High-Fat Diet-Induced Obese Effects of Adipocyte-Specific CXCR2 Conditional Knockout in the Peritoneal Tumor Microenvironment of Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Adipocyte-Specific CXCR2 cKO Mice
2.2. Western Blots
2.3. Histological Analysis of Adipose Tissues
2.4. Proteomic Array for Chemokine Signature
2.5. Mouse Peritoneal Dissemination of OC in a Postmenopausal Obese Mouse Model
2.6. Cellular Characteristics in OC-Induced Ascites
2.7. Biochemical Analyses in OC-Induced Ascites
2.8. Histological and Immunohistochemical Evaluation of Tumor Tissues
2.9. Statistical Analysis
3. Results
3.1. Adipocyte-Specific CXCR2 cKO Mice Have Low Levels of CCL2/6 in Adipose Tissues Compared to Those in CXCR2 WT Mice
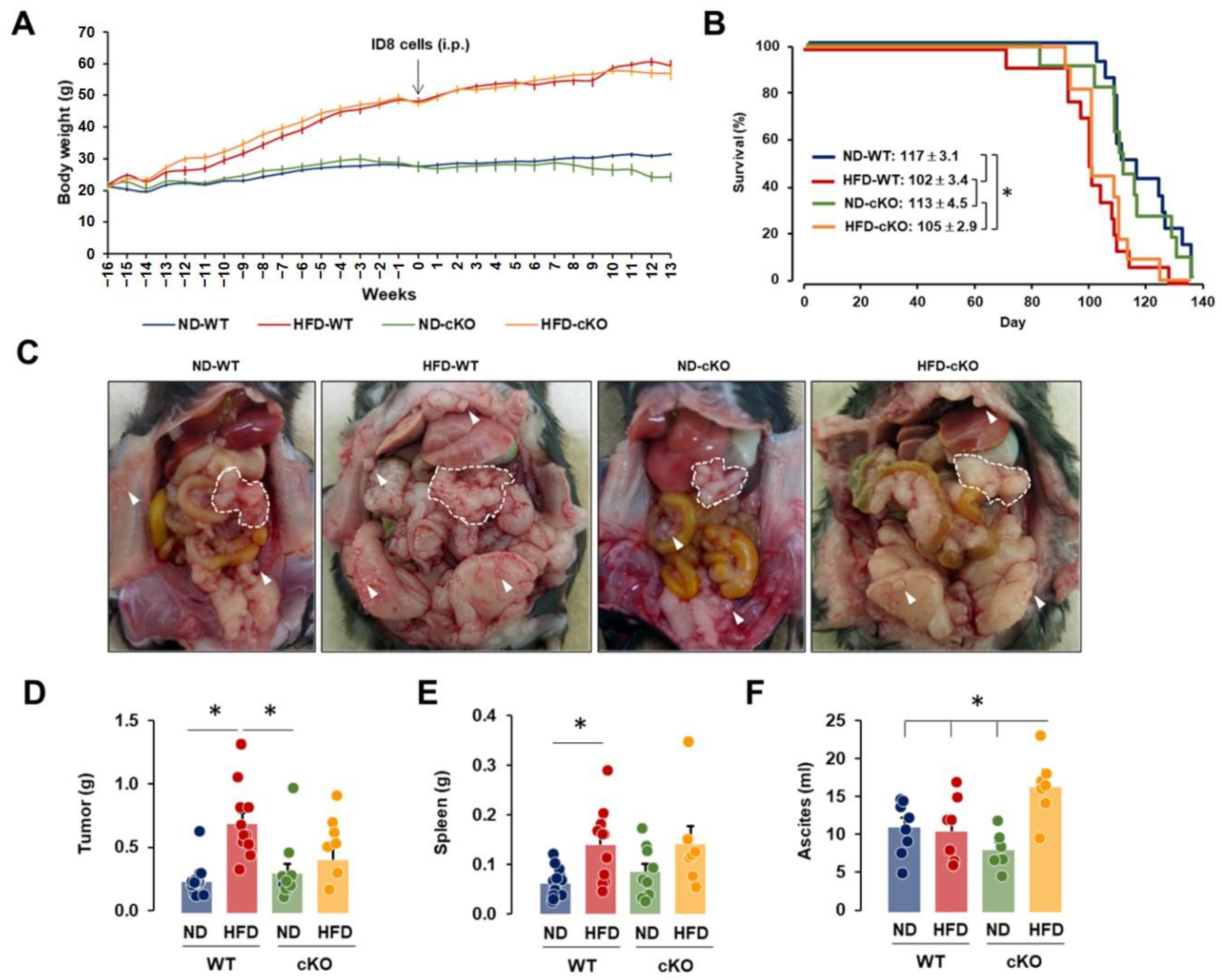
3.2. Obese CXCR2 WT Mice Have Greater Tumor Burdens, While Obese CXCR2 cKO Mice Have Greater Ascites Burdens
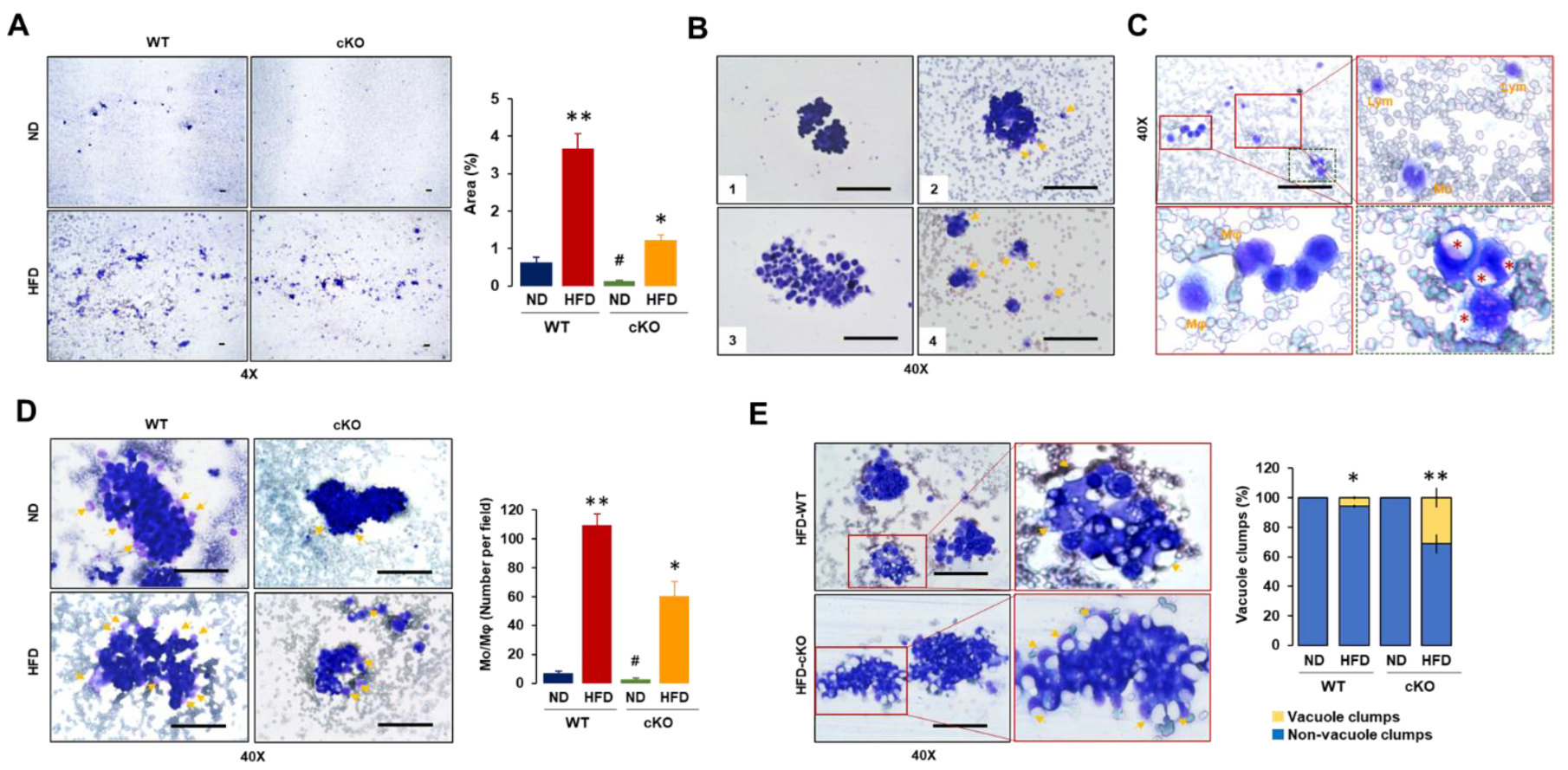
3.3. Adipocyte-Specific CXCR2 cKO Mice Have Lower Floating Tumor Burdens and Tumor-Attached Monocytes/Macrophages but Increased Vacuole Clumps in OC-Induced Ascites after Diet-Induced Obesity
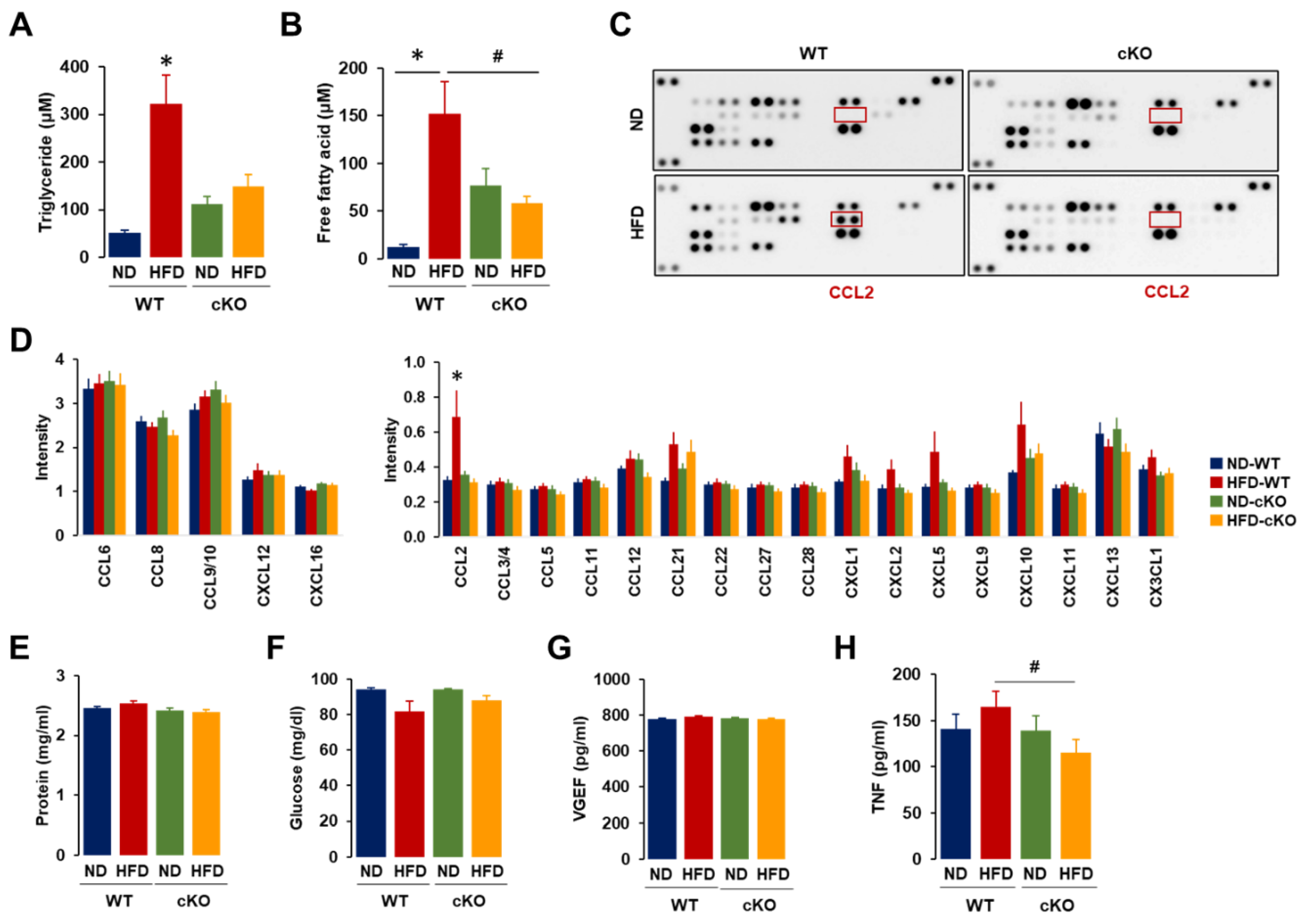
3.4. Ascites in Obese CXCR2 cKO Mice Showed Lower Levels of Triglycerides, Free Fatty Acids, CCL2, and TNF Compared to Those in Obese WT Mice
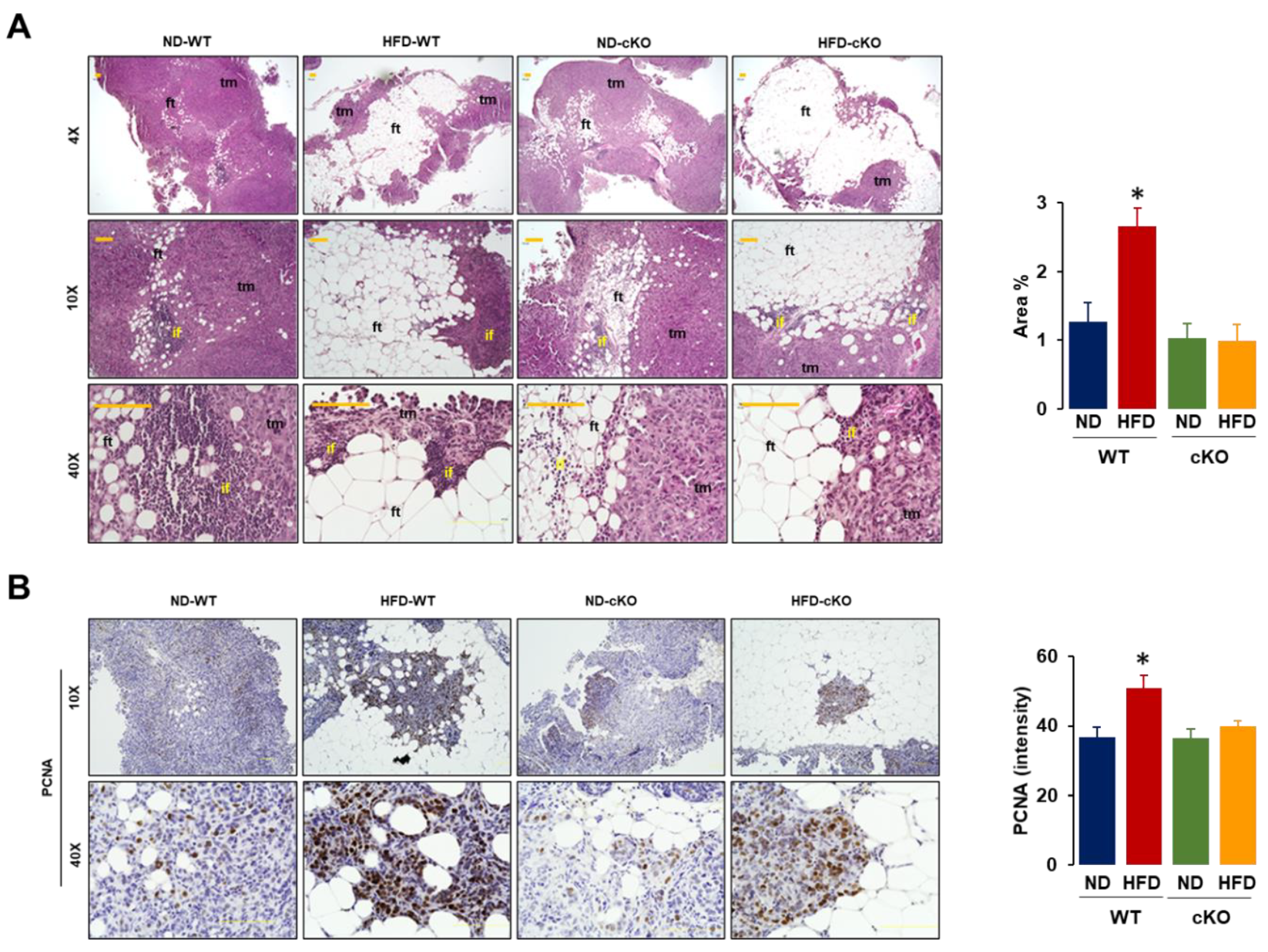
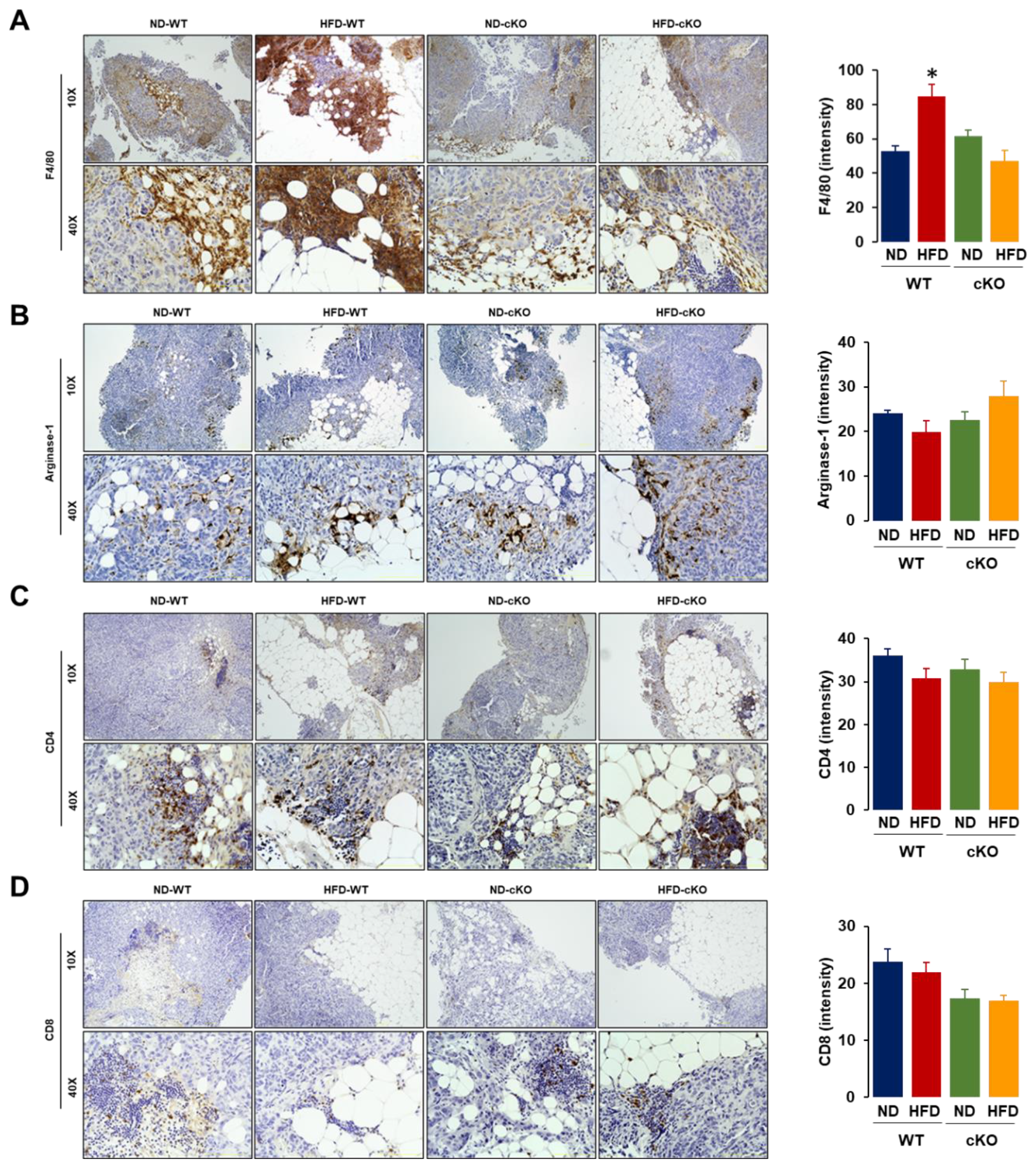
3.5. Obese CXCR2 cKO Mice Show Attenuated Inflammatory Area, PCNA, and F4/80 in Tumor Tissues Compared to Obese WT Mice
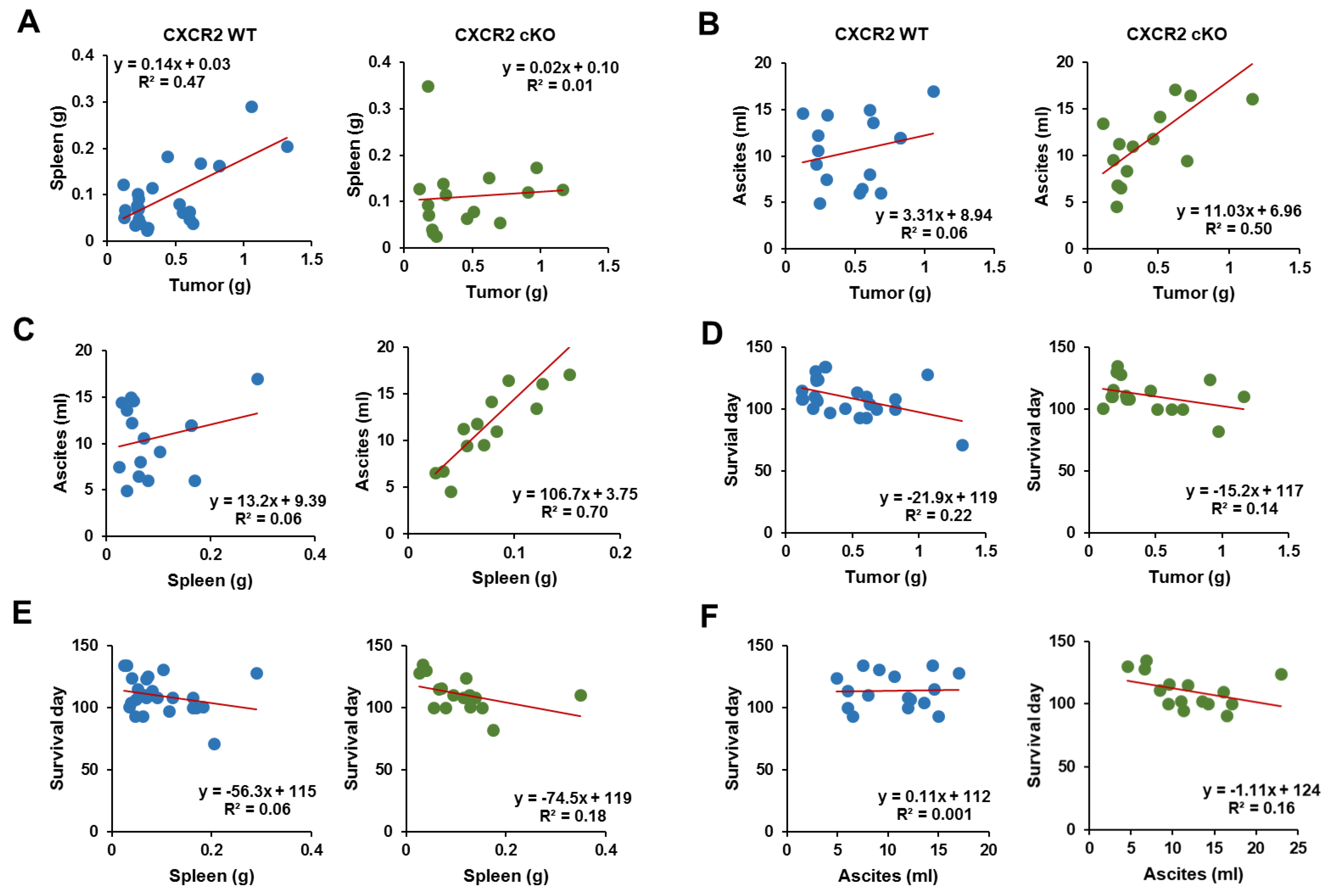
