A Highly Reliable Convolutional Neural Network Based Soft Tissue Sarcoma Metastasis Detection from Chest X-ray Images: A Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
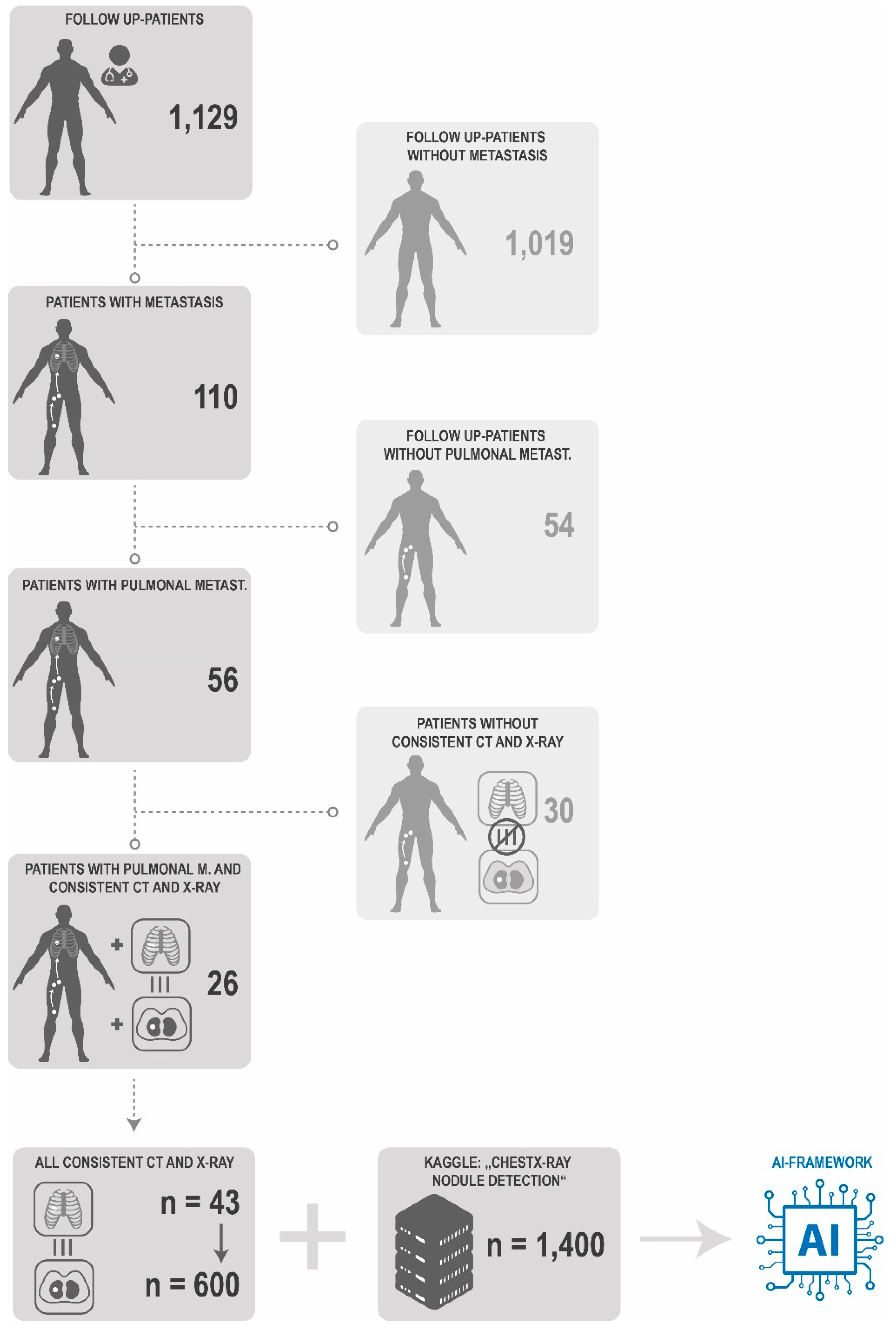
2.1. Acquisition of Patient X-ray
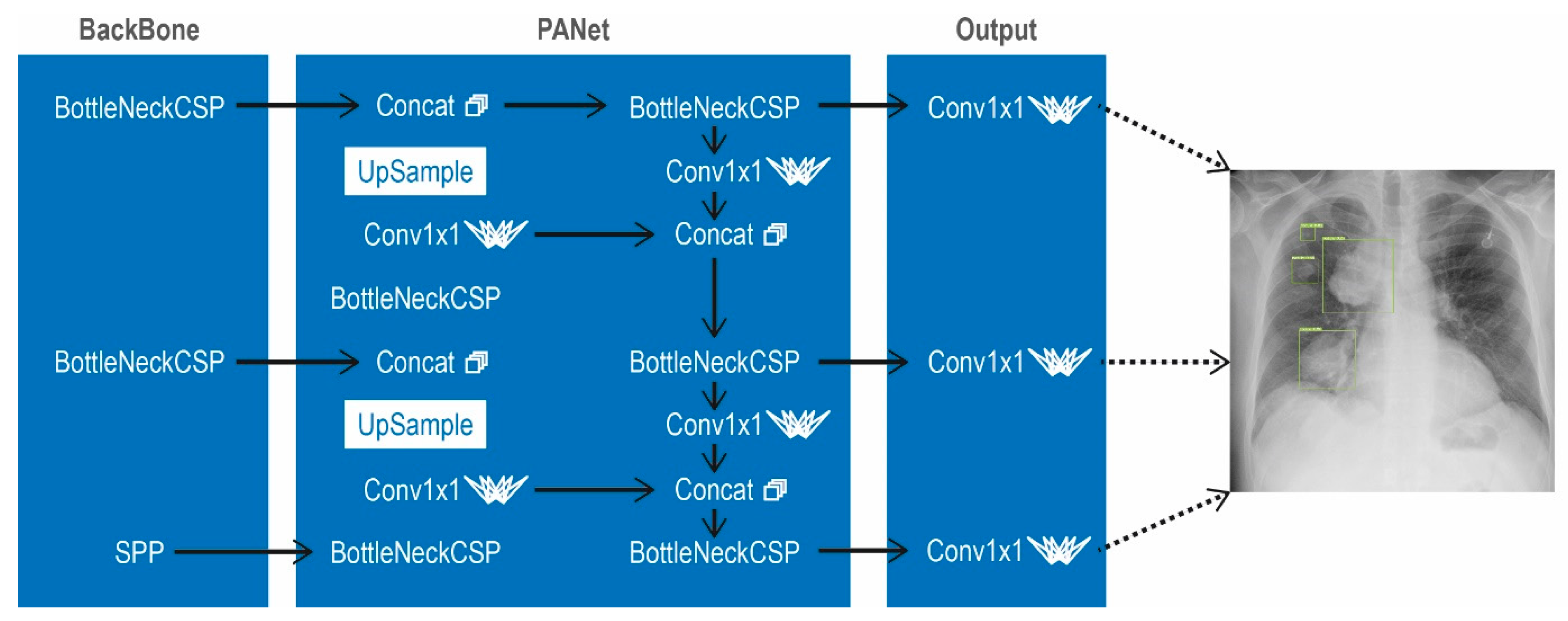
2.2. Implementation in Python
2.3. Validation of the Model
2.4. Data Managment
3. Results
3.1. Patient Data
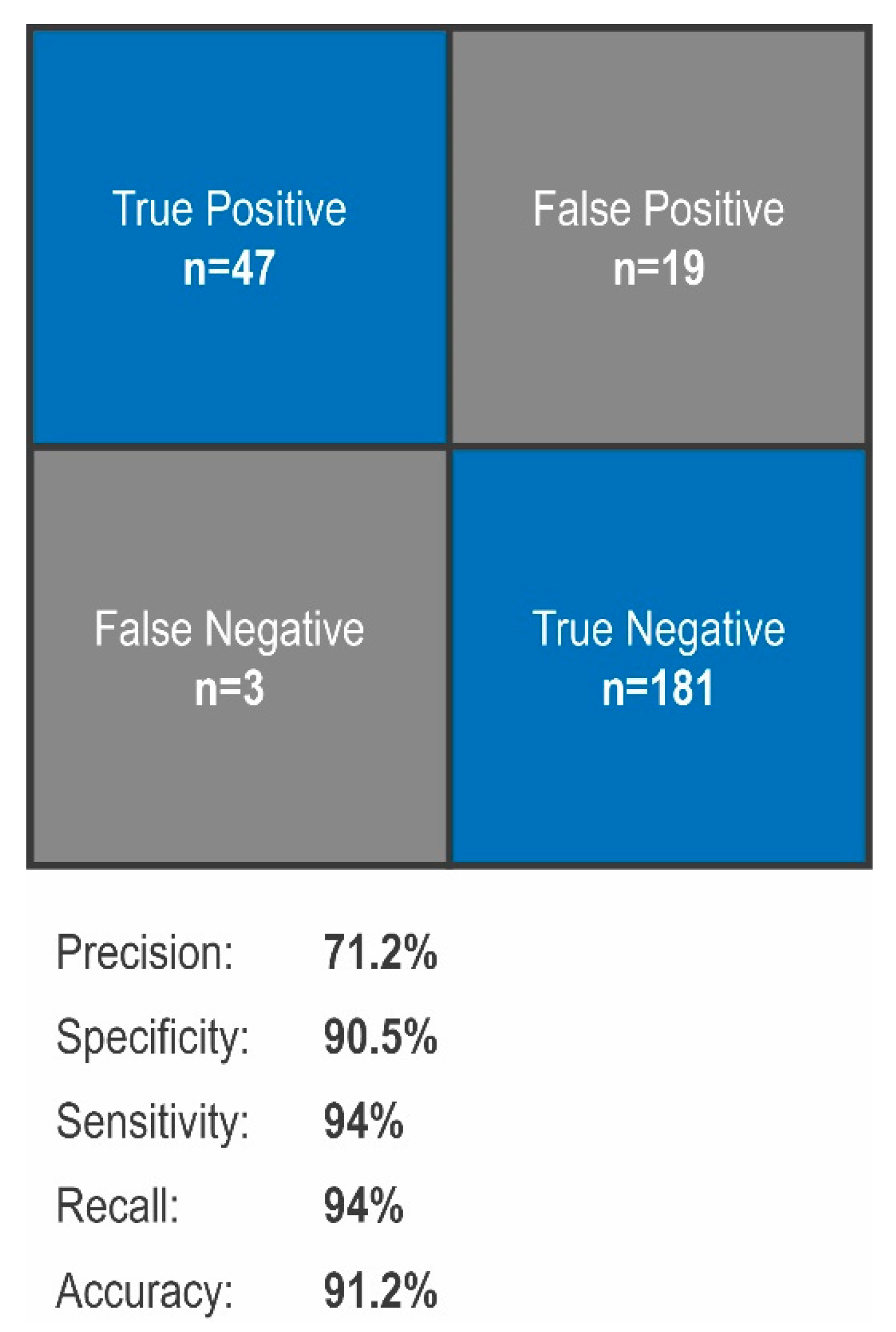
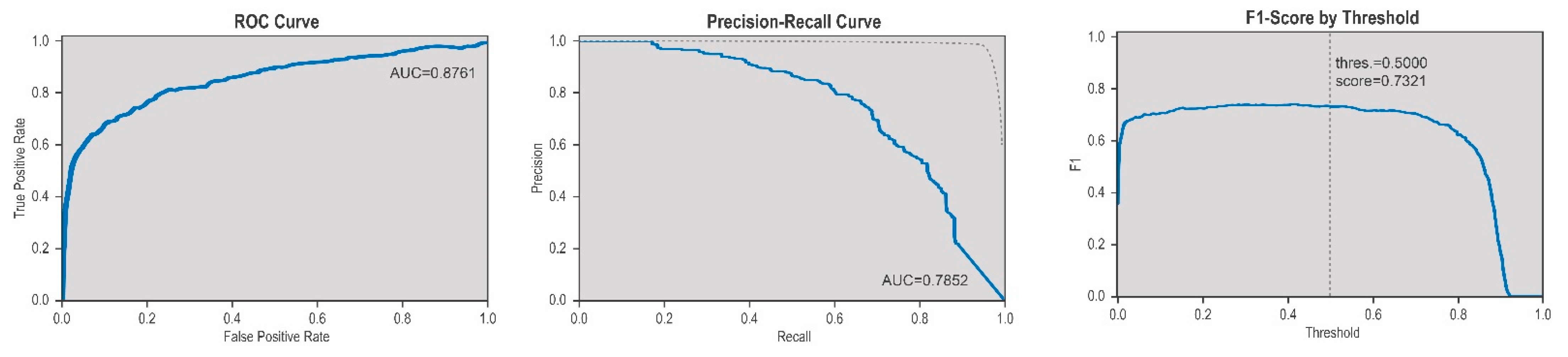
3.2. Evaluation of Sensitivy, Specificity, Precision and Accuracy
3.3. Smallest Reliably Detectable Size
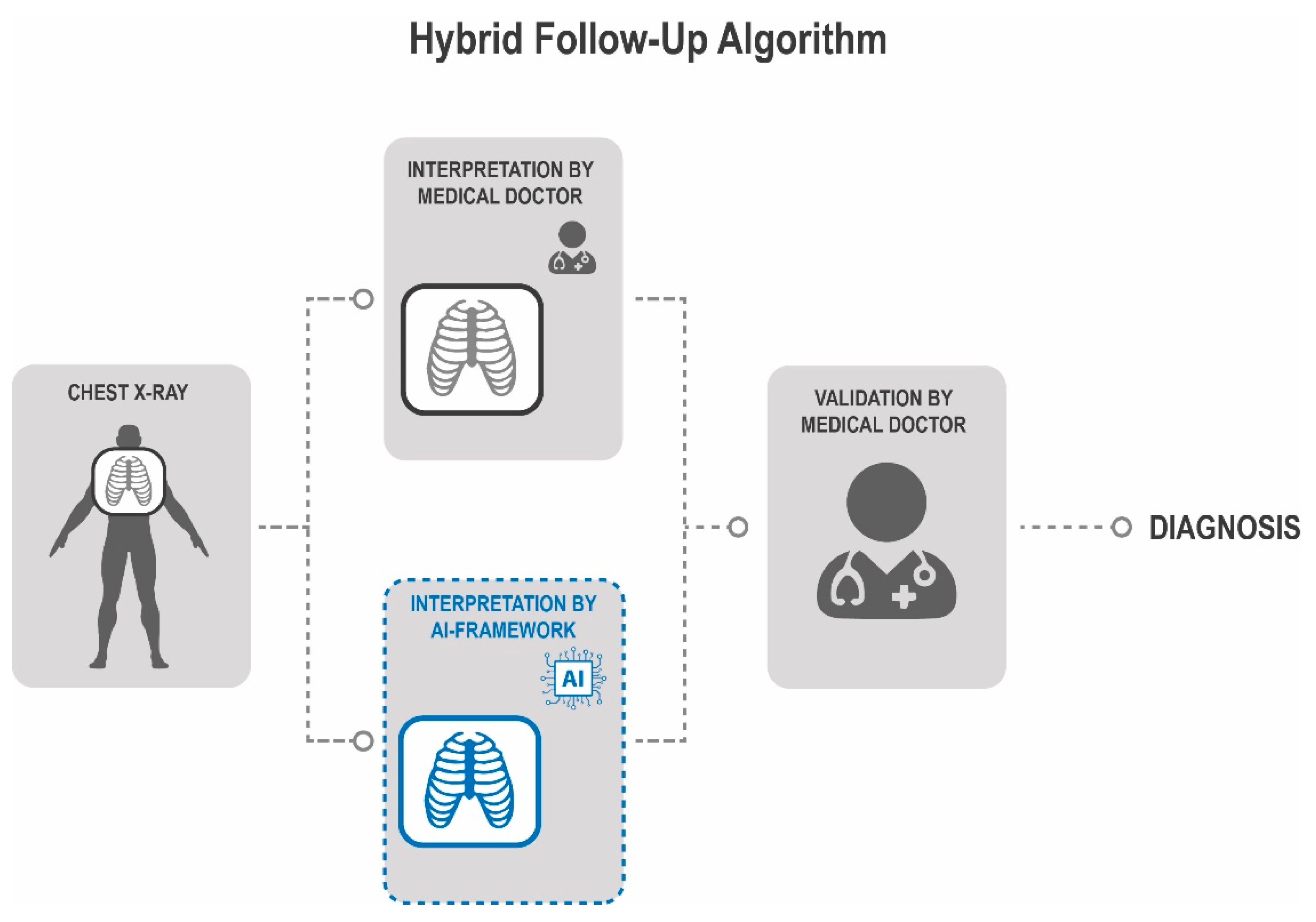
3.4. Implementation of a Hybrid Follow-Up Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, C.E. Overview of Sarcomas in the Adolescent and Young Adult Population. J. Pediatr. Hematol. Oncol. 2005, 27, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ressing, M.; Wardelmann, E.; Hohenberger, P.; Jakob, J.; Kasper, B.; Emrich, K.; Eberle, A.; Blettner, M.; Zeissig, S.R. Strengthening health data on a rare and heterogeneous disease: Sarcoma incidence and histological subtypes in Germany. BMC Public Health 2018, 18, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damerell, V.; Pepper, M.S.; Prince, S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct. Target. Ther. 2021, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Flugstad, D.L.; Wilke, C.P.; McNutt, M.A.; Welk, R.A.; Hart, M.J.; McQuinn, W.C. Importance of Surgical Resection in the Successful Management of Soft Tissue Sarcoma. Arch. Surg. 1999, 134, 856. [Google Scholar] [CrossRef] [Green Version]
