Validation of microRNA-199b as A Promising Predictor of Outcome and Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Samples
2.2. Determination of Pathological Response
2.3. Total RNA Isolation
2.4. Quantification of miRNA Expression Levels
2.5. Statistical Analysis
3. Results
3.1. MiR-199b Is Downregulated in a Subgroup of Patients and Associates with Molecular and Clinical Parameters
3.2. MiR-199b Downregulation Determines Poor Pathological Response to Neoadjuvant CRT in Locally Advanced Rectal Cancer Patients
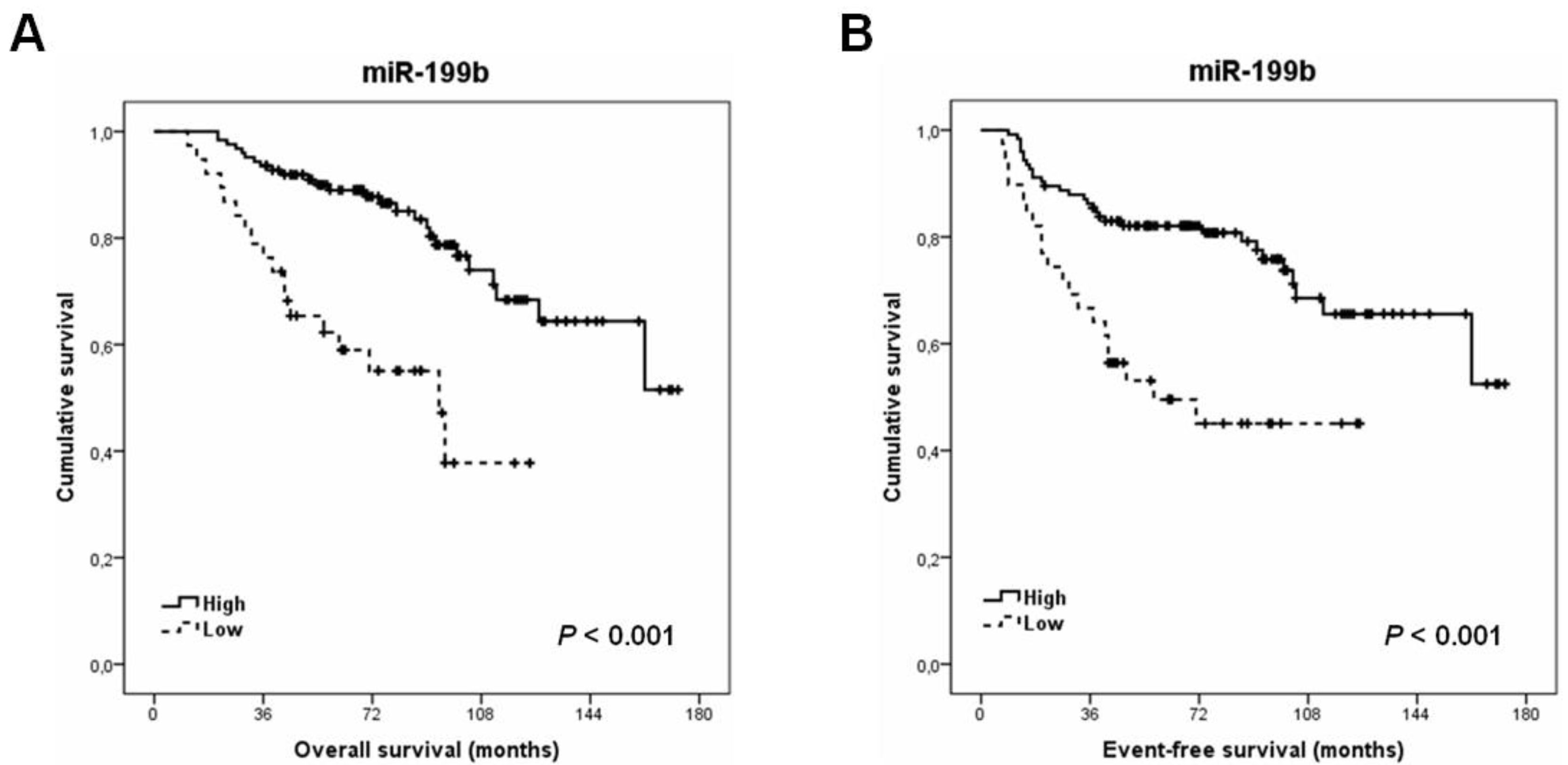
3.3. MiR-199b Downregulation Is an Alteration That Predicts Poor Outcome and Recurrence in Locally Advanced Rectal Cancer Patients
3.4. MiR-199b Retains Its Downregulated Levels in Post-Treatment Samples from Those Cases with Lack of Response to Neoadjuvant CRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Spanish Society of Medical Oncology. Cancer Data in Spain. Available online: https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf (accessed on 13 August 2021).