3.6. CXCR2 cKO Mice Showed a Higher Positive Relationship between Ascites and Tumor Parameters Compared to WT Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the iarc working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; O’Flanagan, C.H.; Bowers, L.W.; Allott, E.H.; Hursting, S.D. Translating mechanism-based strategies to break the obesity-cancer link: A narrative review. J. Acad. Nutr. Diet. 2018, 118, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Reguero, M.; Gómez de Cedrón, M.; Wagner, S.; Reglero, G.; Quintela, J.C.; Ramírez de Molina, A. Precision nutrition to activate thermogenesis as a complementary approach to target obesity and associated-metabolic-disorders. Cancers 2021, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Delphan, M.; Scherer, D.; Bowers, L.W.; Hursting, S.; Ulrich, C.M. Signals from the Adipose microenvironment and the obesity-cancer link—A systematic review. Cancer Prev. Res. 2017, 10, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021. [Google Scholar]
- Mogi, K.; Yoshihara, M.; Iyoshi, S.; Kitami, K.; Uno, K.; Tano, S.; Koya, Y.; Sugiyama, M.; Yamakita, Y.; Nawa, A.; et al. Ovarian cancer-associated mesothelial cells: Transdifferentiation to minions of cancer and orchestrate developing peritoneal dissemination. Cancers 2021, 13, 1352. [Google Scholar] [CrossRef]
- Uruski, P.; Mikuła-Pietrasik, J.; Pakuła, M.; Budkiewicz, S.; Drzewiecki, M.; Gaiday, A.N.; Wierzowiecka, M.; Naumowicz, E.; Moszyński, R.; Tykarski, A.; et al. Malignant ascites promote adhesion of ovarian cancer cells to peritoneal mesothelium and fibroblasts. Int. J. Mol. Sci. 2021, 22, 4222. [Google Scholar] [CrossRef]