- Harati, K.; Goertz, O.; Pieper, A.; Daigeler, A.; Lehnhardt, M. Soft tissue sarcomas of the extremities: What is the best surgical margin? Oncol. Res. Treat. 2018, 41, 155. [Google Scholar]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2017; NIH National Cancer Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Daigeler, A.; Harati, K.; Goertz, O.; Hirsch, T.; Steinau, H.-U.; Lehnhardt, M. Prognostic Factors and Surgical Tactics in Patients with Locally Recurrent Soft Tissue Sarcomas. Handchir. Mikrochir. Plast. Chir. 2015, 47, 118–127. [Google Scholar] [CrossRef]
- Billingsley, K.G.; Burt, M.E.; Jara, E.; Ginsberg, R.J.; Woodruff, J.M.; Leung, D.H.Y.; Brennan, M.F. Pulmonary Metastases From Soft Tissue Sarcoma. Ann. Surg. 1999, 229, 602. [Google Scholar] [CrossRef]
- Acem, I.; Smit, M.M.; Verhoef, C.; van Houdt, W.J.; Haas, R.L.; van der Hage, J.A.; Grünhagen, D.J.; van de Sande, M.A.J. Management of Soft Tissue Sarcomas in Extremities: Variation in Treatment Recommendations and Surveillance According to Specialty and Continent. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Rutkowski, P.; Ługowska, I. Follow-up in soft tissue sarcomas. Memo-Mag. Eur. Med. Oncol. 