Quantifying the Effects of Medical Examination and Possible Risk Factors against the Incidence of Cervical Cancer in a Low Human Papillomavirus Vaccination Coverage: An Ecological Study in Japan
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
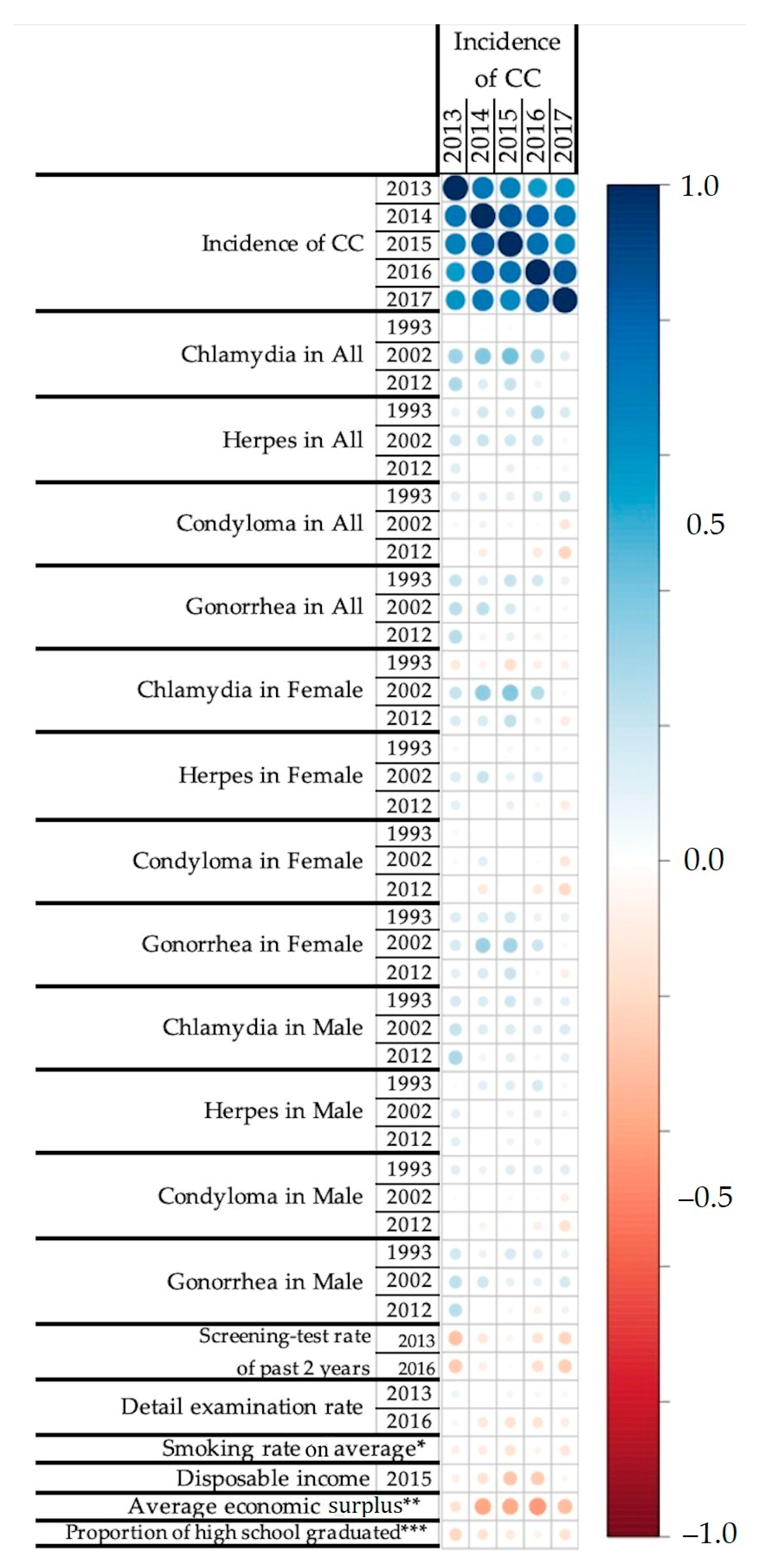
3.1. Correlation Analysis
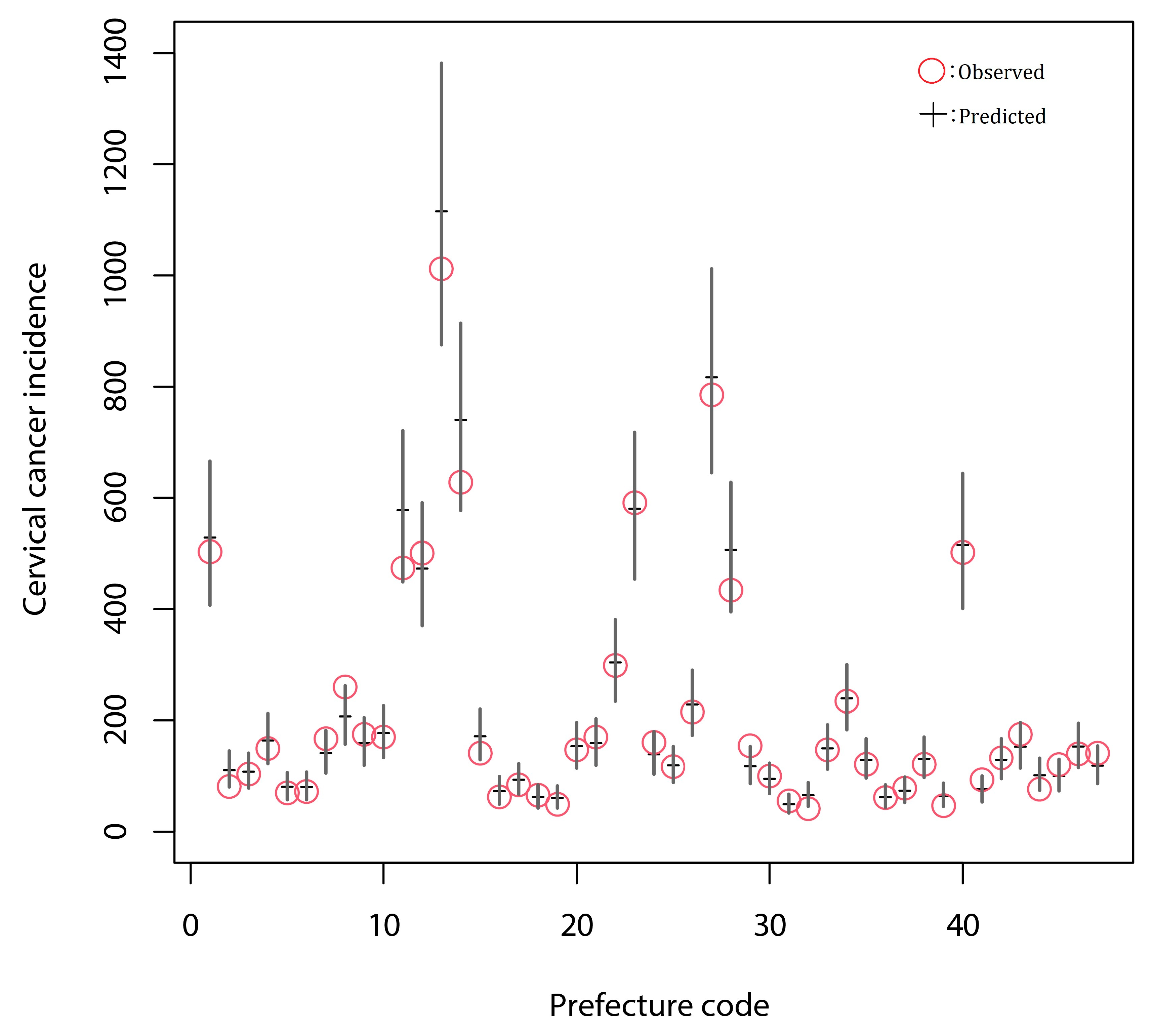
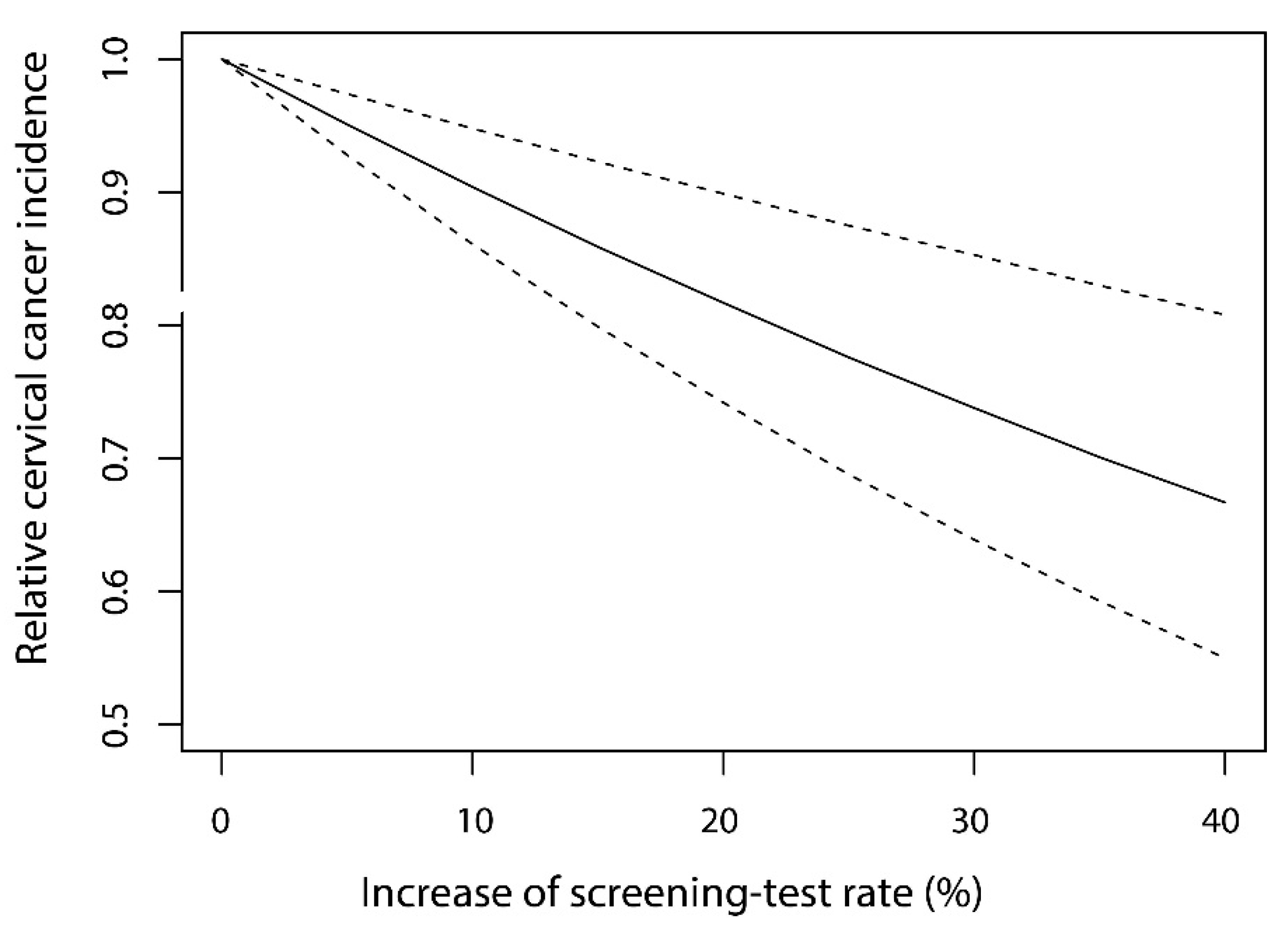
3.2. Generalized Linear Modeling and the Estimation of the Effect of Screening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Andersson, S.; Safari, H.; Mints, M.; Lewensohn-Fuchs, I.; Gyllensten, U.; Johansson, B. Type distribution, viral load and integration status of high-risk human papillomaviruses in pre-stages of cervical cancer (CIN). Br. J. Cancer 2005, 92, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous detection and viral DNA load quantification of different human papillomavirus types in clinical specimens by the high analytical droplet digital PCR method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Bharadwaj, M. A step towards resolving the mystery of HPV vaccination: Breakthrough of cervical cancer. Adv. Cytol. Pathol. 2018, 3, 1. [Google Scholar] [CrossRef]
- Janicek, M.F.; Averette, H.E. Cervical cancer: Prevention, diagnosis, and therapeutics. CA Cancer. J. Clin. 2001, 51, 92–114. [Google Scholar] [CrossRef]
- World Health Organization. The Immunological Basis for Immunization Series: Module 19: Human Papillomavirus Infection; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human papillomavirus-attributable cancers—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- GARDASIL. Available online: https://www.fda.gov/media/74350/download (accessed on 20 October 2020).
