Clinical Significance of Circulating Tumor Cell Induced Epithelial-Mesenchymal Transition in Patients with Metastatic Colorectal Cancer by Single-Cell RNA-Sequencing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
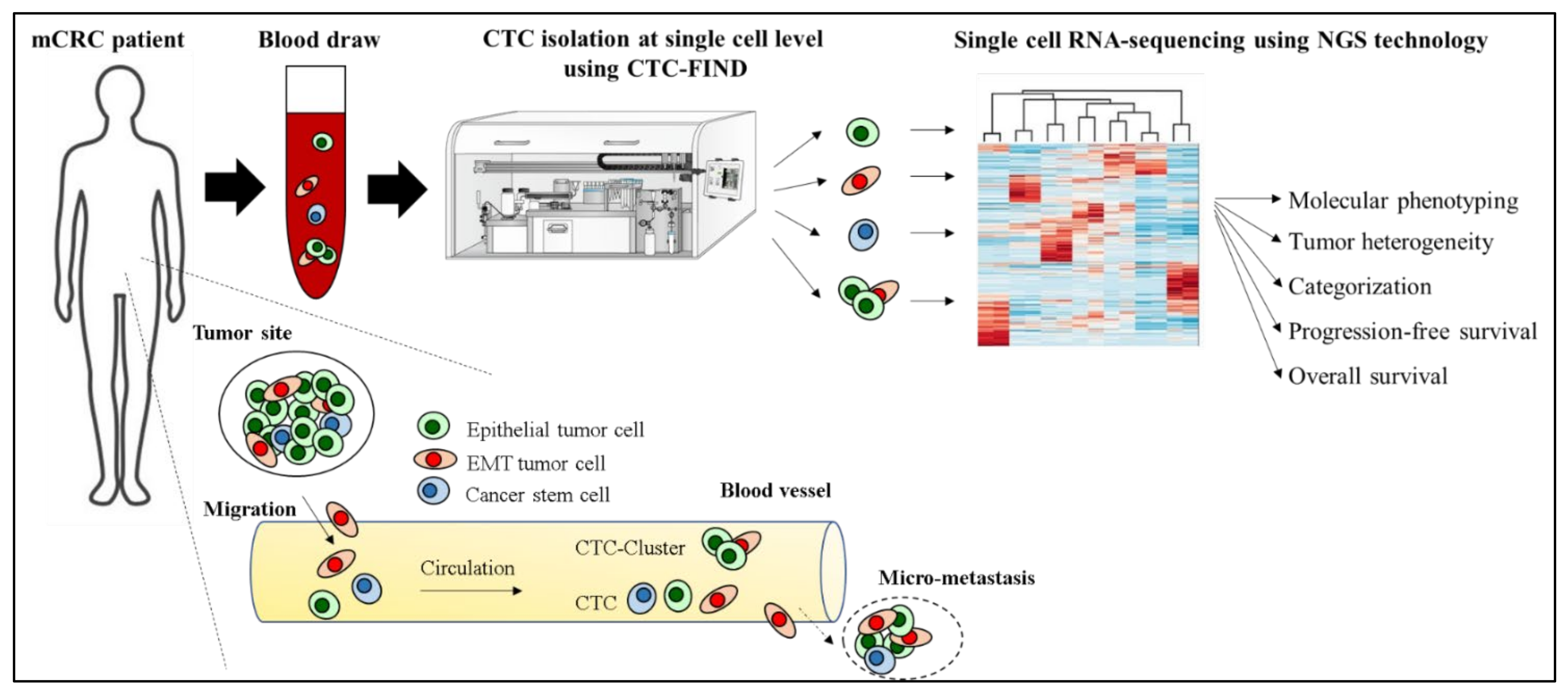
2.1. Sample Collection
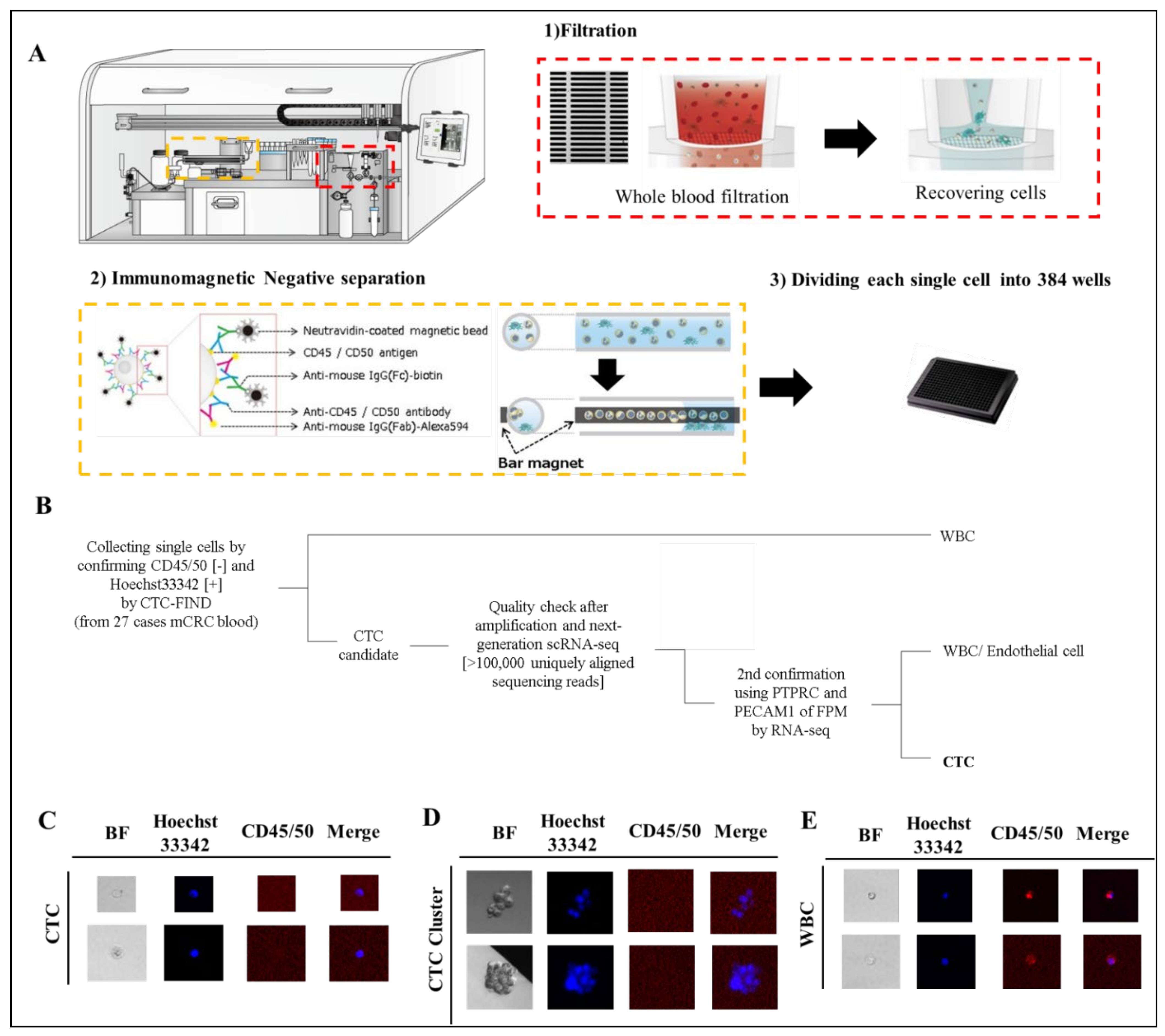
2.2. CTC Enrichment
2.3. Leukocyte Depletion
2.4. Isolating Single CTC Candidates
2.5. Single Cell RNA Sequencing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Detection and Isolation of CTC Candidates from mCRC Patients
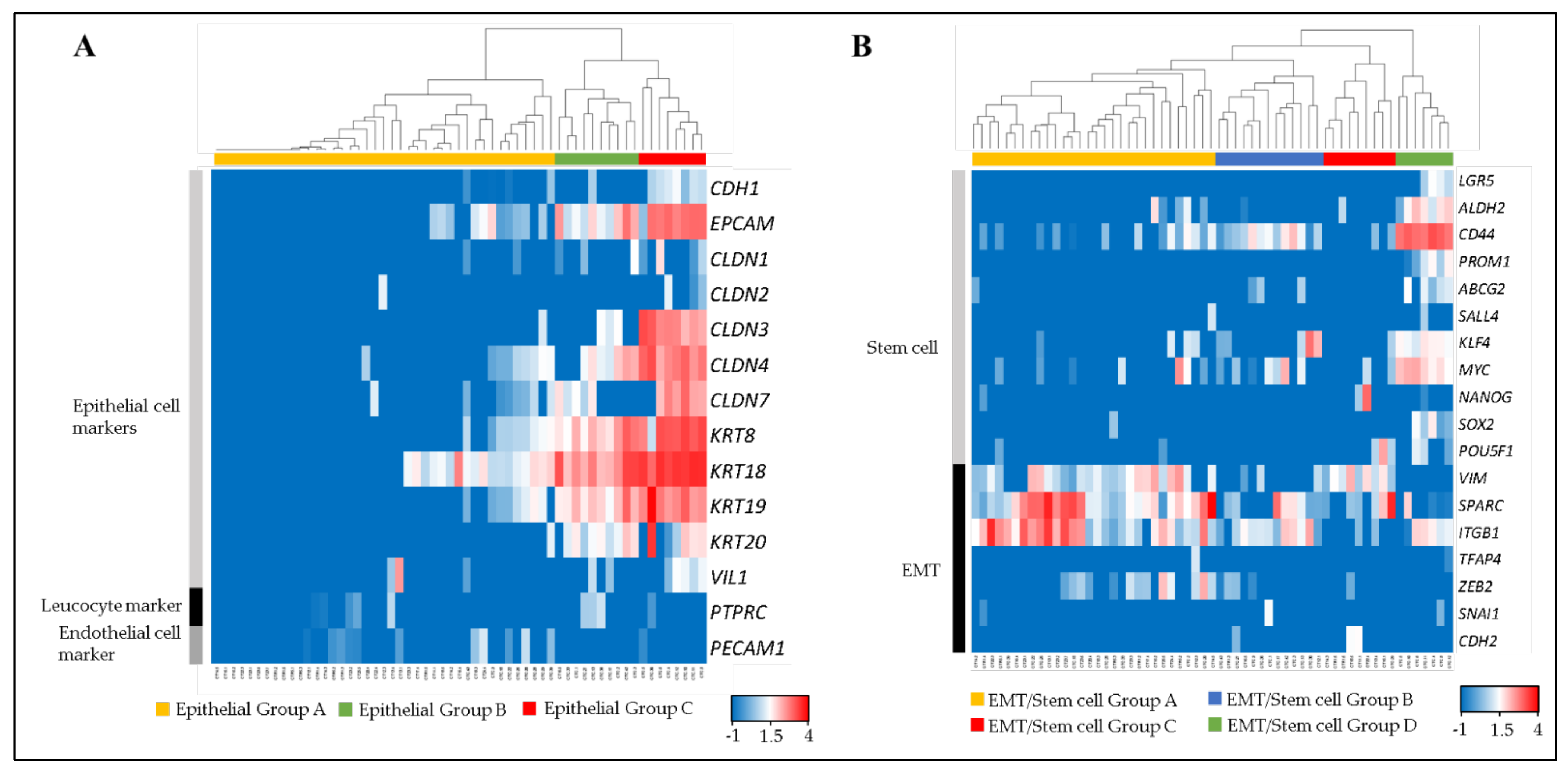
3.2. Epithelial Phenotyping of Single CTCs and CTC-Clusters
3.3. EMT and Stem Cell Related Phenotyping
3.4. Categorization of CTC Phenotypes
3.5. Phenotypic Heterogeneity of CTCs in mCRC
3.6. Phenotypic Frequency
3.7. Prognostic Relevance of CTC Phenotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vatandoust, S.; Price, T.J.; Karapetis, C.S. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol. 2015, 21, 11767–11776. [Google Scholar] [CrossRef]
- Mao, C.; Wu, X.-Y.; Yang, Z.-Y.; Threapleton, D.E.; Yuan, J.-Q.; Yu, Y.-Y.; Tang, J.-L. Concordant analysis of KRAS, BRAF, PIK3CA mutations and PTEN expression between primary colorectal cancer and matched metastases. Sci. Rep. 2015, 5, 8065. [Google Scholar] [CrossRef] [PubMed]
- Komor, M.A.; Bosch, L.J.; Bounova, G.; Bolijn, A.S.; Diemen, P.M.D.-V.; Rausch, C.; Hoogstrate, Y.; Stubbs, A.P.; De Jong, M.; Jenster, G.; et al. Consensus molecular subtype classification of colorectal adenomas. J. Pathol. 2018, 246, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, H.-J.; Argiles, G.; Yoshino, T.; Tejpar, S.; Ciardiello, F.; Braunger, J.; Salnikov, A.V.; Gabrielyan, O.; Schmid, R.; Höfler, J.; et al. Association of Consensus Molecular Subtypes and Molecular Markers with Clinical Outcomes in Patients With Metastatic Colorectal Cancer: Biomarker Analyses From LUME-Colon 1. Clin. Color. Cancer 2021, 20, 84–95.e8. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Ou, F.-S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of Consensus Molecular Subtype on Survival in Patients With Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2019, 37, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Hou, Y.; Zhou, X.; Li, X.; Yong, J.; Wang, Y.; Wang, W.; Yan, J.; Hu, B.; Guo, H.; et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science 2018, 362, 1060–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Zhou, F.; Tang, D.; Lin, L.; Zou, C.; Tan, W.; Dai, Y. Single-cell transcriptional profiling reveals the heterogenicity in colorectal cancer. Medicine 2019, 98, e16916. [Google Scholar] [CrossRef] [PubMed]
- Tieng, F.Y.F.; Baharudin, R.; Abu, N.; Yunos, R.-I.M.; Lee, L.-H.; Ab Mutalib, N.-S. Single Cell Transcriptome in Colorectal Cancer—Current Updates on Its Application in Metastasis, Chemoresistance and the Roles of Circulating Tumor Cells. Front. Pharmacol. 2020, 11, 135. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Meropol, N.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 2013, 24, 420–428. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, W.; Hanna, D.L.; Yang, D.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Suenaga, M.; Schirripa, M.; El-Khoueiry, A.; et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectal cancer patients. Pharm. J. 2018, 18, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [Green Version]
- Matsusaka, S.; Kozuka, M.; Takagi, H.; Ito, H.; Minowa, S.; Hirai, M.; Hatake, K. A novel detection strategy for living circulating tumor cells using 5-aminolevulinic acid. Cancer Lett. 2014, 355, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ito, H.; Kozuka, M.; Takagi, H.; Hirai, M.; Fujii, T. Cancer marker-free enrichment and direct mutation detection in rare cancer cells by combining multi-property isolation and microfluidic concentration. Lab Chip 2019, 19, 757–766. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in anti-androgen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Cherradi, S.; Martineau, P.; Gongora, C.; Del Rio, M. Claudin gene expression profiles and clinical value in colorectal tumors classified according to their molecular subtype. Cancer Manag. Res. 2019, 11, 1337–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostert, B.; Sieuwerts, A.M.; Vries, J.B.-D.; Kraan, J.; Lalmahomed, Z.; Van Galen, A.; Van Der Spoel, P.; De Weerd, V.; Ramírez-Moreno, R.; Smid, M.; et al. mRNA expression profiles in circulating tumor cells of metastatic colorectal cancer patients. Mol. Oncol. 2015, 9, 920–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoursheed, M.; Mathew, T.; Makar, R.; Louis, S.; Asfar, S.; Al-Sayer, H.; Dashti, H.; Al-Bader, A. Expression of E-cadherin in human colorectal cancer. Surgeon 2003, 1, 86–91. [Google Scholar] [CrossRef]
- Ferrary, E.; Cohen-Tannoudji, M.; Pehau-Arnaudet, G.; Lapillonne, A.; Athman, R.; Ruiz, T.; Boulouha, L.; El Marjou, F.; Doye, A.; Fontaine, J.-J.; et al. In Vivo, Villin Is Required for Ca2+-Dependent F-Actin Disruption in Intestinal Brush Borders. J. Cell Biol. 1999, 146, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef]
- Du, L.; Wang, H.; He, L.; Zhang, J.; Ni, B.; Wang, X.; Jin, H.; Cahuzac, N.; Mehrpour, M.; Lu, Y.; et al. CD44 is of Functional Importance for Colorectal Cancer Stem Cells. Clin. Cancer Res. 2008, 14, 6751–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.-W.; Wu, J.; Jiang, C.-P. ABCG2: A potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010, 86, 631–637. [Google Scholar] [CrossRef]
- Forghanifard, M.M.; Moghbeli, M.; Raeisossadati, R.; Tavassoli, A.; Mallak, A.J.; Boroumand-Noughabi, S.; Abbaszadegan, M.R. Role of SALL4 in the progression and metastasis of colorectal cancer. J. Biomed. Sci. 2013, 20, 6. [Google Scholar] [CrossRef] [Green Version]