- Ryan, É.J.; Creavin, B.; Sheahan, K. Delivery of Personalized Care for Locally Advanced Rectal Cancer: Incorporating Pathological, Molecular Genetic, and Immunological Biomarkers into the Multimodal Paradigm. Front. Oncol. 2020, 10, 1369. [Google Scholar] [CrossRef]
- Lichthardt, S.; Wagner, J.; Löb, S.; Matthes, N.; Kastner, C.; Anger, F.; Germer, C.-T.; Wiegering, A. Pathological complete response due to a prolonged time interval between preoperative chemoradiation and surgery in locally advanced rectal cancer: Analysis from the German StuDoQ|Rectalcarcinoma registry. BMC Cancer 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Isbert, C.; Dietz, U.A.; Kunzmann, V.; Ackermann, S.; Kerscher, A.; Maeder, U.; Flentje, M.; Schlegel, N.; Reibetanz, C.-T.G.; et al. Multimodal therapy in treatment of rectal cancer is associated with improved survival and reduced local recurrence—a retrospective analysis over two decades. BMC Cancer 2014, 14, 816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar]
- Canto, L.M.D.; Cury, S.S.; Barros-Filho, M.C.; Kupper, B.E.C.; Begnami, M.D.F.S.; Scapulatempo-Neto, C.; Carvalho, R.F.; Marchi, F.A.; Olsen, D.A.; Madsen, J.S.; et al. Locally advanced rectal cancer transcriptomic-based secretome analysis reveals novel biomarkers useful to identify patients according to neoadjuvant chemoradiotherapy response. Sci. Rep. 2019, 9, 8702. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, J.; Peng, L.; Zhang, D.; Jin, M.; Wang, J.; Xue, J.; Liu, H.; Zhang, T. Complete pathological response following neoadjuvant FOLFOX chemotherapy in BRCA2-mutant locally advanced rectal cancer: A case report. BMC Cancer 2018, 18, 1253. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Mathis, K. Novelties in treatment of locally advanced rectal cancer. F1000Res 2018, 7, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, F.D.E.; Luglio, G.; Tropeano, F.P.; Pagano, G.; D’Armiento, M.; Kroemer, G.; Maiuri, M.C.; De Palma, G.D. The Role of Micro-RNAs and Circulating Tumor Markers as Predictors of Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2020, 21, 7040. [Google Scholar] [CrossRef] [PubMed]
- Imedio, L.; Cristóbal, I.; Rubio, J.; Santos, A.; Rojo, F.; García-Foncillas, J. MicroRNAs in Rectal Cancer: Functional Significance and Promising Therapeutic Value. Cancers 2020, 12, 2040. [Google Scholar] [CrossRef]
- Azizian, A.; Epping, I.; Kramer, F.; Jo, P.; Bernhardt, M.; Kitz, J.; Salinas, G.; Wolff, H.A.; Grade, M.; Beißbarth, T.; et al. Prognostic Value of MicroRNAs in Preoperative Treated Rectal Cancer. Int. J. Mol. Sci. 2016, 17, 568. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. MicroRNAs in cancer management. Lancet Oncol. 2012, 13, e249–e258. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Azizian, A.; Gruber, J.; Ghadimi, B.M.; Gaedcke, J. MicroRNA in rectal cancer. World J. Gastrointest. Oncol. 2016, 8, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Zhao, Y.; Guo, B. MiR-199b-5p targets HER2 in breast cancer cells. J. Cell. Biochem. 2013, 114, 1457–1463. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, W.; Xiao, Y.; Ma, J.; Xu, F.; Zhang, K.; Gao, Y.; Chen, Q.; Li, Y.; Li, H.; et al. MiR-199b-5p Suppresses Tumor Angiogenesis Mediated by Vascular Endothelial Cells in Breast Cancer by Targeting ALK1. Front. Genet. 2020, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Garzia, L.; Andolfo, I.; Cusanelli, E.; Marino, N.; Petrosino, G.; De Martino, D.; Esposito, V.; Galeone, A.; Navas, L.; Esposito, S.; et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS ONE 2009, 4, e4998. [Google Scholar] [CrossRef]