2014, 7, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, J.H.; Romney, B.M.; Goldsmith, L.S. Missed bronchogenic carcinoma: Radiographic findings in 27 patients with a potentially resectable lesion evident in retrospect. Radiology 1992, 182, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gavelli, G.; Giampalma, E. Sensitivity and specificity of chest x-ray screening for lung cancer. Cancer 2000, 89, 2453–2456. [Google Scholar] [CrossRef]
- Kakinuma, R.; Ashizawa, K.; Kobayashi, T.; Fukushima, A.; Hayashi, H.; Kondo, T.; Machida, M.; Matsusako, M.; Minami, K.; Oikado, K.; et al. Comparison of sensitivity of lung nodule detection between radiologists and technologists on low-dose CT lung cancer screening images. Br. J. Radiol. 2012, 85, e603–e608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, Y.; Chung, M.J.; Kotter, E.; Yune, S.; Kim, M.; Do, S.; Han, K.; Kim, H.; Yang, S.; Lee, D.-J.; et al. Deep Convolutional Neural Network–based Software Improves Radiologist Detection of Malignant Lung Nodules on Chest Radiographs. Radiology 2020, 294, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, A.; Prabhu, G.A.; Rajinikanth, V.; Thanaraj, K.P.; Satapathy, S.C.; Robbins, D.E.; Shasky, C.; Zhang, Y.-D.; Tavares, J.M.R.S.; Raja, N.S.M. Deep-learning framework to detect lung abnormality—A study with chest X-Ray and lung CT scan images. Pattern Recognit. Lett. 2020, 129, 271–278. [Google Scholar] [CrossRef]
- Konnik, M.; Ahmadi, B.; May, N.; Favata, J.; Shahbazi, Z.; Shahbazmohamadi, S.; Tavousi, P. Training AI-Based Feature Extraction Algorithms, for Micro CT Images, Using Synthesized Data. J. Nondestruct. Eval. 2021, 40, 25. [Google Scholar] [CrossRef]