- Quan, Q.; Zhou, S.; Liu, Y.; Yin, W.; Liao, Q.; Ren, S.; Zhang, F.; Meng, Y.; Mu, X. Relationship between ascites volume and clinical outcomes in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2021, 47, 1527–1535. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Lee, E.S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.S. Chemokine network and overall survival in TP53 wild-type and mutant ovarian cancer. Immune Netw. 2018, 18, e29. [Google Scholar] [CrossRef]
- Son, D.S.; Kabir, S.M.; Dong, Y.L.; Lee, E.; Adunyah, S.E. Inhibitory effect of tumor suppressor p53 on proinflammatory chemokine expression in ovarian cancer cells by reducing proteasomal degradation of IκB. PLoS ONE 2012, 7, e51116. [Google Scholar] [CrossRef]
- Son, D.S.; Kabir, S.M.; Dong, Y.; Lee, E.; Adunyah, S.E. Characteristics of chemokine signatures elicited by EGF and TNF in ovarian cancer cells. J. Inflamm. 2013, 10, 25. [Google Scholar] [CrossRef]
- Ignacio, R.M.; Kabir, S.M.; Lee, E.S.; Adunyah, S.E.; Son, D.S. NF-κB-mediated CCL20 reigns dominantly in CXCR2-driven ovarian cancer progression. PLoS ONE 2016, 11, e0164189. [Google Scholar] [CrossRef]
- Yung, M.M.; Tang, H.W.; Cai, P.C.; Leung, T.H.; Ngu, S.F.; Chan, K.K.; Xu, D.; Yang, H.; Ngan, H.Y.; Chan, D.W. GRO-α and IL-8 enhance ovarian cancer metastatic potential via the CXCR2-mediated TAK1/NFκB signaling cascade. Theranostics 2018, 8, 1270–1285. [Google Scholar] [CrossRef]