- GARDASIL 9. Available online: https://www.fda.gov/media/90064/download (accessed on 20 October 2020).
- Simms, K.T.; Hanley, S.J.B.; Smith, M.A.; Keane, A.; Canfell, K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: A modelling study. Lancet Public Health 2020, 5, e223–e234. [Google Scholar] [CrossRef]
- Pharmaceutical Products Interview Form of Cervarix. Available online: https://gskpro.com/content/dam/global/hcpportal/ja_JP/products-info/cervarix/cervarix-if.pdf (accessed on 20 October 2020).
- Pharmaceutical Products Interview Form of GARDASIL. Available online: https://www.msdconnect.jp/static/mcijapan/images/if_gardasil.pdf (accessed on 20 October 2020).
- Pharmaceutical Products Interview Form of SILGARD 9. Available online: https://www.msdconnect.jp/static/mcijapan/images/if_silgard9.pdf (accessed on 20 October 2020).
- Implementation of Revised Part of Immunization Act. Available online: https://www.mhlw.go.jp/stf/shingi/2r98520000033079-att/2r985200000330hr_1.pdf (accessed on 20 October 2020).
- Tsuda, K.; Yamamoto, K.; Leppold, C.; Tanimoto, T.; Kusumi, E.; Komatsu, T.; Kami, M. Trends of media coverage on human papillomavirus vaccination in Japanese newspapers. Clin. Infect. Dis. 2016, 63, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Hanley, S.J.; Yoshioka, E.; Ito, Y.; Kishi, R. HPV vaccination crisis in Japan. Lancet 2015, 385, 2571. [Google Scholar] [CrossRef]
- Ueda, Y.; Enomoto, T.; Sekine, M.; Egawa-Takata, T.; Morimoto, A.; Kimura, T. Japan’s failure to vaccinate girls against human papillomavirus. Am. J. Obstet. Gynecol. 2015, 212, 405–406. [Google Scholar] [CrossRef]
- Anttila, T.; Saikku, P.; Koskela, P.; Bloigu, A.; Dillner, J.; Ikaheimo, I.; Jellum, E.; Lehtinen, M.; Lenner, P.; Hakulinen, T.; et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 2001, 285, 47–51. [Google Scholar] [CrossRef]
- Arnheim Dahlstrom, L.; Andersson, K.; Luostarinen, T.; Thoresen, S.; Ogmundsdottir, H.; Tryggvadottir, L.; Wiklund, F.; Skare, G.B.; Eklund, C.; Sjolin, K.; et al. Prospective seroepidemiologic study of human papillomavirus and other risk factors in cervical cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2541–2550. [Google Scholar] [CrossRef]
- Munoz, N.; Castellsague, X.; Berrington de Gonzalez, A.; Gissmann, L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006, 24 (Suppl. S3), 1–10. [Google Scholar] [CrossRef]
- Smith, J.S.; Herrero, R.; Bosetti, C.; Munoz, N.; Bosch, F.X.; Eluf-Neto, J.; Castellsague, X.; Meijer, C.J.; Van den Brule, A.J.; Franceschi, S.; et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 2002, 94, 1604–1613. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 83, 1–1438. [Google Scholar]
- Vaccarella, S.; Herrero, R.; Snijders, P.J.; Dai, M.; Thomas, J.O.; Hieu, N.T.; Ferreccio, C.; Matos, E.; Posso, H.; de Sanjose, S.; et al. Smoking and human papillomavirus infection: Pooled analysis of the international agency for research on cancer HPV Prevalence Surveys. Int. J. Epidemiol. 2008, 37, 536–546. [Google Scholar] [CrossRef]