- Leng, Z.; Tao, K.; Xia, Q.; Tan, J.; Yue, Z.; Chen, J.; Xi, H.; Li, J.; Zheng, H. Krüppel-Like Factor 4 Acts as an Oncogene in Colon Cancer Stem Cell-Enriched Spheroid Cells. PLoS ONE 2013, 8, e56082. [Google Scholar] [CrossRef] [Green Version]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target Against Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2340. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.E.; Babaei-Jadidi, R.; Saadeddin, A.; Spencer-Dene, B.; Hossaini, S.; Abuzinadah, M.; Li, N.; Fadhil, W.; Ilyas, M.; Bonnet, D.; et al. Embryonic NANOG Activity Defines Colorectal Cancer Stem Cells and Modulates through AP1- and TCF-dependent Mechanisms. Stem Cells 2012, 30, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Kianersi, K.; Beiraghdar, M. Comparison of gene expression of SOX2 and OCT4 in normal tissue, polyps, and colon adenocarcinoma using immunohistochemical staining. Adv. Biomed. Res. 2015, 4, 234. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ge, J.; Wang, X.; Qian, X.; Zhang, C.; Li, X. OCT4 regulates epithelial-mesenchymal transition and its knockdown inhibits colorectal cancer cell migration and invasion. Oncol. Rep. 2012, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [Green Version]
- Carriere, P.; Calvo, N.; Díaz, M.B.N.; Lopez-Moncada, F.; Herrera, A.; Torres, M.J.; Alonso, E.; Gandini, N.A.; Gigola, G.; Contreras, H.R.; et al. Role of SPARC in the epithelial-mesenchymal transition induced by PTHrP in human colon cancer cells. Mol. Cell. Endocrinol. 2021, 530, 111253. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.J.; Tak, K.H.; Kim, S.K.; Kim, C.W.; Lee, J.L.; Roh, S.A.; Cho, D.-H.; Kim, S.-Y.; Kim, Y.S.; Kim, J.C. Biological Characteristics and Clinical Significance of ITGB1 and RHOC in Patients with Re-current Colorectal Cancer. Anticancer Res. 2019, 39, 4853–4864. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jackstadt, R.; Siemens, H.; Li, H.; Kirchner, T.; Hermeking, H. Abstract 1136: p53-induced miR-15a/16-1 and AP4 form a double-negative feedback loop to regulate epithelial-mesenchymal transition and metastasis in colorectal cancer. Tumor Biol. 2014, 74, 532–542. [Google Scholar] [CrossRef]