- Yang, G.; Rosen, D.G.; Liu, G.; Yang, F.; Guo, X.; Xiao, X.; Xue, F.; Mercado-Uribe, I.; Huang, J.; Lin, S.H.; et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin. Cancer Res. 2010, 16, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, R.M.C.; Dong, Y.L.; Kabir, S.M.; Choi, H.; Lee, E.S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.S. CXCR2 is a negative regulator of p21 in p53-dependent and independent manner via Akt-mediated Mdm2 in ovarian cancer. Oncotarget 2018, 9, 9751–9765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, Y.L.; Kabir, S.M.; Lee, E.S.; Son, D.S. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS ONE 2013, 8, e83789. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.; Lee, E.S.; Son, D.S. Chemokine network during adipogenesis in 3T3-L1 cells: Differential response between growth and proinflammatory factor in preadipocytes vs. adipocytes. Adipocyte 2014, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, R.M.C.; Lee, E.S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.S. Obesity-induced peritoneal dissemination of ovarian cancer and dominant recruitment of macrophages in ascites. Immune Netw. 2018, 18, e47. [Google Scholar] [CrossRef]
- Shen, H.; Schuster, R.; Lu, B.; Waltz, S.E.; Lentsch, A.B. Critical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growth. Prostate 2006, 66, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.P.; Belperio, J.A.; Xue, Y.Y.; Burdick, M.D.; Strieter, R.M. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 2004, 172, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Nebot, J.B.; Kelly, C.J.; Medina-Ruiz, L.; Schuette, F.; Graham, G.J. The chemokine receptor CXCR2 contributes to murine adipocyte development. J. Leukoc. Biol. 2019, 105, 497–506. [Google Scholar] [CrossRef]
