of 10–100 nm can effectively protect the drug-loaded core from interaction with the complement system and macrophage uptake, which contributes to their prolonged circulation with a long half-life of more than 10 h [118,119]. Poly (caprolactone), poly (D,L-lactide), poly (D,L-lactide-co-glycolide), and long-chain alkyl derivatives are biodegradable polyesters and commonly used as the core-forming polymer [117]. Poly (ethylene glycol) (PEG) is the ideal shell-forming polymer, which can avoid interaction with serum proteins [117,118].
After resolving previous weakness of inadequate drug-circulation time, the major obstacle to the implementation of polymeric micelles-based GBM therapy is the lack of targeting moieties that could allow for greater GBM specific accumulation [117]. Therefore, further effects of targeting specific receptors expressed on GBM cells are ongoing to improve the efficacy of current formulation. For example, polymeric mixed micelles composed of Pluronic P-123 and F-127 containing 17-Allylamino-17-demethoxy geldanamycin (17-AAG) can be a good nanomaterials-based drug delivery carrier because 17-AAG is a potent inhibitor of heat shock protein 90 (Hsp90) and can cause destabilization of Hsp90 related client proteins in cancer cells [119,120]. The design of 17-AAG loaded Pluronic P-123 and F-127 mixed micelles is favorable, and the targeting ability of 17-AAG, controlled release rate and high drug loading have also been documented as a potential delivery system for GBM treatment [119]. Transferrin receptor (TfR) is the promising target site because it is overexpressed on both the BBB and GBM cells. Sun et al. designed the TfR-PEG polymeric micelles, which could be absorbed rapidly by tumor cells, and traversed effectively the BBB [121]. TfR-PEG polymeric micelles loaded with paclitaxel can effectively inhibit the proliferation of U87 GBM cells in vitro, and prolong median survival of nude mice bearing GBMs [121].
4.3. Dendrimers
Dendrimers are the smallest molecules with sizes less than 12 nm and have highly branched and compact scaffolds architecture, which is suitable for transporting short interfering RNA (siRNA) and protecting it from degradation in the circulation [122,123]. Additional advantages of dendrimers loaded with methotrexate include increased drug potency and high efficiency of crossing the BBB [124]. However, dendrimers also have some disadvantages, such as rapid clearance of the RES, toxicity to normal tissue because of interaction with cell membrane, and relatively poorer controlled release behavior [122,125]. Therefore, numerous functionalized strategies, such as attachment of lipid, amino acid, peptide, or aptamer, have been used for modification of dendrimers [123,126].
Recently, poly (amidoamine) (PAMAM) dendrimer-entrapped gold (Au) nanoparticles has been prepared to compact two different siRNA for oncogene silencing. In the newly novel approach, the PAMAM-Au dendrimers are coated with beta-cyclodextrin (β-CD), which has been demonstrated to be efficient carrier for delivery of siRNA to glioma cells [125,126]. The endogenous amino acids improve the biocompatibility and endosomal escape of amino acid functionalized dendrimers, while phosphate dendrimers with hydrophobic backbone and hydrophilic surface can have better penetration through the BBB [127,128]. Another example is the arginine-glycine-aspartic functionalized dendrimer-entrapped gold nanoparticles, which have good cytocompatibility and highly efficient transfection capacity and have been demonstrated as potentially efficient gene therapy for GBMs [127]. Polyether-copolyester (PEPE) dendrimers conjugated with d-glucosamine have been designed to enhance the drug delivery across the BBB and tumor targeting [124]. The in vitro model has showed that glycosylation of the PEPE dendrimers not only increase the rapid accumulation around the tumor spheroids but also overcome MTX resistance because methotrexate-loaded glucosylated PEPE dendrimers was able to kill even MTX-resistant cells [124].
4.4. Metal Particles
Metal particles can enhance radiosensitization of GBM tumor cells and significant DNA damage of tumor cells have been observed in animal models treated with metal particles prior to radiation therapy [129]. The metal particles own characteristics of high X-ray absorption, synthetic versatility, and unique electronic properties, which accounts for their good candidates as radiosensitizers [130]. Among noble metal inorganic nanoparticles, gold nanoparticles (AuNPs) are characterized by easy modification, controllable diameters, and large surface/volume ratios, and are one of the most ideal nanomedicine materials for GBM therapy [131]. The controlled size of AuNPs makes it easily cross the BBB, but its clinical application is limited by lack of targeting ability [131,132].
Recently, a DNA aptamer selected from a large random single-stranded DNA has been prepared to target EGFRvIII of GBMs [132]. The targeting efficiency of aptamer is further enhanced by entrapped into AuNPs through a gold-sulfur covalent bond [132]. The aptamer-AuNP complexes have been demonstrated as a new type of drug candidate for GBM therapy because they showed efficient antitumor effects in vivo and in vitro inhibition of tumor proliferation [132]. The weak transmembrane penetration of aptamer is overcome by the appropriately sized AuNPs. Nanoparticle can also help delivery of the therapeutic gene targets. A novel polyfunctional gold-iron oxide nanoparticle to deliver therapeutic miR-100, the tumor suppressor, was recently designed and proved to enhance sensitization of GMB cells to the systemically administered TMZ in mice [92].