- Kahlert, C.; Lahes, S.; Radhakrishnan, P.; Dutta, S.; Mogler, C.; Herpel, E.; Brand, K.; Steinert, G.; Schneider, M.; Mollenhauer, M.; et al. Overexpression of ZEB2 at the Invasion Front of Colorectal Cancer Is an Independent Prognostic Marker and Regulates Tumor Invasion In Vitro. Clin. Cancer Res. 2011, 17, 7654–7663. [Google Scholar] [CrossRef] [Green Version]
- Francí, C.; Gallén, M.; Alameda, F.; Baró, T.; Iglesias, M.; Virtanen, I.; De Herreros, A.G. Snail1 Protein in the Stroma as a New Putative Prognosis Marker for Colon Tumours. PLoS ONE 2009, 4, e5595. [Google Scholar] [CrossRef]
- Yan, X.; Yan, L.; Liu, S.; Shan, Z.; Tian, Y.; Jin, Z. N-cadherin, a novel prognostic biomarker, drives malignant progression of colorectal cancer. Mol. Med. Rep. 2015, 12, 2999–3006. [Google Scholar] [CrossRef] [Green Version]
- Habli, Z.; Alchamaa, W.; Saab, R.; Kadara, H.; Khraiche, M.L. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Nanduri, S.L.K.; Hissa, B.; Weitz, J.; Schölch, S.; Bork, U. The prognostic role of circulating tumor cells in colorectal cancer. Expert Rev. Anticancer Ther. 2019, 19, 1077–1088. [Google Scholar] [CrossRef]
- Yadavalli, S.; Jayaram, S.; Manda, S.S.; Madugundu, A.K.; Nayakanti, D.S.; Tan, T.Z.; Bhat, R.; Rangarajan, A.; Chatterjee, A.; Gowda, H.; et al. Data-Driven Discovery of Extravasation Pathway in Circulating Tumor Cells. Sci. Rep. 2017, 7, 43710. [Google Scholar] [CrossRef] [Green Version]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, X.; Lu, C.; Ma, W.; Wang, Y.; Xia, G.; Wang, C.; Yang, G. Cancer stem-like cells with hybrid epithelial/mesenchymal phenotype leading the collective invasion. Cancer Sci. 2019, 111, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, J.; Wang, J.; Cao, Z.; Wang, J.; Guo, X.; Dong, W. beta1 integrin modulates tumor growth and apoptosis of human colorectal cancer. Oncol. Rep. 2014, 32, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.K.; Gnanamony, M.; Gondi, C.S. The ‘SPARC’ of life: Analysis of the role of osteonectin/SPARC in pancreatic cancer (Review). Int. J. Oncol. 