- Lucijanić, M. Survival analysis in clinical practice: Analyze your own data using an Excel workbook. Croat. Med. J. 2016, 57, 77–79. [Google Scholar] [CrossRef]
- Cui, S.; Qiao, L.; Yu, S.; Men, L.; Li, Y.; Li, F.; Du, J. The antagonist of CXCR1 and CXCR2 protects db/db mice from metabolic diseases through modulating inflammation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1205–E1217. [Google Scholar] [CrossRef]
- Mann, S.N.; Pitel, K.S.; Nelson-Holte, M.H.; Iwaniec, U.T.; Turner, R.T.; Sathiaseelan, R.; Kirkland, J.L.; Schneider, A.; Morris, K.T.; Malayannan, S.; et al. 17α-Estradiol prevents ovariectomy-mediated obesity and bone loss. Exp. Gerontol. 2020, 142, 111113. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Du, B.; Zhou, X.K.; Sue, E.; Giri, D.; Harbus, M.D.; Falcone, D.J.; Hudis, C.A.; Subbaramaiah, K.; Dannenberg, A.J. Estrogen protects against obesity-induced mammary gland inflammation in mice. Cancer Prev. Res. 2015, 8, 751–759. [Google Scholar] [CrossRef]
- Rosen, B.S.; Cook, K.S.; Yaglom, J.; Groves, D.L.; Volanakis, J.E.; Damm, D.; White, T.; Spiegelman, B.M. Adipsin and complement factor D activity: An immune-related defect in obesity. Science 1989, 244, 1483–1487. [Google Scholar] [CrossRef]
- Pavelka, J.C.; Brown, R.S.; Karlan, B.Y.; Cass, I.; Leuchter, R.S.; Lagasse, L.D.; Li, A.J. Effect of obesity on survival in epithelial ovarian cancer. Cancer 2006, 107, 1520–1524. [Google Scholar] [CrossRef]