- Chuang, L.T.; Temin, S.; Camacho, R.; Duenas-Gonzalez, A.; Feldman, S.; Gultekin, M.; Gupta, V.; Horton, S.; Jacob, G.; Kidd, E.A.; et al. Management and care of women with invasive cervical cancer: American society of clinical oncology resource-stratified clinical practice guideline. J. Glob. Oncol. 2016, 2, 311–340. [Google Scholar] [CrossRef]
- Randall, T.C.; Ghebre, R. Challenges in prevention and care delivery for women with cervical cancer in Sub-Saharan Africa. Front. Oncol. 2016, 6, 160. [Google Scholar] [CrossRef]
- Parikh, S.; Brennan, P.; Boffetta, P. Meta-analysis of social inequality and the risk of cervical cancer. Int. J. Cancer 2003, 105, 687–691. [Google Scholar] [CrossRef]
- Hamashima, C.; Aoki, D.; Miyagi, E.; Saito, E.; Nakayama, T.; Sagawa, M.; Saito, H.; Sobue, T.; Japanese Research Group for Development of Cervical Cancer Screening. The Japanese guideline for cervical cancer screening. Jpn. J. Clin. Oncol. 2010, 40, 485–502. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Human Papillomavirus (HPV) and Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 16 May 2021).
- Monitoring of Cancer Incidence in Japan. Available online: https://ganjoho.jp/reg_stat/statistics/brochure/monitoring.html (accessed on 20 October 2020).
- Cancer Registry and Statistics. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450173&tstat=000001133323&cycle=7&tclass1=000001133363&tclass2=000001133368&tclass3=000001133369&tclass4val=0 (accessed on 20 October 2020).
- Annual Report of National Epidemiological Surveillance of Infectious Diseases Program. Available online: https://www.niid.go.jp/niid/ja/idsc/8218-surveillance-nenpou.html (accessed on 20 October 2020).
- Past Annual Report of National Epidemiological Surveillance of Infectious Diseases Program. Available online: https://www.niid.go.jp/niid/ja/allarticles/surveillance/2270-idwr/nenpou/10116-kako2019.html (accessed on 20 October 2020).
- Download Page of Statistical Data on Cancer Screening. Available online: https://ganjoho.jp/reg_stat/statistics/stat/screening/dl_screening.html (accessed on 20 October 2020).
- System of Social and Demographic Statistics Data Prefectural Basic Data. Available online: https://www.e-stat.go.jp/dbview?sid=0000010112 (accessed on 20 October 2020).
- Average Economic Surplus by Prefecture (The Difference between Disposable Income and The Average Expenditure). Available online: https://www.mlit.go.jp/policy/shingikai/content/001389727.pdf (accessed on 20 October 2020).
- Employment Status Survey. Available online: https://www.e-stat.go.jp/stat-search/database?page=1&toukei=00200532 (accessed on 20 October 2020).
- Summary of Result of Estimate Population. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00200241&tstat=000001039591&cycle=7&tclass1=000001039601&tclass2val=0 (accessed on 20 October 2020).