2016, 48, 1765–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, E.; Reduzzi, C.; Motta, R.; Di Cosimo, S.; Bianchi, G.V.; Martinetti, A.; Wechsler, J.; Cappelletti, V.; Daidone, M.G. Did Circulating Tumor Cells Tell us all they Could? The Missed Circulating Tumor Cell Message in Breast Cancer. Int. J. Biol. Markers 2015, 30, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Cai, Z.; Li, S.; Cheng, Y.; Gao, H.; Liu, F.; Wu, S.; Liu, S.; Dong, Y.; Zheng, L.; et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget 2016, 8, 9293–9302. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| EMT/Stem Group A: | EMT/Stem Group B: | EMT/Stem Group C: | EMT/Stem Group D: | |
|---|---|---|---|---|
| High Stem/Low EMT | Low Stem/Middle EMT | Low Stem/Low EMT | Low Stem/High EMT | |
| Epithelial Group A: High | 7 | 0 | 1 | 0 |
| ↑ ** | ns | ns | ↓ ** | |
| Epithelial Group B: Middle | 0 | 0 | 7 | 3 |
| ns | ns | ↑ ** | ns | |
| Epithelial Group C: Low | 0 | 9 | 4 | 28 |
| ↓ ** | ↑ ** | ↓ ** | ↑ ** |
| Type of CTC | Patient # | Detection Rate | |
|---|---|---|---|
| Positive | Negative | ||
| Epitherial | 8 | 19 | 30% **,*** |
| EMT | 20 | 7 | 74% ** |
| Epitherial/EMT | 24 | 3 | 89% *** |
| CTC Type | CTC # | n | PFS | OS | ||||
|---|---|---|---|---|---|---|---|---|
| Median | (95% CI), Days | p-Value | Median | (95% CI), Days | p-Value | |||
| EMT | ≥1 | 0.075 | 0.212 | |||||
| − | 8 | 180 | (−272, 632) | 230 | (−306, 766) | |||
| + | 14 | 48 | (43, 53) | 110 | (38, 182) | |||
| EMT | ≥2 | 0.034 * | 0.04 * | |||||
| − | 14 | 76 | (−20, 172) | 383 | (−139, 905) | |||
| + | 8 | 40 | (28, 52) | 64 | (−47, 175) | |||
| Epithelial | ≥1 | 0.825 | 0.496 | |||||
| − | 20 | 52 | (42, 62) | 230 | (−116, 577) | |||
| + | 2 | 41 | - | 194 | - | |||