Previous concerns of metal particles include their cytotoxicity and physical damage to the normal tissue after long-term accumulation in the circulation [133]. The mechanisms of metal particle toxicity include induction of oxidative stress, inflammatory cytokine release, lysosome degradation, and DNA destruction [133,134]. However, several gold and silver nanoformulations entrapped with chemotherapeutic agents are already approved by the American Food and Drug Administration for clinical trials, since their biodistribution and mode of clearance are now well understood [135].
4.5. Silica
Silica nanoparticles (SiNPs) have several benefits commonly used in various medical applications, including good biocompatibility, large surface area for drug loading, stability, and inexpensive costs [136]. Previous concerns of their cytotoxicity, DNA destruction, and production of reactive oxygen species limit the clinical application of SiNPs as biomarkers, cancer therapeutics, or drug delivery system [136,137]. Later SiNPs have been investigated in many research areas for their clinical safety and potential applications. Because the SiNPs-induced toxicity can be controlled by appropriate size, dose, and cell type [137,138], researchers now can try multimodal modifications of SiNPs to make it clinically applicable. The greater toxicity of smaller-sized SiNPs can be modified by synthetic modification of SiNPs [139].
For GBM treatment, transferrin-modified porous silica nanoparticles are current popular formulation, which can have high biocompatibility, degradability, and high drug-loaded capacity [140,141]. The transferrin-functionalized pSiNPs can achieve a sustained release of the drug (such as doxorubicin) at the targeted site because transferrin receptor is often overexpressed on the BBB and the surface of GBM cell only. A multicomponent nanoparticle composed of a mesoporous silica shell and an iron oxide core with fibronectin-targeting ligands has also been developed, which can have an efficient, large amount, and widespread drug delivery into the GBM after an external low-power radiofrequency field [142].
4.6. Nanoparticle-Induced Hyperthermia
The combination of hyperthermia and modern radiation and/or chemotherapy has been used for nearly half a century. The mechanisms of hyperthermia-induced radiosensitization and chemosensitization include impaired DNA repair, increased apoptotic pathways, heat-induced inhibition of the AKT signaling pathway, and disruption of the BBB [143,144,145]. Local temperatures up to 45 °C induce GMB cell apoptosis in a murine animal model [144]. Although various techniques, including radiofrequency, ultrasonic waves, water baths or heat blankets, microwaves, laser-induced interstitial thermotherapy, and magnetic nanoparticles (MNPs)—have been used to exert hyperthermic effects on tumors, MNPs have the advantages of direct intratumoral administration, high localized accumulation to create sufficient heat generation in tumors, and good efficacy [144].
MNPs are ideal candidates for CED application for the treatment of GBMs. Real time MRI-guided MNP delivery into the brain via CED has been investigated for decades [69,146]. Iron oxide MNPs are preferred for magnetic hyperthermia due to their high heating capacity and have been designed to therapeutically target cancer cells [146]. Recently, the targeting effects of iron-oxide nanoparticles conjugated with the EGFR inhibitor, cetuximab, were found to have a significant antitumor effect in EGFRvIII-expressing GSCs [146]. Fan et al. also demonstrated a novel theranostic complex of superparamagnetic iron oxide-loaded microbubbles for drug delivery to the brain, and the distribution and quantitative deposition of the agent was also accurately estimated [147].
Although the safety and effectiveness of MNPs can be confirmed by an accurate and reliable treatment plan, the heterogeneous response to magnetic hyperthermia within the GBM mass limits their clinical applications. For example, a transient increase in the growth of the CD133 subtype of gliomas after hyperthermic preconditioning was noted in a recent xenograft model [148]. Furthermore, the issue of MNP toxicity deserves to be further investigated and depends on the chemical composition, surface coatings, physical characteristics of MNPs, and local concentration. For example, MNPs containing iron oxide and titanium are less toxic than those composed of heavy metals, including gold, silver, cobalt, zinc, and cadmium. Recent researchers made use of dextran and bovine serum albumin as the surface coatings of MNPs, which have been demonstrated to reduce toxicity and prevent intravascular coagulation [144,149].
4.7. Nanoparticles as Carriers of Antitumor Antibiotics
Various chemotherapeutic agents including doxorubicin, bleomycin, epirubicin, daunorubicin, and actinomycin D are classified as antitumor antibiotics because they are produced by Streptomyces bacteria and cause cell death by interfering with DNA replication and damaging DNA in GBM cells [150]. These antitumor antibiotics show great antitumor effects on GBM cells in vitro, but are ineffective in vivo due to their poor ability to penetrate the BBB [50]. The resolution is to encapsulate these chemotherapeutic agents in PEGylated liposomes and apply effective drug delivery strategies [61]. For example, the loading of doxorubicin in poly (lactide-co-glycolide) nanoparticles coated with poloxamer 188 (Dox-PLGA) enables enhanced brain delivery [61,151]. Another example is ultrasound-induced microbubbles, which effectively deliver drugs, such as liposomal doxorubicin, to enter the brain through a transient opening of the BBB in a rat glioma model [152].
5. Clinical Trials
Although numerous in vivo and in vitro studies have been conducted to prove the efficiency and therapeutic potential of nanotechnology and/or nanocarriers-based treatment of GBM, few clinical trials using nanotherapies to target GBM have been completed. The information about clinical trials focusing on GBM treatment is summarized in Table 1.