- Stekhoven, D.J. Miss Forest: Nonparametric Missing Value Imputation using Random Forest. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- R Development Core Team R. The R Foundation for Statistical Computing; R Development Core Team R: Vienna, Austria, 2020. [Google Scholar]
- Ripley, B.D.; Venables, W.N. Modern Applied Statistics with S; Springer: Berlin, Germany, 2002. [Google Scholar]
- Harrell, F.E. RMS: Regression Modeling Strategies; Springer: Berlin, Germany, 2020. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Franceschi, S.; Smith, J.S.; van den Brule, A.; Herrero, R.; Arslan, A.; Anh, P.T.; Bosch, F.X.; Hieu, N.T.; Matos, E.; Posso, H.; et al. Cervical infection with Chlamydia trachomatis and Neisseria gonorrhoeae in women from ten areas in four continents. A cross-sectional study. Sex. Transm. Dis. 2007, 34, 563–569. [Google Scholar] [CrossRef]
- Friis, S.; Kjaer, S.K.; Frisch, M.; Mellemkjaer, L.; Olsen, J.H. Cervical intraepithelial neoplasia, anogenital cancer, and other cancer types in women after hospitalization for condylomata acuminata. J. Infect. Dis. 1997, 175, 743–748. [Google Scholar] [CrossRef][Green Version]
- Furgyik, S. Gonorrhea infection as a risk indicator for cervix cancer. Geburtshilfe Frauenheilkd 1987, 47, 320–323. [Google Scholar] [CrossRef]
- Lehtinen, M.; Koskela, P.; Jellum, E.; Bloigu, A.; Anttila, T.; Hallmans, G.; Luukkaala, T.; Thoresen, S.; Youngman, L.; Dillner, J.; et al. Herpes simplex virus and risk of cervical cancer: A longitudinal, nested case-control study in the nordic countries. Am. J. Epidemiol. 2002, 156, 687–692. [Google Scholar] [CrossRef]
- Sadan, O.; Bilevsky, E.; Shejter, E.; Levy, T.; Bachar, R.; Yarden, H.; Glezerman, M.; Lurie, S. Occurrence of cervical intraepithelial neoplasia in generally healthy women with exophytic vulvar condyloma acuminata. Infect. Dis. Obstet. Gynecol. 2005, 13, 141–143. [Google Scholar] [CrossRef]
- Wallin, K.L.; Wiklund, F.; Luostarinen, T.; Angstrom, T.; Anttila, T.; Bergman, F.; Hallmans, G.; Ikaheimo, I.; Koskela, P.; Lehtinen, M.; et al. A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int. J. Cancer 2002, 101, 371–374. [Google Scholar] [CrossRef]
- Svare, E.I.; Kjaer, S.K.; Worm, A.M.; Osterlind, A.; Moi, H.; Christensen, R.B.; Meijer, C.J.; Walboomers, J.M.; van den Brule, A.J. Risk factors for HPV infection in women from sexually transmitted disease clinics: Comparison between two areas with different cervical cancer incidence. Int. J. Cancer 1998, 75, 1–8. [Google Scholar] [CrossRef]
- Luostarinen, T.; af Geijersstam, V.; Bjorge, T.; Eklund, C.; Hakama, M.; Hakulinen, T.; Jellum, E.; Koskela, P.; Paavonen, J.; Pukkala, E.; et al. No excess risk of cervical carcinoma among women seropositive for both HPV16 and HPV6/11. Int. J. Cancer 1999, 80, 818–822. [Google Scholar] [CrossRef]
- Naucler, P.; Chen, H.C.; Persson, K.; You, S.L.; Hsieh, C.Y.; Sun, C.A.; Dillner, J.; Chen, C.J. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: Nested case-control study. J. Gen. Virol. 2007, 88, 814–822. [Google Scholar] [CrossRef]
- Wu, S.; Chen, H. Anti-Condyloma acuminata mechanism of microRNAs-375 modulates HPV in cervical cancer cells via the UBE3A and IGF-1R pathway. Oncol. Lett. 2018, 16, 3241–3247. [Google Scholar] [CrossRef]
- Landy, R.; Pesola, F.; Castanon, A.; Sasieni, P. Impact of cervical screening on cervical cancer mortality: Estimation using stage-specific results from a nested case-control study. Br. J. Cancer 2016, 115, 1140–1146. [Google Scholar] [CrossRef]