- Jocher, G.; Stoken, A.; Borovec, J.; Christopher, S.T.; Laughing, L.C. Ultralytics/yolov5: v4.0-nn.SiLU() activations, Weights & Biases logging, PyTorch Hub integration. Zenodo 2021. [Google Scholar] [CrossRef]
- Dipu, N.M.; Shohan, S.A.; Salam, K.M.A. Deep Learning Based Brain Tumor Detection and Classification. In Proceedings of the 2021 International Conference on Intelligent Technologies (CONIT), Hubli, India, 25–27 June 2021; pp. 1–6. [Google Scholar]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021. [Google Scholar] [CrossRef]
- Jakob, J. Update Therapie von Weichgewebesarkomen. Forum Fam. Plan. West. Hemisph. 2021, 36, 52–57. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Z.; Kuen, J.; Ma, L.; Shahroudy, A.; Shuai, B.; Liu, T.; Wang, X.; Wang, G.; Cai, J.; et al. Recent advances in convolutional neural networks. Pattern Recognit. 2018, 77, 354–377. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-C.; Roth, H.R.; Gao, M.; Lu, L.; Xu, Z.; Nogues, I.; Yao, J.; Mollura, D.; Summers, R.M. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016, 35, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, A.; Khan, M.A.; Saba, T.; Mehmood, Z.; Tariq, U.; Ayesha, N. Microscopic brain tumor detection and classification using 3D CNN and feature selection architecture. Microsc. Res. Tech. 2021, 84, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Chen, H.; Yu, L.; Qin, J.; Heng, P.