Table 1.
Clinical trials using nanotechnology and nanocarrier-based delivery systems for treating glioblastoma multiforme.
A combination of TAZ and pegylated liposomal doxorubicin (PEG-Dox) has been commonly used as the post-operative treatment for newly diagnosed GBM patients following chemo-radiotherapy and also for patients with high grade recurrent GBMs since nearly two decades ago [157,158,159,160,161]. These clinical trials found that liposomal doxorubicin was tolerable and feasible, with the main side effects being palmaroplantar erythrodysesthesia and myelosupression. The 12-month progression free survival after combined TAZ and PEG-Dox regimen was reported to range between 15–30.2% and the median overall survival was 13.4–17.6 months. Although combination of TAZ and PEG-Dox is well tolerated, it does not add significant benefit regarding patients’ outcomes [157,158].
The EnGenelC delivery vehicle (EDV) is a novel nanocellular (minicell) compound that can encapsulate adequate concentrations of chemotherapeutic agents to target EGFR, overexpressed in 40–50% of patients with GBMs [154]. Therefore, a phase I study of EDVDox (EDV containing doxorubicin) was conducted in 14 GBM patients. This new regimen is well tolerated with only nausea, fever, and chills or rigors experienced in some of patients [154]. No previous side effects of palmaroplantar erythrodysesthesia and myelosupression were observed in these patients. Although all of these combination regimens are effective and well tolerated in clinical trials, none has been documented to significantly result in a meaningful improvement of a patient’s outcome [154,157,158,159,160,161]. It can be hypothesized that these nanoformulations would help to improve GBM treatment, but the clinical significance is limited by inadequate case numbers.
Hyperthermia can increase the cytotoxic effects of radiotherapy. Therefore, magnetic iron-oxide nanoparticles are used and directly injected into the tumor and subsequently stimulated by an alternating magnetic field to generate heat [153]. This approach has been demonstrated feasible and effective in animal models [144,146], and later in clinical trials [153,162]. In Europe, a MNP compound (NanoThem® AS1; MagForce Nanotechnologies AG, Berlin, Germany) for magnetic hyperthermia application in combination with radiotherapy for patients with recurrent GBM has been approved [154]. However, there are several obstacles for clinical application of MNPs, including the difficulty of accurate intratumoural heating and precise temperature control at the GBM site, the presences of pacemakers and defibrillators as the contraindication, and the removal of dental filling, implants, and crowns [153,162].
Currently some new nanoformulations, including EnGenelC delivery vehicle (EDV)-doxorubicin, 5-fluorouracil-releasing microspheres, or Semliki Forest virus vector carrying IL-12 gene encapsulated in cationic liposomes have been proven safe and efficient in GBM patients [154,155,156], but the beneficial effects of these regimens require further clinical trials. In addition, a phase II study of combined TAZ and targeted P53 gene therapy (SGT-53, previously conducted on other solid tumors [163]) for treatment of patients with recurrent GBM is currently recruiting participants.
6. Conclusions
GBMs are well known for the poor prognosis and current therapeutic strategies have not improved overall survival or progression free survival. Because of their characteristic size, shape, and surface properties, nanoparticles are capable of encapsulating and delivering therapeutic molecules to the brain. In clinical trials, these nanoformulations combined with oral TAZ and radiotherapy have been used in GBM patients after maximal resection and are well tolerated in most patients. The MNPs for hyperthermia allow for a tumor-specific and sustained effect, and GBM cells can be sensitized to radio-chemotherapy through hyperthermia effects. However, none of these regimens do significantly improve patients’ outcome in terms of progression free survival or overall survival. The limitation of these clinical trials may be due to a lack of large number or at least sufficient patients to reach statistical significance.
Future direction of nanotechnology and clinical applications may consider monoclonal antibodies, combining GSC-targeting SGT-53 with traditional TMZ, or novel nanoformulations loaded with therapeutic miRNAs to improve immunotherapy and antiangiogenic processes. MNPs are the promising nanoparticles for intratumoral hyperthermia therapy in patients with GBM. However, most effects of various nanoformulations on GBM cell models cannot be replicated in actual clinical trials because tumor heterogeneity remains unpredictable and the major obstacle for successful GBM treatment.
Author Contributions
Conceptualization: J.-F.H. and S.-M.C. writing—original drift: J.-F.H., C.-C.L., M.-Y.L., H.-C.W., and H.-R.H. writing—images, figures, and figure legends: C.-J.W. and Y.-S.W. writing—review, revise, and editing: M.-H.T. All authors have read and approved the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Chang Gung Memorial Hospital (CMRPG3H1631).
