Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia
Abstract
:Simple Summary
Abstract
1. Introduction
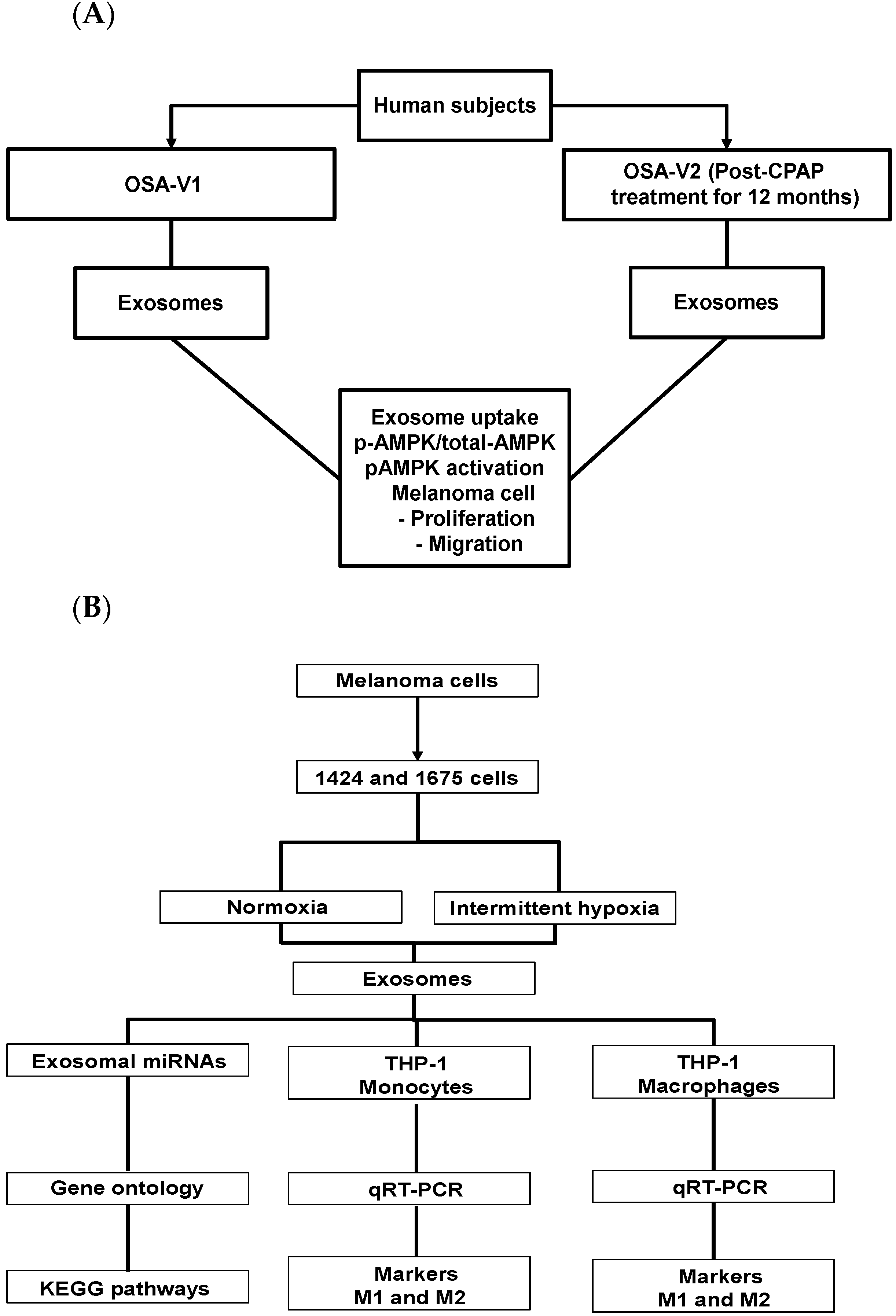
2. Materials and Methods
2.1. Human Subjects
2.2. Cell Lines and Culture Reagents
2.3. Exosome Isolation
2.3.1. Plasma Exosomes
2.3.2. Cell Culture Exosomes
2.4. Exosome Uptake by Melanoma Cells
2.5. Exosome Size Determination and Quantification
2.6. Proliferation and Migration Assays
2.7. p-AMPK and p-AMPK Activator Western Blots
2.8. Cell Cultures and Intermittent Hypoxia
2.9. qRT-PCR
2.10. Exosomal miRNA Isolation from Melanoma Cells
2.10.1. miRNAs Microarray Processing and Analysis
2.10.2. Target Gene Prediction and Function Enrichment Analysis
2.11. THP-1 Monocytes and Differentiation to Macrophages
2.12. Data Analyses
3. Results
3.1. Human Subjects
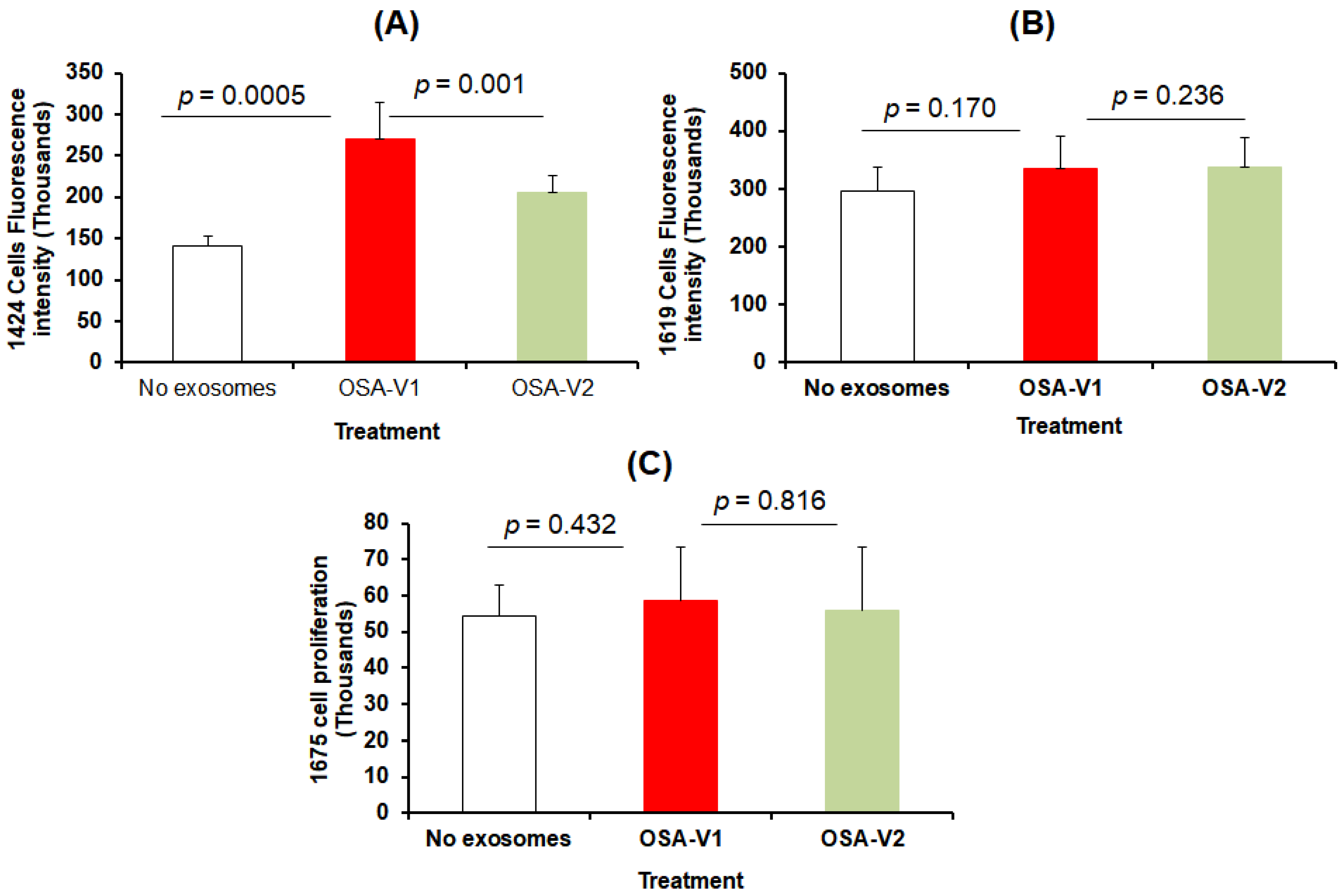
3.2. Exosome Characterization and Uptake
3.3. Cell Proliferation and Migration
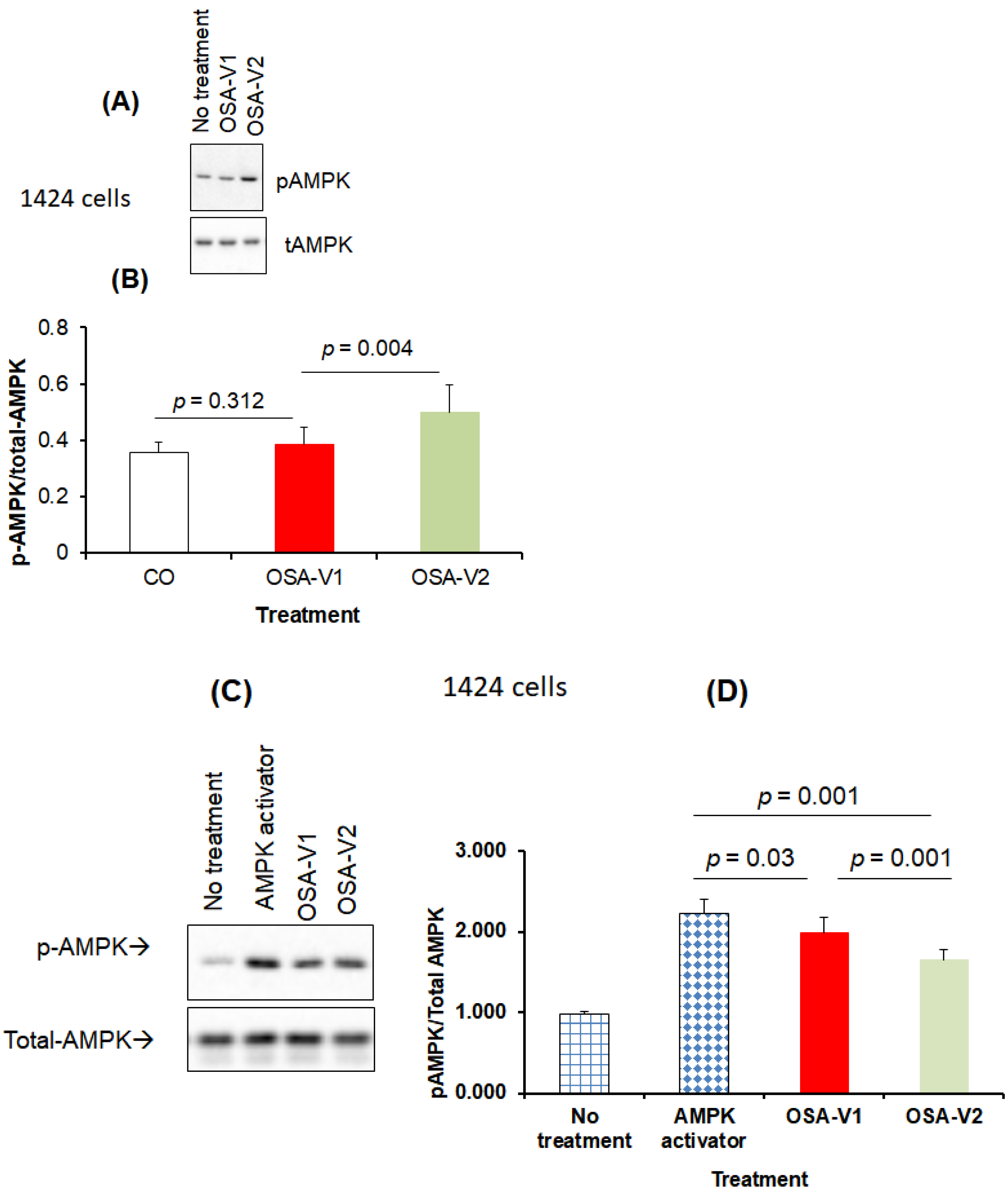
3.4. Effect of Exosomes on AMPK
3.5. Effect of Intermittent Hypoxia on Exosomes Released by Melanoma Cells
3.6. Melanoma Gene Ontology and KEGG Pathways
3.7. Effects of Exosomes Derived from Melanoma Cells on Naïve THP1 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perez-Warnisher, M.T.; Cabezas, E.; Troncoso, M.F.; Gomez, T.; Melchor, R.; Pinillos, E.J.; El Hachem, A.; Gotera, C.; Rodriguez, P.; Mahillo, I.; et al. Sleep disordered breathing and nocturnal hypoxemia are very prevalent in a lung cancer screening population and may condition lung cancer screening findings: Results of the prospective Sleep Apnea in Lung Cancer Screening (SAILS) study. Sleep Med. 2019, 54, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Barbe, F. Cancer and OSA: Current Evidence from Human Studies. Chest 2016, 150, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Peppard, P.E.; Young, T.; Finn, L.; Hla, K.M.; Farre, R. Sleep-disordered breathing and cancer mortality: Results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012, 186, 190–194. [Google Scholar] [CrossRef]
- Christensen, A.S.; Clark, A.; Salo, P.; Nymann, P.; Lange, P.; Prescott, E.; Rod, N.H. Symptoms of sleep disordered breathing and risk of cancer: A prospective cohort study. Sleep 2013, 36, 1429–1435. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Martinez, M.; Duran-Cantolla, J.; Pena Mde, L.; Masdeu, M.J.; Gonzalez, M.; Campo, F.; Gallego, I.; Marin, J.M.; et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Garcia, M.A.; Martorell-Calatayud, A.; Nagore, E.; Valero, I.; Selma, M.J.; Chiner, E.; Landete, P.; Montserrat, J.M.; Carrera, C.; Perez-Gil, A.; et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur. Respir J. 2014, 43, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farre, R.; Nieto, F.J. Putative Links between Sleep Apnea and Cancer: From Hypotheses to Evolving Evidence. Chest 2015, 148, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Gozal, D.; Almendros, I.; Phipps, A.I.; Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Farre, R. Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? Int. J. Mol. Sci. 2020, 21, 8779. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Nagore, E.; Martorell, A.; Rodriguez-Peralto, J.L.; Riveiro-Falkenbach, E.; Hernandez, L.; Banuls, J.; Arias, E.; Ortiz, P.; et al. Sleep-Disordered Breathing is Independently Associated with Increased Aggressiveness of Cutaneous Melanoma: A Multicenter Observational Study in 443 Patients. Chest 2018, 154, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Leung, R.S.; Hawker, G.; Tomlinson, G.; Gershon, A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014, 186, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Gozal, D.; Ham, S.A.; Mokhlesi, B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Dalmases, M.; Cabanas, M.L.; Campos-Rodriguez, F.; Navajas, D.; Farre, R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol. Neurobiol. 2013, 186, 303–307. [Google Scholar] [CrossRef]
- Hakim, F.; Wang, Y.; Zhang, S.X.; Zheng, J.; Yolcu, E.S.; Carreras, A.; Khalyfa, A.; Shirwan, H.; Almendros, I.; Gozal, D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014, 74, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Montserrat, J.M.; Ramirez, J.; Torres, M.; Duran-Cantolla, J.; Navajas, D.; Farre, R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur. Respir J. 2012, 39, 215–217. [Google Scholar] [CrossRef]
- Li, L.; Ren, F.; Qi, C.; Xu, L.; Fang, Y.; Liang, M.; Feng, J.; Chen, B.; Ning, W.; Cao, J. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir Res. 2018, 19, 28. [Google Scholar] [CrossRef] [Green Version]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Bonsignore, M.R.; Chimenti, L.; Navajas, D.; Farre, R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012, 13, 1254–1260. [Google Scholar] [CrossRef]