-A. Multilevel Contextual 3-D CNNs for False Positive Reduction in Pulmonary Nodule Detection. IEEE Trans. Biomed. Eng. 2017, 64, 1558–1567. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Ethun, C.G.; Switchenko, J.M.; Lipscomb, J.; Poultsides, G.A.; Grignol, V.; Howard, J.H.; Gamblin, T.C.; Roggin, K.K.; Votanopoulos, K.; et al. Lung Surveillance Strategy for High-Grade Soft Tissue Sarcomas: Chest X-Ray or CT Scan? J. Am. Coll. Surg. 2019, 229, 449–457. [Google Scholar] [CrossRef]
- Jang, S.; Song, H.; Shin, Y.J.; Kim, J.; Kim, J.; Lee, K.W.; Lee, S.S.; Lee, W.; Lee, S.; Lee, K.H. Deep Learning–based Automatic Detection Algorithm for Reducing Overlooked Lung Cancers on Chest Radiographs. Radiology 2020, 296, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef]
- Hayakawa, K.; Matsumoto, S.; Ae, K.; Tanizawa, T.; Gokita, T.; Funauchi, Y.; Motoi, N. Risk factors for distant metastasis of dermatofibrosarcoma protuberans. J. Orthop. Traumatol. 2016, 17, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahles, A.; Ong, C.S.; Zhong, Y.; Rätsch, G. SplAdder: Identification, quantification and testing of alternative splicing events from RNA-Seq data. Bioinformatics 2016, 32, 1840–1847. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F.; Dapper, H.; Asadpour, R.; Knebel, C.; Spraker, M.B.; Schwarze, V.; Schaub, S.K.; Mayr, N.A.; Specht, K.; Woodruff, H.C.; et al. Development and External Validation of Deep-Learning-Based Tumor Grading Models in Soft-Tissue Sarcoma Patients Using MR Imaging. Cancers 2021, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ghani, M.U.; Saba, T.; Rehman, A. Brain tumor segmentation in multi-spectral MRI using convolutional neural networks (CNN). Microsc. Res. Tech. 2018, 81, 419–427. [Google Scholar] [CrossRef]
- Bielak, L.; Wiedenmann, N.; Berlin, A.; Nicolay, N.H.; Gunashekar, D.D.; Hägele, L.; Lottner, T.; Grosu, A.-L.; Bock, M. Convolutional neural networks for head and neck tumor segmentation on 7-channel multiparametric MRI: A leave-one-out analysis. Radiat. Oncol. 2020, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, Z. Comparison of CT and MRI images for the prediction of soft-tissue sarcoma grading and lung metastasis via a convolutional neural networks model. Clin. Radiol. 2020, 75, 64–69. [Google Scholar] [CrossRef] [Green Version]

| Value | |
|---|---|
| Age at X-ray (years) | 62.8 ± 12.2 |
| Sex | |
| Male | 15 (58%) |
| Female | 11 (42%) |
| Sarcoma Entity | |
| Undiff. Pleomorph. Sarcoma | 13 (50%) |
| Liposarcoma | 2 (8%) |
| Myoxid Sarcoma | 3 (12%) |
| Leiomyosarcoma | 3 (12%) |
| Malignant fibrous histiocytoma | 4 (15%) |
| Other Sarcoma Entitiy | 1 (3%) |
| Tumor Grade | |
| G1 | 2 (8%) |
| G2 | 5 (18%) |