Data Availability Statement
The datasets used/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
All authors thank Chiao-Ching Chiang for help English editing, which contributed greatly to the completion of this research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AuNPs | gold nanoparticles |
| BBB | blood brain barrier |
| EGFR | epidermal growth factor receptor |
| GBM | glioblastoma multiforme |
| GSC | glioblastoma stem cell |
| IDH | isocitrate dehydrogenase |
| MNPs | magnetic nanoparticles |
| PEG-Dox | pegylated liposomal doxorubicin |
| PEG | Poly (ethylene glycol) |
| TMZ | temozolomide |
| 17-AAG | 17-Allylamino-17-demethoxy geldanamycin |
| TfR | Transferrin receptor |
| Pgp | P-glycoprotein |
| RES | the reticuloendothelial system |
| SiNPs | silica nanoparticles |
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20 (Suppl 4), iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Karcher, S.; Steiner, H.H.; Ahmadi, R.; Zoubaa, S.; Vasvari, G.; Bauer, H.; Unterberg, A.; Herold-Mende, C. Different angiogenic phenotypes in primary and secondary glioblastomas. Int. J. Cancer 2006, 118, 2182–2189. [Google Scholar] [PubMed]
- Renault, I.Z.; Golgher, D. Molecular genetics of glioblastomas: Defining subtypes and understanding the biology. Neuroimaging Clin. N. Am. 2015, 25, 97–103. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, W.; Cao, W.D.; Cheng, G.; Zhang, Y.Q. Glioblastoma multiforme: Molecular characterization and current treatment strategy. Exp. Ther. Med. 2012, 3, 9–14. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Chakrabarti, I.; Cockburn, M.; Cozen, W.; Wang, Y.P.; Preston-Martin, S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer 2005, 104, 2798–2805. [Google Scholar] [CrossRef]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of gender on the survival of patients with glioblastoma. Biosci. Rep. 2018, 38, BSR20180752. [Google Scholar] [CrossRef]
- Korja, M.; Raj, R.; Seppä, K.; Luostarinen, T.; Malila, N.; Seppälä, M.; Mäenpää, H.; Pitkäniemi, J. Glioblastoma survival is improving despite increasing incidence rates: A nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2019, 21, 370–379. [Google Scholar] [CrossRef]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain tumors: Rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor. J. Environ. Public Health 2018, 2018, 7910754. [Google Scholar]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the brain and CNS: Global patterns and trends in incidence. Neuro Oncol. 2017, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Itakura, H.; Achrol, A.S.; Mitchell, L.A.; Loya, J.J.; Liu, T.; Westbroek, E.M.; Feroze, A.H.; Rodriguez, S.; Echegaray, S.; Azad, T.D.; et al. Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci. Transl. Med. 2015, 7, 303ra138. [Google Scholar] [CrossRef]
- Johnson, D.R.; Guerin, J.B.; Giannini, C.; Morris, J.M.; Eckel, L.J.; Kaufmann, T.J. 2016 Updates to the WHO brain tumor classification system: What the radiologist needs to know. Radiographics 2017, 37, 2164–2180. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.M.; Huang, R.Y. An update on the approach to the imaging of brain tumors. Curr. Neurol. Neurosci. Rep. 2017, 17, 53. [Google Scholar] [CrossRef]
- Smirniotopoulos, J.G.; Murphy, F.M.; Rushing, E.J.; Rees, J.H.; Schroeder, J.W. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007, 27, 525–551. [Google Scholar] [CrossRef]
- Palanichamy, K.; Chakravarti, A. Diagnostic and prognostic significance of methionine uptake and methionine positron emission tomography imaging in Gliomas. Front. Oncol. 2017, 7, 257. [Google Scholar] [CrossRef]
- Novy, Z.; Stepankova, J.; Hola, M.; Flasarova, D.; Popper, M.; Petrik, M. Preclinical evaluation of radiolabeled peptides for PET imaging of Glioblastoma Multiforme. Molecules 2019, 24, 2496. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [PubMed]
- Choi, S.W.; Cho, H.H.; Koo, H.; Cho, K.R.; Nenning, K.H.; Langs, G.; Furtner, J.; Baumann, B.; Woehrer, A.; Cho, H.J.; et al. Multi-Habitat radiomics unravels distinct phenotypic subtypes of glioblastoma with clinical and genomic signature. Cancers 2020, 12, 1707. [Google Scholar] [CrossRef]
- Gevaert, O.; Mitchell, L.A.; Achorl, A.S.; Xu, J.; Echegaray, S.; Steinberg, G.K.; Cheshier, S.H.; Nape, S.; Zaharchuk, G.; Plevritis, S.K. Glioblastoma multiforme: Exploratory radiogenomic analysis by using quantitative image features. Radiology 2014, 273, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Narita, Y.; Miyakita, Y.; Ohno, M.; Fukushima, S.; Kayama, T.; Shibui, S. Pathological findings and prognostic factors in recurrent glioblastomas. Brain Tumor Pathol. 2012, 29, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, M.; Kajiwara, K.; Goto, H.; Sugimoto, K.; Nomura, S.; Ikeda, E.; Suzuki, M. MRI findings and pathological features in early-stage glioblastoma. J. Neurooncol. 2015, 123, 289–297. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Mock, A.; Louis, D.N. The 2016 WHO classification of central nervous system tumors: What neurologists need to know. Curr. Opin. Neurol. 2017, 30, 643–649. [Google Scholar] [CrossRef]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the treatment of Glioblastoma: Multisystem mechanisms of therapeutic resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef]