- Landy, R.; Sasieni, P.D.; Mathews, C.; Wiggins, C.L.; Robertson, M.; McDonald, Y.J.; Goldberg, D.W.; Scarinci, I.C.; Cuzick, J.; Wheeler, C.M.; et al. Impact of screening on cervical cancer incidence: A population-based case-control study in the United States. Int. J. Cancer 2020, 147, 887–896. [Google Scholar] [CrossRef]
- Sasieni, P.; Castanon, A.; Cuzick, J. Effectiveness of cervical screening with age: Population based case-control study of prospectively recorded data. BMJ 2009, 339, b2968. [Google Scholar] [CrossRef]
- Yang, B.; Morrell, S.; Zuo, Y.; Roder, D.; Tracey, E.; Jelfs, P. A case-control study of the protective benefit of cervical screening against invasive cervical cancer in NSW women. Cancer Causes Control 2008, 19, 569–576. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Screening for squamous cervical cancer: Duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on evaluation of cervical cancer screening programmes. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 659–664. [Google Scholar] [CrossRef] [PubMed]
- About the Screening Test of Cervical Cancer. Available online: https://ganjoho.jp/public/pre_scr/screening/about_scr02.html (accessed on 20 October 2020).
- National Basic Plan to Promote Cancer Control Program in Japan. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000183313.html (accessed on 20 October 2020).
- Sano, H.; Goto, R.; Hamashima, C. Does lack of resources impair access to breast and cervical cancer screening in Japan? PLoS ONE 2017, 12, e0180819. [Google Scholar] [CrossRef]
- Drolet, M.; Benard, E.; Boily, M.C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.; Cummings, T.; et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef]
- Drolet, M.; Benard, E.; Perez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Konno, R.; Konishi, H.; Sauvaget, C.; Ohashi, Y.; Kakizoe, T. Effectiveness of HPV vaccination against high grade cervical lesions in Japan. Vaccine 2018, 36, 7913–7915. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.O.; Schuz, J.; Engholm, G.; Johansen, C.; Kjaer, S.K.; Steding-Jessen, M.; Storm, H.H.; Olsen, J.H. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994-2003: Summary of findings. Eur. J. Cancer 2008, 44, 2074–2085. [Google Scholar] [CrossRef]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.V., Jr.; Frisch, M.; Brinton, L.A.; Abbas, F.M.; Barnes, W.A.; Gravitt, P.E.; Greenberg, M.D.; Greene, S.M.; Hadjimichael, O.C.; McGowan, L.; et al. Associations between smoking and adenocarcinomas and squamous cell carcinomas of the uterine cervix (United States). Cancer Causes Control 2001, 12, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Tsuji, I.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; Kitamura, Y.; et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn. J. Clin. Oncol. 2019, 49, 77–86. [Google Scholar] [CrossRef]
- Wei, L.; Griego, A.M.; Chu, M.; Ozbun, M.A. Tobacco exposure results in increased E6 and E7 oncogene expression, DNA damage and mutation rates in cells maintaining episomal human papillomavirus 16 genomes. Carcinogenesis 2014, 35, 2373–2381. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef]
| Data | Period | Source |
|---|---|---|
| Incidence of CC | 2013–2017 | Cancer Registry and Statistics [33,34] |
| Incidence of STDs Condyloma, Chlamydia, Gonorrhea, Herpes | 1993–2012 | National Epidemiological Surveillance of Infectious Diseases [35,36] |