- Yang, H.S.; Yoon, C.; Myung, S.K.; Park, S.M. Effect of obesity on survival of women with epithelial ovarian cancer: A systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 2011, 21, 1525–1532. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, X.; Prabhu, S.; Shahoei, S.H.; Nelson, E.R.; Swanson, K.S.; Anastasio, M.A.; Smith, A.M. 3D microscopy and deep learning reveal the heterogeneity of crown-like structure microenvironments in intact adipose tissue. Sci. Adv. 2021, 7, eabe2480. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Dong, Z.; Liu, R.; Huang, J.; Xu, G. Caloric restriction and Roux-en-Y gastric bypass promote white adipose tissue browning in mice. J. Endocrinol. Investig. in press. 2021. [Google Scholar] [CrossRef]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar] [CrossRef]
- Said, S.B.; Loo, G.H.; Kosai, N.R.; Rajan, R.; Mohd, R.; Wahab, A.A.; Shah, S.A. Reduction in urinary chemokine (c-c motif) ligand 2 (CCL2) after surgery-induced weight loss. Sci. Rep. 2020, 10, 790. [Google Scholar] [CrossRef]
- Yang, J.; Stack, M.S. Lipid regulatory proteins as potential therapeutic targets for ovarian cancer in obese women. Cancers 2020, 12, 3469. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Nannuru, K.C.; Varney, M.L.; Singh, R.K. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin. Exp. Metastasis 2015, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.D.; Bricio-Moreno, L.; Sorensen, E.W.; Miyabe, Y.; Lian, J.; Solomon, T.; Kurt-Jones, E.A.; Luster, A.D. Astrocyte- and neuron-derived CXCL1 drives neutrophil transmigration and blood-brain barrier permeability in viral encephalitis. Cell Rep. 2020, 32, 108150. [Google Scholar] [CrossRef]