- Orringer, D.; Lau, D.; Khatri, S.; Zamora-Berridi, G.J.; Zhang, K.; Wu, C.; Chaudhary, N.; Sagher, O. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012, 117, 851–859. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Graffeo, C.S.; Perry, A.; Burns, T.C.; Parney, I.F.; Alfredo Quinones-Hinojosa, A.; Chaichana, K.L. Survival benefit of maximal resection for Glioblastoma reoperation in the Temozolomide era: A meta-analysis. World Neurosurg. 2019, 127, 31–37. [Google Scholar] [CrossRef]
- Corso, C.D.; Bindra, R.S.; Mehta, M.P. The role of radiation in treating glioblastoma: Here to stay. J. Neurooncol. 2017, 134, 479–485. [Google Scholar] [CrossRef]
- Lopez Perez, R.; Nicolay, N.H.; Wolf, J.C.; Frister, M.; Schmezer, P.; Weber, K.J.; Huber, P.E. DNA damage response of clinical carbon ion versus photon radiation in human glioblastoma cells. Radiother. Oncol. 2019, 133, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; McEllin, B.; Camacho, C.V.; Tomimatsu, N.; Sirasanagandala, S.; Nannepaga, S.; Hatanpaa, K.J.; Mickey, B.; Madden, C.; Maher, E.; et al. EGFRvIII and DNA double-strand break repair: A molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009, 69, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Todorova, P.K.; Fletcher-Sananikone, E.; Mukherjee, B.; Kollipara, R.; Vemireddy, V.; Xie, X.J.; Guida, P.M.; Story, M.D.; Hatanpaa, K.; Habib, A.A.; et al. Radiation-induced DNA damage cooperates with heterozygosity of TP53 and PTEN to generate high-grade gliomas. Cancer Res. 2019, 79, 3749–3761. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg. Rev. 2018, 41, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef]
- Sharma, M.; Schroeder, J.L.; Elson, P.; Meola, A.; Barnett, G.H.; Vogelbaum, M.A.; Suh, J.H.; Chao, S.T.; Mohammadi, A.M.; Stevens, G.H.J.; et al. Outcomes and prognostic stratification of patients with recurrent glioblastoma treated with salvage stereotactic radiosurgery. J. Neurosurg. 2018, 131, 489–499. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoom, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhou, C.F.; Lin, Z.X. Temozolomide and radiotherapy for newly diagnosed glioblastoma multiforme: A systematic review. Cancer Investig. 2014, 32, 31–36. [Google Scholar] [CrossRef]
- Kole, A.J.; Park, H.S.; Yeboa, D.N.; Rutter, C.E.; Corso, C.D.; Aneja, S.; Lester-Coll, N.H.; Mancini, B.R.; Knisely, J.P.; Yu, J.B. Concurrent chemoradiotherapy versus radiotherapy alone for “biopsy only” glioblastoma multiforme. Cancer 2016, 12, 2364–2370. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Q.; Wang, Y.A.; Hua, S.; Sauvé, C.G.; Ong, D.; Lan, Z.D.; Chang, Q.; Ho, Y.W.; Monasterio, M.M.; et al. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell 2016, 167, 1281–1295.e18. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.B.; Sievers, C.; Donohue, L.K.; Gillespie, S.M.; Flavahan, W.A.; Miller, T.E.; Venteicher, A.S.; Hebert, C.H.; Carey, C.D.; Rodig, S.J.; et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell 2017, 20, 233–246.e7. [Google Scholar] [CrossRef] [PubMed]
- Nakod, P.S.; Kim, Y.; Rao, S.S. Biomimetic models to examine microenvironmental regulation of glioblastoma stem cells. Cancer Lett. 2018, 429, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef]
- Eskilsson, E.; Røsland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol. 2018, 20, 743–752. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef]
- Jin, X.; Kim, L.J.Y.; Wu, Q.; Wallace, L.C.; Prager, B.C.; Sanvoranart, T.; Gimple, R.C.; Wang, X.; Mack, S.C.; Miller, T.E.; et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat. Med. 2017, 23, 1352–1361. [Google Scholar] [CrossRef]
- Pointer, K.B.; Clark, P.A.; Zorniak, M.; Alrfaei, B.M.; Kuo, J.S. Glioblastoma cancer stem cells: Biomarker and therapeutic advances. Neurochem. Int. 2014, 71, 1–7. [Google Scholar] [CrossRef]
- Dréan, A.; Goldwirt, L.; Verreault, M.; Canney, M.; Schmitt, C.; Guehennec, J.; Delattre, J.Y.; Carpentier, A.; Idbaih, A. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev. Neurother. 2016, 16, 1285–1300. [Google Scholar] [CrossRef]
- Papademetriou, I.T.; Porter, T. Promising approaches to circumvent the blood-brain barrier: Progress, pitfalls and clinical prospects in brain cancer. Ther. Deliv. 2015, 6, 989–1016. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumor barrier in brain tumors and metastasis. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sprowls, S.A.; Arsiwala, T.A.; Bumgarner, J.R.; Shah, N.; Lateef, S.S.; Kielkowski, B.N.; Lockman, P.R. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer 2019, 5, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, C.F.; Wu, S.K.; Chen, L.F.; Hsieh, W.Y.; Lin, W.L. Targeting microbubbles-carrying TGFβ1 inhibitor combined with ultrasound sonication induce BBB/BTB disruption to enhance nanomedicine treatment for brain tumors. J. Control. Release 2015, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Straten, D.V.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Fan, S.; Zheng, Y.; Liao, S.; Xiong, Y.; Li, Y.; Liu, J. Recent advances on glioblastoma multiforme and nano-drug carriers: A review. Curr. Med. Chem. 2019, 26, 5862–5874. [Google Scholar] [CrossRef]