- Campillo, N.; Falcones, B.; Otero, J.; Colina, R.; Gozal, D.; Navajas, D.; Farre, R.; Almendros, I. Differential Oxygenation in Tumor Microenvironment Modulates Macrophage and Cancer Cell Crosstalk: Novel Experimental Setting and Proof of Concept. Front. Oncol. 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubillos-Zapata, C.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Sanchez de la Torre, M.; Nagore, E.; Martorell-Calatayud, A.; Hernandez Blasco, L.; Chiner Vives, E.; Abad-Capa, J.; Montserrat, J.M.; et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur. Respir J. 2019, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Almendros, I.; Garcia-Rio, F.; Sanchez-de-la-Torre, M.; Farre, R.; Gozal, D. Cancer and Sleep Apnea: Cutaneous Melanoma as a Case Study. Am. J. Respir. Crit. Care Med. 2019, 200, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- D’Aguanno, S.; Mallone, F.; Marenco, M.; Del Bufalo, D.; Moramarco, A. Hypoxia-dependent drivers of melanoma progression. J. Exp. Clin. Cancer Res. 2021, 40, 159. [Google Scholar] [CrossRef] [PubMed]
- Dratkiewicz, E.; Simiczyjew, A.; Mazurkiewicz, J.; Zietek, M.; Matkowski, R.; Nowak, D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells 2021, 10, 862. [Google Scholar] [CrossRef]
- Marhuenda, E.; Campillo, N.; Gabasa, M.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Gozal, D.; Navajas, D.; Alcaraz, J.; Farre, R.; Almendros, I. Effects of Sustained and Intermittent Hypoxia on Human Lung Cancer Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 540–544. [Google Scholar] [CrossRef]
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef]
- Deep, G.; Agarwal, R. Targeting tumor microenvironment with silibinin: Promise and potential for a translational cancer chemopreventive strategy. Curr. Cancer Drug Targets 2013, 13, 486–499. [Google Scholar] [CrossRef] [Green Version]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sceneay, J.; Smyth, M.J.; Moller, A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef]
- Khalyfa, A.; Almendros, I.; Gileles-Hillel, A.; Akbarpour, M.; Trzepizur, W.; Mokhlesi, B.; Huang, L.; Andrade, J.; Farre, R.; Gozal, D. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget 2016, 7, 54676–54690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Huang, L.; Akbarpour, M.; Andrade, J.; Farre, R.; Gozal, D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest 2016, 150, 1030–1041. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2018, 19, 3383. [Google Scholar] [CrossRef] [Green Version]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Alvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Teran-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Artal, J.; Martin, T.; Carrizo, S.J.; Andres, M.; Martin-Burriel, I.; Bolea, R.; Sanz, A.; Varona, L.; Godino, J.; et al. Epigenetics modifications and Subclinical Atherosclerosis in Obstructive Sleep Apnea: The EPIOSA study. BMC Pulm. Med. 2014, 14, 114. [Google Scholar]
- Campos-Rodriguez, F.; Cordero-Guevara, J.; Asensio-Cruz, M.I.; Sanchez-Armengol, A.; Sanchez-Lopez, V.; Arellano-Orden, E.; Gozal, D.; Martinez-Garcia, M.A. Interleukin 6 as a marker of depression in women with sleep apnea. J. Sleep Res. 2021, 30, e13035. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Queipo-Corona, C.; Carmona-Bernal, C.; Jurado-Gamez, B.; Cordero-Guevara, J.; Reyes-Nunez, N.; Troncoso-Acevedo, F.; Abad-Fernandez, A.; Teran-Santos, J.; Caballero-Rodriguez, J.; et al. Continuous Positive Airway Pressure Improves Quality of Life in Women with Obstructive Sleep Apnea. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; American Academy of Sleep Medicine; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masa, J.F.; Jimenez, A.; Duran, J.; Capote, F.; Monasterio, C.; Mayos, M.; Teran, J.; Hernandez, L.; Barbe, F.; Maimo, A.; et al. Alternative methods of titrating continuous positive airway pressure: A large multicenter study. Am. J. Respir. Crit Care Med. 2004, 170, 1218–1224. [Google Scholar]
- Khalyfa, A.; Khalyfa, A.A.; Akbarpour, M.; Connes, P.; Romana, M.; Laping-Carr, G.; Zhang, C.; Andrade, J.; Gozal, D. Extracellular microvesicle microRNAs in children with sickle cell anemia with divergent clinical phenotypes. Br. J. Haematol. in press. 2016. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gaddameedhi, S.; Crooks, E.; Zhang, C.; Li, Y.; Qiao, Z.; Trzepizur, W.; Kay, S.A.; Andrade, J.; Satterfield, B.C.; et al. Circulating Exosomal miRNAs Signal Circadian Misalignment to Peripheral Metabolic Tissues. Int. J. Mol. Sci. 2020, 21, 6396. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Khalyfa, A.; Qiao, Z.; Ullate, J.; Marin, J.M.; Kheirandish-Gozal, L.; Gozal, D. Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 5604. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalyfa, A.; Youssefnia, N.; Foster, G.E.; Beaudin, A.E.; Qiao, Z.; Pialoux, V.; Pun, M.; Hanly, P.J.; Kheirandish-Gozal, L.; Poulin, M.J.; et al. Plasma Exosomes and Improvements in Endothelial Function by Angiotensin 2 Type 1 Receptor or Cyclooxygenase 2 Blockade following Intermittent Hypoxia. Front. Neurol. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiang, B.; Wang, X.; Xiang, C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. 2017, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Farre, R.; Almendros, I.; Montserrat, J.M.; Gozal, D.; Navajas, D. Gas Partial Pressure in Cultured Cells: Patho-Physiological Importance and Methodological Approaches. Front. Physiol. 2018, 9, 1803. [Google Scholar] [CrossRef]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, W.T. Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J. Thorac. Dis. 2018, 10 (Suppl. 34), S4253–S4261. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Marin, J.M.; Agusti, A.; Villar, I.; Forner, M.; Nieto, D.; Carrizo, S.J.; Barbe, F.; Vicente, E.; Wei, Y.; Nieto, F.J.; et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012, 307, 2169–2176. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-de-la-Torre, M.; Khalyfa, A.; Sanchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-Garcia, M.A.; Barcelo, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Feng, J.; Li, L.; Chen, B. Obstructive sleep apnea promotes cancer development and progression: A concise review. Sleep Breath 2015, 19, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farre, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [PubMed] [Green Version]
- Almendros, I.; Wang, Y.; Becker, L.; Lennon, F.E.; Zheng, J.; Coats, B.R.; Schoenfelt, K.S.; Carreras, A.; Hakim, F.; Zhang, S.X.; et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 593–601. [Google Scholar] [CrossRef]
- Nogues, L.; Benito-Martin, A.; Hergueta-Redondo, M.; Peinado, H. The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Mol. Asp. Med. 2018, 60, 15–26. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qin, H.; Poon, T.C.; Sze, S.C.; Ding, X.; Co, N.N.; Ngai, S.M.; Chan, T.F.; Wong, N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 2015, 36, 1008–1018. [Google Scholar] [CrossRef] [Green Version]