- Semple, B.D.; Bye, N.; Ziebell, J.M.; Morganti-Kossmann, M.C. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol. Dis. 2010, 40, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ignacio, R.M.C.; Lee, E.S.; Wilson, A.J.; Khabele, D.; Son, D.S. Augmented serum amyloid A1/2 mediated by TNF-induced NF-κB in human serous ovarian epithelial tumors. Immune Netw. 2017, 17, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Son, D.S.; Roby, K.F. Interleukin-1alpha-induced chemokines in mouse granulosa cells: Impact on keratinocyte chemoattractant chemokine, a CXC subfamily. Mol. Endocrinol. 2006, 20, 2999–3013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Son, D.S.; Parl, A.K.; Rice, V.M.; Khabele, D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol. Ther. 2007, 6, 1302–1312. [Google Scholar] [CrossRef]
- Dwyer, J.; Hebda, J.K.; Le Guelte, A.; Galan-Moya, E.M.; Smith, S.S.; Azzi, S.; Bidere, N.; Gavard, J. Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS ONE 2012, 7, e45562. [Google Scholar] [CrossRef] [PubMed]
- Hol, J.; Wilhelmsen, L.; Haraldsen, G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J. Leukoc. Biol. 2010, 87, 501–508. [Google Scholar] [CrossRef]
- Yasui, H.; Kajiyama, H.; Tamauchi, S.; Suzuki, S.; Peng, Y.; Yoshikawa, N.; Sugiyama, M.; Nakamura, K.; Kikkawa, F. CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clin. Exp. Metastasis 2020, 37, 145–158. [Google Scholar] [CrossRef]
- Fader, A.N.; Rasool, N.; Vaziri, S.A.; Kozuki, T.; Faber, P.W.; Elson, P.; Biscotti, C.V.; Michener, C.M.; Rose, P.G.; Rojas-Espaillat, L.; et al. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res. 2010, 30, 4791–4798. [Google Scholar]
- O’Connor, T.; Heikenwalder, M. CCL2 in the tumor microenvironment. Adv. Exp. Med. Biol. 2021, 1302, 1–14. [Google Scholar]