| Screening test rate for cervix | 2013, 2016 | Comprehensive Survey of Living Conditions [37] |
| Detailed examination rate for cervical diseases | 2013, 2016 | Comprehensive Survey of Living Conditions [37] |
| Smoking rate | 2001, 2004, 2007, 2010, 2013 | Comprehensive Survey of Living Conditions [37] |
| Disposable income | 2015 | Statistics Bureau of Japan [38] |
| Average economic surplus | 2014 | Ministry of Land, Infrastructure, Transport and Tourism [39] |
| Proportion whose educational level is equal to or under high school graduate (education level) | 2012 | Population Census [40] |
| Equal to or over 25 years old female population in each prefecture | 2015 | Statistics Bureau of Japan [41] |
| Years of STD Incidences that Were Averaged * | Type of STD Data | ||||
|---|---|---|---|---|---|
| All STDs | Female STDs Only | Male STDs Only | STDs in Both Genders | ||
| 1 ** | Time-lag | 14 | 14 | 15 | 15 |
| AIC | 2155.1 | 2177.3 | 2162.9 | 2146.5 *** | |
| 3 | Time-lag | 16 | 15 | 16 | 16 |
| AIC | 2150.8 | 2174.5 | 2160.8 | 2151.0 | |
| 5 | Time-lag | 16 | 16 | 18 | 16 |
| AIC | 2150.0 | 2171.5 | 2160.9 | 2150.7 | |
| 7 | Time-lag | 18 | 17 | 19 | 17 |
| AIC | 2150.6 | 2170.0 | 2162.2 | 2149.9 | |
| 9 | Time-lag | 19 | 19 | 20 | 19 |
| AIC | 2151.5 | 2168.6 | 2163.8 | 2149.6 | |
| 11 | Time-lag | 17 | 20 | 20 | 20 |
| AIC | 2153.1 | 2168.9 | 2166.7 | 2151.1 | |
| 13 | Time-lag | 18 | 20 | 20 | 18 |
| AIC | 2153.1 | 2169.4 | 2168.8 | 2152.4 | |
| 15 | Time-lag | 19 | 20 | 19 | 19 |
| AIC | 2154.1 | 2169.9 | 2168.8 | 2152.5 | |
| Variables | Regression Coefficient | 95%CI | p |
|---|---|---|---|
| Intercept | −7.0399 | −7.3886–−6.6912 | <0.001 |
| Chlamydia (female) | 0.0028 | 0.0011–0.0044 | <0.001 |
| Condyloma (female) | −0.0128 | −0.0260–0.0003 | 0.056 |
| Condyloma (male) | −0.0207 | −0.0330–−0.0084 | 0.001 |
| Gonorrhea (male) | 0.0061 | 0.0039–0.0084 | <0.001 |
| Average economic surplus | −0.0025 | −0.0034–−0.0016 | <0.001 |
| Smoking rate | −0.0209 | −0.0301–−0.0116 | <0.001 |
| Screening test rate | −0.0101 | −0.0150–−0.0053 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Matsuyama, R.; Tsunematsu, M.; Kakehashi, M. Quantifying the Effects of Medical Examination and Possible Risk Factors against the Incidence of Cervical Cancer in a Low Human Papillomavirus Vaccination Coverage: An Ecological Study in Japan. Cancers 2021, 13, 4784. https://doi.org/10.3390/cancers13194784
Yu Y, Matsuyama R, Tsunematsu M, Kakehashi M. Quantifying the Effects of Medical Examination and Possible Risk Factors against the Incidence of Cervical Cancer in a Low Human Papillomavirus Vaccination Coverage: An Ecological Study in Japan. Cancers. 2021; 13(19):4784. https://doi.org/10.3390/cancers13194784
Chicago/Turabian StyleYu, Yueming, Ryota Matsuyama, Miwako Tsunematsu, and Masayuki Kakehashi. 2021. "Quantifying the Effects of Medical Examination and Possible Risk Factors against the Incidence of Cervical Cancer in a Low Human Papillomavirus Vaccination Coverage: An Ecological Study in Japan" Cancers 13, no. 19: 4784. https://doi.org/10.3390/cancers13194784
APA StyleYu, Y., Matsuyama, R., Tsunematsu, M., & Kakehashi, M. (2021). Quantifying the Effects of Medical Examination and Possible Risk Factors against the Incidence of Cervical Cancer in a Low Human Papillomavirus Vaccination Coverage: An Ecological Study in Japan. Cancers, 13(19), 4784. https://doi.org/10.3390/cancers13194784