- Zhou, J.; Patel, T.R.; Sirianni, R.W.; Strohbehn, G.; Zheng, M.Q.; Duong, N.; Schafbauer, T.; Huttner, A.J.; Huang, Y.; Carson, R.E.; et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. USA 2013, 110, 11751–11756. [Google Scholar] [CrossRef]
- Laquintana, V.; Denora, N.; Cutrignelli, A.; Perrone, M.; Iacobazzi, R.M.; Annese, C.; Lopalco, A.; Lopedota, A.A.; Franco, M. TSPO ligand-methotrexate prodrug conjugates: Design, synthesis, and biological evaluation. Int. J. Mol. Sci. 2016, 17, 967. [Google Scholar] [CrossRef]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Wilson Miller, G.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef]
- Lundy, D.J.; Lee, K.J.; Peng, I.C.; Hsu, C.H.; Lin, J.H.; Chen, K.H.; Tien, Y.W.; Hsieh, P.C.H. Inducing a transient increase in blood-brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme. ACS Nano 2019, 13, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tan, Y.; Dai, S.; Zhu, Y.; Meng, T.; Yang, X.; Liu, Y.; Liu, X.; Yuan, H.; Hu, F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalvass, J.C.; Polli, J.W.; Bourdet, D.L.; Feng, B.; Huang, S.M.; Liu, X.; Smith, Q.R.; Zhang, L.K.; Zamek-Gliszczynski, M.J. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: The ITC evidence-based position. Clin. Pharmacol. Ther. 2013, 94, 80–94. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Abate, C.; Rolando, B.; Battaglia, L.; Gazzano, E.; Colombino, E.; Costanzo Costamagna, C.; Annovazzi, L.; Mellai, M.; Berardi, F.; et al. Validation of thiosemicarbazone compounds as P-Glycoprotein inhibitors in human primary brain-blood barrier and glioblastoma stem cells. Mol. Pharm. 2019, 16, 3361–3373. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Chung, A.H.; Parrish, K.E.; Crabtree, D.; Halvorson, K.G.; Hu, G.; Elmquist, W.F.; Becher, O.J. ABCG2 and ABCB1 limit the efficacy of Dasatinib in a PDGF-B-Driven brainstem glioma model. Mol. Cancer Ther. 2016, 15, 819–829. [Google Scholar] [CrossRef]
- Pinzon-Daza, M.; Garzon, R.; Couraud, P.; Romero Ia Weksler, B.; Ghigo, D.; Bosia, A.; Riganti, C. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier. Br. J. Pharmacol. 2012, 167, 1431–1447. [Google Scholar] [CrossRef]
- Brown, C.B.; Jacobs, S.; Johnson, M.P.; Southerland, C.; Threatt, S. Convection-enhanced delivery in the treatment of glioblastoma. Semin. Oncol. Nurs. 2018, 34, 494–500. [Google Scholar] [CrossRef]
- Ung, T.H.; Malone, H.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery for glioblastoma: Targeted delivery of antitumor therapeutics. CNS Oncol. 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-enhanced delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Frellsen, A.F.; Hansen, A.E.; Jolck, R.I.; Kempen, P.J.; Severin, G.W.; Rasmussen, P.H.; Kjær, A.; Jensen, A.T.I.; Andresen, T.L. Mouse positron emission tomography study of the biodistribution of gold nanoparticles with different surface coating using embedded copper-64. ACS Nano 2016, 10, 9887–9898. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Davoodi, P.; Zhan, W.; Chow, P.K.; Wang, C.H. Development of nanoparticles for drug delivery to brain tumor: The effect of surface materials on penetration into brain tissue. J. Pharm. Sci. 2019, 108, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater. 2015, 24, 140–151. [Google Scholar] [CrossRef]
- Gagliardi, M.; Borri, C. Polymer nanoparticles as smart carriers for the enhanced release of therapeutic agents to the CNS. Curr. Pharm. Des. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Gang Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoE-Reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedcine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; López, B.L.; Ligia Sierra, L. PEG-g-chitosan nanoparticles functionalized with monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervious system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016, 8, 749–764. [Google Scholar]
- Chen, E.M.; Quijano, A.R.; Seo, Y.E.; Jackson, C.; Josowitz, A.D.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Zhaozhong Jiang, Z.; et al. Biodegradable PEG-poly (omega-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Preat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Alphandery, E.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Development of non-pyrogenic magnetosome minerals coated with poly-I-lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials 2017, 141, 210–222. [Google Scholar] [CrossRef]
- Alphandery, E.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R.; et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef] [PubMed]
- Bruinsmann, F.A.; Richter Vaz, G.; de Cristo Soares Alves, A.; Aguirre, T.; Raffin Pohlmann, A.; Staniscuaski Guterres, S.; Sonvico, F. Nasal drug delivery of anticancer drugs for the treatment of glioblastoma: Preclinical and clinical trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Rudzinska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Singh, M.S.; Lamprecht, A. Cargoing P-gp inhibitors via nanoparticle sensitizes tumor cells against doxorubicin. Int. J. Pharm. 2015, 478, 745–752. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.D.P.; Espinha, P.L.; et al. Therapeutic efficacy of multiple applications of magnetic hyperthermia technique in glioblastoma using aminosilane coated iron oxide nanoparticles: In vitro and in vivo study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef]