- Steele, C.W.; Karim, S.A.; Foth, M.; Rishi, L.; Leach, J.D.; Porter, R.J.; Nixon, C.; Jeffry Evans, T.R.; Carter, C.R.; Nibbs, R.J.; et al. CXCR2 inhibition suppresses acute and chronic pancreatic inflammation. J. Pathol. 2015, 237, 85–97. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, D.; Lee, E.-S.; Beeghly-Fadiel, A.; Wilson, A.J.; Whalen, M.M.; Adunyah, S.E.; Son, D.-S. High-Fat Diet-Induced Obese Effects of Adipocyte-Specific CXCR2 Conditional Knockout in the Peritoneal Tumor Microenvironment of Ovarian Cancer. Cancers 2021, 13, 5033. https://doi.org/10.3390/cancers13195033
Choe D, Lee E-S, Beeghly-Fadiel A, Wilson AJ, Whalen MM, Adunyah SE, Son D-S. High-Fat Diet-Induced Obese Effects of Adipocyte-Specific CXCR2 Conditional Knockout in the Peritoneal Tumor Microenvironment of Ovarian Cancer. Cancers. 2021; 13(19):5033. https://doi.org/10.3390/cancers13195033
Chicago/Turabian StyleChoe, Deokyeong, Eun-Sook Lee, Alicia Beeghly-Fadiel, Andrew J. Wilson, Margaret M. Whalen, Samuel E. Adunyah, and Deok-Soo Son. 2021. "High-Fat Diet-Induced Obese Effects of Adipocyte-Specific CXCR2 Conditional Knockout in the Peritoneal Tumor Microenvironment of Ovarian Cancer" Cancers 13, no. 19: 5033. https://doi.org/10.3390/cancers13195033
APA StyleChoe, D., Lee, E.-S., Beeghly-Fadiel, A., Wilson, A. J., Whalen, M. M., Adunyah, S. E., & Son, D.-S. (2021). High-Fat Diet-Induced Obese Effects of Adipocyte-Specific CXCR2 Conditional Knockout in the Peritoneal Tumor Microenvironment of Ovarian Cancer. Cancers, 13(19), 5033. https://doi.org/10.3390/cancers13195033