| Epithelial | ≥2 | 0.336 | 0.327 | |||||
| − | 21 | 55 | (45, 65) | 194 | (−29, 359) | |||
| + | 1 | 464 | - | - | - | |||
| Epithelial/EMT | ≥1 | 0.643 | 0.74 | |||||
| − | 17 | 52 | (43, 61) | 230 | (−232, 692) | |||
| + | 5 | 105 | (32, 242) | 194 | (2, 385) | |||
| Epithelial/EMT | ≥2 | 0.347 | 0.685 | |||||
| − | 18 | 49 | (40, 57) | 230 | (−94, 554) | |||
| + | 4 | 105 | (−265, 475) | 105 | (105, 105) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozuka, M.; Battaglin, F.; Jayachandran, P.; Wang, J.; Arai, H.; Soni, S.; Zhang, W.; Hirai, M.; Matsusaka, S.; Lenz, H.-J. Clinical Significance of Circulating Tumor Cell Induced Epithelial-Mesenchymal Transition in Patients with Metastatic Colorectal Cancer by Single-Cell RNA-Sequencing. Cancers 2021, 13, 4862. https://doi.org/10.3390/cancers13194862
Kozuka M, Battaglin F, Jayachandran P, Wang J, Arai H, Soni S, Zhang W, Hirai M, Matsusaka S, Lenz H-J. Clinical Significance of Circulating Tumor Cell Induced Epithelial-Mesenchymal Transition in Patients with Metastatic Colorectal Cancer by Single-Cell RNA-Sequencing. Cancers. 2021; 13(19):4862. https://doi.org/10.3390/cancers13194862
Chicago/Turabian StyleKozuka, Masahiro, Francesca Battaglin, Priya Jayachandran, Jingyuan Wang, Hiroyuki Arai, Shivani Soni, Wu Zhang, Mitsuharu Hirai, Satoshi Matsusaka, and Heinz-Josef Lenz. 2021. "Clinical Significance of Circulating Tumor Cell Induced Epithelial-Mesenchymal Transition in Patients with Metastatic Colorectal Cancer by Single-Cell RNA-Sequencing" Cancers 13, no. 19: 4862. https://doi.org/10.3390/cancers13194862
APA StyleKozuka, M., Battaglin, F., Jayachandran, P., Wang, J., Arai, H., Soni, S., Zhang, W., Hirai, M., Matsusaka, S., & Lenz, H.-J. (2021). Clinical Significance of Circulating Tumor Cell Induced Epithelial-Mesenchymal Transition in Patients with Metastatic Colorectal Cancer by Single-Cell RNA-Sequencing. Cancers, 13(19), 4862. https://doi.org/10.3390/cancers13194862