- Shang, W.; Chen, X.; Nie, L.; Xu, M.; Chen, N.; Zeng, H.; Zhou, Q. MiR199b suppresses expression of hypoxia-inducible factor 1α (HIF-1α) in prostate cancer cells. Int. J. Mol. Sci. 2013, 14, 8422–8436. [Google Scholar] [CrossRef] [Green Version]
- Li, G.L.; Yuan, J.H.; Zhuang, G.D.; Wu, D.Q. MiR-199b exerts tumor suppressive functions in hepatocellular carcinoma by directly targeting JAG1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7679–7687. [Google Scholar] [PubMed]
- Wang, C.; Song, B.; Song, W.; Liu, J.; Sun, A.; Wu, D.; Yu, H.; Lian, J.; Chen, L.; Han, J. Underexpressed microRNA-199b-5p targets hypoxia-inducible factor-1α in hepatocellular carcinoma and predicts prognosis of hepatocellular carcinoma patients. J. Gastroenterol. Hepatol. 2011, 26, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Favreau, A.J.; McGlauflin, R.E.; Duarte, C.W.; Sathyanarayana, P. miR-199b, a novel tumor suppressor miRNA in acute myeloid leukemia with prognostic implications. Exp. Hematol. Oncol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.L.; Wang, B.; Jiang, K.W.; Ye, C.X.; Cheng, C.; Yan, Y.C.; Zhang, J.-Z.; Yang, Y.; Gao, Z.-D.; Ye, Y.-J.; et al. Downregulation of miR-199b is associated with distant metastasis in colorectal cancer via activation of SIRT1 and inhibition of CREB/KISS1 signaling. Oncotarget 2016, 7, 35092–35105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Y.; Zhi, Z.; Wang, L.; Zhao, Y.Y.; Deng, M.; Liu, Y.H.; Qin, Y.; Tian, M.-M.; Liu, Y.; Shen, T.; et al. NSD2 circular RNA promotes metastasis of colorectal cancer by targeting miR-199b-5p-mediated DDR1 and JAG1 signalling. J. Pathol. 2019, 248, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Makkinje, A.; Damuni, Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 1996, 271, 11059–11062. [Google Scholar] [CrossRef] [Green Version]
- Cristóbal, I.; Rubio, J.; Torrejón, B.; Santos, A.; Caramés, C.; Luque, M.; Sanz-Álvarez, M.; Alonso, R.; Zazo, S.; Madoz-Gúrpide, J.; et al. MicroRNA-199b Deregulation Shows a Strong SET-Independent Prognostic Value in Early-Stage Colorectal Cancer. J. Clin. Med. 2020, 9, 2419. [Google Scholar] [CrossRef]
- Cristóbal, I.; Caramés, C.; Rincón, R.; Manso, R.; Madoz-Gúrpide, J.; Torrejón, B.; González-Alonso, P.; Rojo, F.; García-Foncillas, J. Downregulation of microRNA-199b predicts unfavorable prognosis and emerges as a novel therapeutic target which contributes to PP2A inhibition in metastatic colorectal cancer. Oncotarget 2017, 8, 40169–40180. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, L.; Zhang, Q.; Yang, W.; Zhang, Y. E2F7 Transcriptionally Inhibits MicroRNA-199b Expression to Promote USP47, Thereby Enhancing Colon Cancer Tumor Stem Cell Activity and Promoting the Occurrence of Colon Cancer. Front. Oncol. 