- Grillone, A.; Battaglini, M.; Moscato, S.; Mattii, L.; de Julian Fernandez, C.; Scarpellini, A.; Giorgi, M.; Sinibaldi, E.; Ciofani, G. Nutlin-loaded magnetic solid lipid nanoparticles for targeted glioblastoma treatment. Nanomedicine 2019, 14, 727–752. [Google Scholar] [CrossRef]
- Pucci, C.; De Pasquale, D.; Marino, A.; Martinelli, C.; Lauciello, S.; Ciofani, G. Hybrid magnetic nanovectors promote selective glioblastoma cell death through a combined effect of lysosomal membrane permeabilization and chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 29037–29055. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Muniyandi, P.; Maekawa, T.; Kumar, D.S. Vasicular systems employing natural substances as promising drug candidates for MMP inhibition in glioblastoma: A nanotechnological approach. Int. J. Pharm. 2018, 551, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.; Liu, X.; Lv, W.; Zhang, H.; Zhang, M.; Li, X.; Xin, H.; Xu, Q. Enhanced antiglioma efficacy of Ultrahigh loading capacity paclitaxel prodrug conjugate self-assembled targeted nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.C.; Roth, I.M.; Wickremesekera, A.C.; Davis, P.F.; Kaye, A.H.; Mantamadiotis, T.; Stylli, S.S.; Tan, S.T. Therapeutic targeting of cancer stem cells in human glioblastoma by manipulating the renin-angiotensin system. Cells 2019, 8, 1364. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, R. Gliobalstoma stem cells as a new therapeutic target for glioblastoma. Clin. Med. Insights Oncol. 2015, 9, 95–103. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Zatsepilova, T.A.; Preferanskaya, N.G.; Stepanova, O.I.; Sokolov, A.V.; Dostdar, S.A.; Minyaeva, N.N.; Neganova, M.E.; et al. Feasibility of targeting glioblastoma stem cells: From concept to clinical trials. Curr. Top. Med. Chem. 2019, 19, 2974–2984. [Google Scholar] [CrossRef]
- Kunoh, T.; Shimura, T.; Kasai, T.; Matsumoto, S.; Mahmud, H.; Khayrani, A.C.; Seno, M.; Kunoh, H.; Takada, J. Use of DNA-generated gold nanoparticles to radiosensitize and eradicate radioresistant glioma stem cells. Nanotechnology 2019, 30, 055101. [Google Scholar] [CrossRef]
- Lepinoux-Chambaud, C.; Eyer, J. The NFL-TBS.40–63 peptide targets and kills glioblastoma stem cells derived from human patients and also targets nanocapsules into these cells. Int. J. Pharm. 2019, 566, 218–228. [Google Scholar] [CrossRef]
- Saalik, P.; Lingasamy, P.; Toome, K.; Mastandrea, I.; Rousso-Noori, L.; Tobi, A.; Simón-Gracia, L.; Hunt, H.; Paiste, P.; Kotamraju, V.R.; et al. Peptide-guided nanoparticles for glioblastoma targeting. J. Control. Release 2019, 308, 109–118. [Google Scholar] [CrossRef]
- Goncalves, D.P.N.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gür, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R.T.; et al. Enhanced targeting of invasive glioblastoma cells by peptide-functionalized gold nanorods in hydrogel-based 3D culture. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, A.R.; Kim, S.H.; Lee, S.J.; Chung, H.; Yoon, M.Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted nanotechnology in glioblastoma multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Slagle-Webb, B.; Wang, X.; Yang, Q.X.; Antonetti, D.A.; Miller, P.A.; Sheehan, J.M.; Connor, J.R. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol. Cancer Ther. 2009, 8, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wong, T.T.; Teng, M.C.; Liu, R.S.; Lu, M.; Liang, H.F.; Wei, M.C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Limasale, Y.D.; Tezcaner, A.; Ozen, C.; Keskin, D.; Banerjee, S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int. J. Pharm. 2015, 479, 364–373. [Google Scholar] [CrossRef]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef]
- Saxena, V.; Hussain, M.D. Formulation and in vitro evaluation of 17-allyamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric mixed micelles for glioblastoma multiforme. Colloids Surf. B Biointerfaces 2013, 112, 350–355. [Google Scholar] [CrossRef]
- Talaei, S.; Mellatyar, H.; Asadi, A.; Akbarzadeh, A.; Sheervalilou, R.; Zarghami, N. Spotlight on 17-AAG as an Hsp90 inhibitor for molecular targeted cancer treatment. Chem. Biol. Drug Des. 2019, 93, 760–786. [Google Scholar] [CrossRef]
- Sun, P.; Xiao, Y.; Di, Q.; Ma, W.; Ma, X.; Wang, Q.; Chen, W. Transferrin receptor-targeted PEG-PLA polymeric micelles for chemotherapy against glioblastoma multiforme. Int. J. Nanomed. 2020, 15, 6673–6688. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of amino-functional polyester dendrimers based on bis-MPA as novel vectors for siRNA delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef] [PubMed]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface engineered dendrimers in siRNA delivery and gene silencing. Curr. Pharm. Des. 2017, 23, 2952–2975. [Google Scholar] [CrossRef] [PubMed]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capacity. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Kong, L.; Cao, X.; Li, A.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Shi, X. Enhanced delivery of therapeutic siRNA into glioblastoma cells using dendrimer-entrapped gold nanoparticles conjugated with beta-cyclodextrin. Nanomaterials 2018, 8, 131. [Google Scholar] [CrossRef]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Dolatabadi, J.E.N.; Losic, D. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Alves, C.S.; Shi, X. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine 2016, 11, 3103–3115. [Google Scholar] [CrossRef]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Estève, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y.; et al. Aptamer-conjugated gold nanoparticles targeting epidermal growth factor receptor variant III for the treatment of glioblastoma. Int. J. Nanomed. 2020, 15, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef]
- Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology 2015, 48, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018, 30, e1704307. [Google Scholar] [CrossRef] [PubMed]
- Kretowski, R.; Kusaczuk, M.; Naumowicz, M.; Kotynska, J.; Szynaka, B.; Cechowska-Pasko, M. The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials 2017, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size dose and cell type. Nanomedicine 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomedicine 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The size-dependent cytotoxicity of amorphous silica nanoparticles: A systemic review of in vitro studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef]
- Luo, M.; Lewik, G.; Ratcliffe, J.C.; Choi, C.H.J.; Makila, E.; Tong, W.Y.; Voelcker, N.H. Systemic evaluation of transferring-modified porous silicon nanoparticles for targeted delivery of doxorubicin to glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 33637–33649. [Google Scholar] [CrossRef]
- Sheykhzadeh, S.; Luo, M.; Peng, B.; White, J.; Abdalla, Y.; Tang, T.; Mäkilä, E.; Voelcker, N.H.; Tong, W.Y. Transferrin-targeted porous silicon nanoparticles reduce glioblastoma cell migration across tight extracellular space. Sci. Rep. 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Turan, O.; Bielecki, P.A.; Perera, V.; Lorkowski, M.; Covarrubias, G.; Tong, K.; Yun, A.; Loutrianakis, G.; Raghunathan, S.; Park, Y.; et al. Treatment of glioblastoma using multicomponent silica nanoparticles. Adv. Ther. 2019, 2, 1900118. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, M.; Stauffer, P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin. Oncol. 2014, 41, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Fan, C.H.; Cheng, Y.H.; Ting, C.Y.; Ho, Y.J.; Hsu, P.H.; Liu, H.L.; Yeh, C.K. Ultrasound/Magmetic targeting with SIPO-DOX-Microbubble complex for image-guided drug delivery in brain tumors. Theranostics 2016, 6, 1542–1556. [Google Scholar] [CrossRef]
- Zeng, X.; Han, I.; Abd-El-Barr, M.; Aljuboori, Z.; Anderson, J.E.; Chi, J.H.; Zafonte, R.D.; Teng, Y.D. The effects of thermal preconditioning on oncogenic and intraspinal cord growth features of human glioma cells. Cell Transplant. 2016, 25, 2099–2109. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorkov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef]
- Shao, R.G. Pharmacology and therapeutic applications of enediyne antitumor antibiotics. Curr. Mol. Pharmacol. 2008, 1, 50–60. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Park, J.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: Evaluation during tumor progression in a rat glioma model. Phys. Med. Biol. 2015, 60, 2511–2527. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J. Clin. Neurosci. 2015, 22, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Boulikas, T.; Lundstrom, K.; Soling, A.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene-a phase I/II clinical protocol. J. Neurooncol. 2003, 64, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Capelle, L.; Guyotat, J.; Fuentes, S.; Assaker, R.; Bataille, B.; François, P.; Dorwling-Carter, D.; Paquis, P.; Bauchet, L.; et al. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: A randomized phase II trial. Neurosurgery 2005, 56, 242–248. [Google Scholar] [CrossRef]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma—A phase II study. BMC Cancer 2009, 9, 308. [Google Scholar] [CrossRef]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A.; COGNO. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef]
- Fabel, K.; Dietrich, J.; Hau, P.; Wismeth, C.; Winner, B.; Przywara, S.; Steinbrecher, A.; Ullrich, W.; Bogdahn, U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 2001, 92, 1936–1942. [Google Scholar] [CrossRef]
- Chua, S.L.; Rosenthal, M.A.; Wong, S.S.; Ashley, D.M.; Woods, A.M.; Dowling, A.; Cher, L.M. Phase 2 study of temozolomide and Caelyx in patients with recurrent glioblastoma multiforme. Neuro Oncol. 2004, 6, 38–43. [Google Scholar] [CrossRef]
- Glas, M.; Koch, H.; Hirschmann, B.; Jauch, T.; Steinbrecher, A.; Herrlinger, U.; Bogdahn, U.; Hau, P. Pegylated liposomal doxorubicin in recurrent malignant glioma: Analysis of a case series. Oncology 2007, 72, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Deimling, A.V.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Nemunaitis, J.; Leung, P.K.; Nunan, R.; Adams, J.; Chang, E.H. Safety and efficacy in advanced solid tumors of a targeted nanocomplex carrying the p53 gene used in combination with Docetaxel: A phase 1b study. Mol. Ther. 2016, 24, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).