| G3 | 19 (74%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallner, C.; Alam, M.; Drysch, M.; Wagner, J.M.; Sogorski, A.; Dadras, M.; von Glinski, M.; Reinkemeier, F.; Becerikli, M.; Heute, C.; et al. A Highly Reliable Convolutional Neural Network Based Soft Tissue Sarcoma Metastasis Detection from Chest X-ray Images: A Retrospective Cohort Study. Cancers 2021, 13, 4961. https://doi.org/10.3390/cancers13194961
Wallner C, Alam M, Drysch M, Wagner JM, Sogorski A, Dadras M, von Glinski M, Reinkemeier F, Becerikli M, Heute C, et al. A Highly Reliable Convolutional Neural Network Based Soft Tissue Sarcoma Metastasis Detection from Chest X-ray Images: A Retrospective Cohort Study. Cancers. 2021; 13(19):4961. https://doi.org/10.3390/cancers13194961
Chicago/Turabian StyleWallner, Christoph, Mansoor Alam, Marius Drysch, Johannes Maximilian Wagner, Alexander Sogorski, Mehran Dadras, Maxi von Glinski, Felix Reinkemeier, Mustafa Becerikli, Christoph Heute, and et al. 2021. "A Highly Reliable Convolutional Neural Network Based Soft Tissue Sarcoma Metastasis Detection from Chest X-ray Images: A Retrospective Cohort Study" Cancers 13, no. 19: 4961. https://doi.org/10.3390/cancers13194961
APA StyleWallner, C., Alam, M., Drysch, M., Wagner, J. M., Sogorski, A., Dadras, M., von Glinski, M., Reinkemeier, F., Becerikli, M., Heute, C., Nicolas, V., Lehnhardt, M., & Behr, B. (2021). A Highly Reliable Convolutional Neural Network Based Soft Tissue Sarcoma Metastasis Detection from Chest X-ray Images: A Retrospective Cohort Study. Cancers, 13(19), 4961. https://doi.org/10.3390/cancers13194961