2021, 10, 565449. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.W.; Kim, G.; Kang, B.W.; Kim, H.J.; Park, S.Y.; Park, J.S.; Choi, G.-S.; Kang, M.K.; Hur, K.; Kim, J.G. High expression of microRNA-199a-5p is associated with superior clinical outcomes in patients with locally advanced rectal cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, I.; Rubio, J.; Santos, A.; Torrejón, B.; Caramés, C.; Imedio, L.; Mariblanca, S.; Luque, M.; Sanz-Alvarez, M.; Zazo, S.; et al. MicroRNA-199b Downregulation Confers Resistance to 5-Fluorouracil Treatment and Predicts Poor Outcome and Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer Patients. Cancers 2020, 12, 1655. [Google Scholar] [CrossRef] [PubMed]


| Parameter | No. Cases | No. miR-199b High (%) | No. miR-199 Low (%) | p | ||
|---|---|---|---|---|---|---|
| MiR-199b | 163 | 124 | (76.1) | 39 | (23.9) | |
| Gender | 163 | 124 | 39 | 0.112 | ||
| Male | 95 | 68 | (71.6) | 27 | (28.4) | |
| Female | 68 | 56 | (82.4) | 12 | (17.6) | |
| Age | 163 | 124 | 39 | 0.504 | ||
| <70 | 87 | 68 | (78.2) | 19 | (21.8) | |
| >70 | 76 | 56 | (73.7) | 20 | (26.3) | |
| ECOG 1 | 163 | 124 | 39 | 0.634 | ||
| 0 | 112 | 84 | (75) | 28 | (25) | |
| 1–2 | 51 | 40 | (78.4) | 11 | (21.6) | |
| Clinical stage pre-CRT 2 | 163 | 124 | 39 | 0.427 | ||
| II | 12 | 8 | (66.7) | 4 | (33.3) | |
| III | 151 | 116 | (76.8) | 35 | (23.2) | |
| Grade pre-CRT | 154 | 116 | 38 | 0.515 | ||
| Low | 58 | 42 | (72.4) | 16 | (27.6) | |
| Moderate-High | 96 | 74 | (77.1) | 22 | (22.9) | |
| ypT 3 | 163 | 124 | 39 | 0.026 | ||
| 0 | 25 | 24 | (96) | 1 | (4) | |
| 1 | 20 | 17 | (85) | 3 | (15) | |
| 2 | 57 | 38 | (66.7) | 19 | (33.3) | |
| 3–4 | 61 | 45 | (73.8) | 16 | (26.2) | |
| ypN 4 | 163 | 124 | 39 | 0.005 | ||
| N0 | 122 | 100 | (82) | 22 | (18) | |
| N1 | 36 | 20 | (55.6) | 16 | (44.4) | |
| N2 | 5 | 4 | (80) | 1 | (20) | |
| Pathological stage | 163 | 124 | 39 | 0.004 | ||
| yp0 | 24 | 23 | (95.8) | 1 | (4.2) | |
| ypI | 62 | 47 | (75.8) | 15 | (24.2) | |
| ypII | 36 | 30 | (83.3) | 6 | (16.7) | |
| ypIII | 41 | 24 | (58.5) | 17 | (41.5) | |
| Adjuvant treatment | 163 | 124 | 39 | 0.085 | ||
| No | 37 | 24 | (64.9) | 13 | (35.1) | |
| 5-FU 5 | 94 | 77 | (81.9) | 17 | (18.1) | |
| FOLFOX 6 | 28 | 19 | (67.9) | 9 | (32.1) | |
| Other | 4 | 4 | (100) | 0 | (0) | |
| Responders vs. Non-Responders | ||||||
|---|---|---|---|---|---|---|
| MiR-199b Expression | No. Cases | MiR-199b High (%) | MiR-199b Low (%) | p | ||
| Response | 163 | 124 | 39 | 0.004 | ||
| Non-Response 1 | 84 | 56 | (45.2) | 28 | (71.8) | |
| Response 2 | 79 | 68 | (54.8) | 11 | (28.2) | |
| Response 1 vs. Non-Response 2 | |||||
|---|---|---|---|---|---|
| Parameter | OR 4 | 95% CI 3 | p | ||
| Lower | Upper | ||||
| Gender | 0.976 | ||||
| Male | 1.000 | ||||
| Female | 1.010 | 0.524 to 1.948 | |||
| Age | 0.221 | ||||
| <70 | 1.000 | ||||
| ≥70 | 1.536 | 0.772 to 3.057 | |||
| Grade pre-CRT 5 | 0.149 | ||||
| Low | 1.000 | ||||
| Moderate-High | 1.486 | 0.868 to 2.545 | |||
| Clinical stage | 0.665 | ||||
| II | 1.000 | ||||
| III | 0.870 | 0.465 to 1.631 | |||
| ECOG 6 | 0.646 | ||||
| 0 | 1.000 | ||||
| 1–2 | 0.841 | 0.401 to 1.762 | |||
| MiR-199b | 0.007 | ||||
| High | 1.000 | ||||
| Low | 3.020 | 1.361 to 6.700 | |||
| Univariate OS 1 Analysis | Multivariate OS Cox Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR 3 | 95% CI 2 | p | HR | 95% CI | p | |||
| Lower | Upper | Lower | Upper | ||||||
| Gender | 0.439 | - | |||||||
| Male | 1.000 | ||||||||
| Female | 0.785 | 0.426 to 1.448 | - | - | |||||
| Age | 0.018 | 0.013 | |||||||
| <70 | 1.000 | 1.000 | |||||||
| ≥70 | 2.062 | 1.123 to 3.758 | 2.138 | 1.171 to 3.904 | |||||
| ypT 4 | 0.051 | - | |||||||
| 0–2 | 1.000 | ||||||||
| 3–4 | 1.306 | 0.998 to 1.710 | - | - | |||||
| ypN 5 | 0.003 | 0.392 | |||||||
| N- | 1.000 | 1.000 | |||||||
| N+ | 2.498 | 1.355 to 4.605 | 1.409 | 0.642 to 3.095 | |||||
| Pathological stage | 0.013 | 0.257 | |||||||
| 0–I | 1.000 | 1.000 | |||||||
| II–III | 2.161 | 1.179 to 3.961 | 1.553 | 0.725 to 3.325 | |||||
| ECOG 6 | 0.094 | - | |||||||
| 0 | 1.000 | ||||||||
| 1–2 | 1.660 | 0.918 to 3.002 | - | - | |||||
| MiR-199b | <0.001 | 0.001 | |||||||
| High | 1.000 | 1.000 | |||||||
| Low | 3.626 | 1.968 to 6.680 | 3.123 | 1.643 to 5.934 | |||||
| MiR-199b Expression | No. Cases | MiR-199b High (%) | MiR-199 Low (%) | p | ||
|---|---|---|---|---|---|---|
| Recurrence | 163 | 124 | 39 | 0.004 | ||
| No | 124 | 101 | (81.5) | 23 | (18.5) | |
| Yes | 39 | 23 | (59) | 16 | (41) | |
| Place of elapse | 31 | 18 | 13 | 0.543 | ||
| Liver | 3 | 2 | (66.7) | 1 | (33.3) | |
| Lung | 15 | 8 | (53.3) | 7 | (46.7) | |
| Retroperitoneum | 2 | 1 | (50) | 1 | (50) | |
| Bone | 2 | 2 | (100) | 0 | (0) | |
| Peritoneum | 1 | 0 | (0) | 1 | (100) | |
| Abdominal wall | 1 | 0 | (0) | 1 | (100) | |
| Liver and lung | 7 | 5 | (71.4) | 2 | (28.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóbal, I.; Santos, A.; Rubio, J.; Caramés, C.; Zazo, S.; Sanz-Álvarez, M.; Luque, M.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Validation of microRNA-199b as A Promising Predictor of Outcome and Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients. Cancers 2021, 13, 5003. https://doi.org/10.3390/cancers13195003
Cristóbal I, Santos A, Rubio J, Caramés C, Zazo S, Sanz-Álvarez M, Luque M, Madoz-Gúrpide J, Rojo F, García-Foncillas J. Validation of microRNA-199b as A Promising Predictor of Outcome and Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients. Cancers. 2021; 13(19):5003. https://doi.org/10.3390/cancers13195003
Chicago/Turabian StyleCristóbal, Ion, Andrea Santos, Jaime Rubio, Cristina Caramés, Sandra Zazo, Marta Sanz-Álvarez, Melani Luque, Juan Madoz-Gúrpide, Federico Rojo, and Jesús García-Foncillas. 2021. "Validation of microRNA-199b as A Promising Predictor of Outcome and Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients" Cancers 13, no. 19: 5003. https://doi.org/10.3390/cancers13195003
APA StyleCristóbal, I., Santos, A., Rubio, J., Caramés, C., Zazo, S., Sanz-Álvarez, M., Luque, M., Madoz-Gúrpide, J., Rojo, F., & García-Foncillas, J. (2021). Validation of microRNA-199b as A Promising Predictor of Outcome and Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients. Cancers, 13(19), 5003. https://doi.org/10.3390/cancers13195003