- Ekstrom, E.J.; Bergenfelz, C.; von Bulow, V.; Serifler, F.; Carlemalm, E.; Jonsson, G.; Andersson, T.; Leandersson, K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 2014, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Barry, S.; Kmetz, D.; Egger, M.; Pan, J.; Rai, S.N.; Qu, J.; McMasters, K.M.; Hao, H. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 2016, 376, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Kawano, K.; Ikeda, Y.; Handa, M.; Kamata, T.; Anbo, H.; Araki, Y.; Kawai, Y.; Watanabe, K.; Itagaki, I.; Kawakami, K.; et al. Enhancing effect by heparin on shear-induced platelet aggregation. Semin. Thromb. Hemost. 1990, 16, 60–65. [Google Scholar]
- Jansen, M.; Ten Klooster, J.P.; Offerhaus, G.J.; Clevers, H. LKB1 and AMPK family signaling: The intimate link between cell polarity and energy metabolism. Physiol. Rev. 2009, 89, 777–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsin, A.S.; Bertrand, L.; Rider, M.H.; Deprez, J.; Beauloye, C.; Vincent, M.F.; Van den Berghe, G.; Carling, D.; Hue, L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000, 10, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Horman, S.; Browne, G.; Krause, U.; Patel, J.; Vertommen, D.; Bertrand, L.; Lavoinne, A.; Hue, L.; Proud, C.; Rider, M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002, 12, 1419–1423. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. Minireview: The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.; Platanias, L.C. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem. Biophys. Res. Commun. 2010, 398, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Eales, K.L.; Hollinshead, K.E.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Garcia, E.; Garcia-Tovar, S.; Casitas, R.; Jaureguizar, A.; Zamarron, E.; Sanchez-Sanchez, B.; Sastre-Perona, A.; Lopez-Collazo, E.; Garcia-Rio, F.; Cubillos-Zapata, C. Intermittent Hypoxia Mediates Paraspeckle Protein-1 Upregulation in Sleep Apnea. Cancers 2021, 13, 3888. [Google Scholar] [CrossRef]
- Hanniford, D.; Segura, M.F.; Zhong, J.; Philips, E.; Jirau-Serrano, X.; Darvishian, F.; Berman, R.S.; Shapiro, R.L.; Pavlick, A.C.; Brown, B.; et al. Identification of metastasis-suppressive microRNAs in primary melanoma. J. Natl. Cancer Inst. 2015, 107, dju494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozubek, J.; Ma, Z.; Fleming, E.; Duggan, T.; Wu, R.; Shin, D.G.; Dadras, S.S. In-depth characterization of microRNA transcriptome in melanoma. PLoS ONE 2013, 8, e72699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, G.; Kwong, L.N. miRNAs, Melanoma and Microenvironment: An Intricate Network. Int. J. Mol. Sci. 2017, 18, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dankort, D.; Curley, D.P.; Cartlidge, R.A.; Nelson, B.; Karnezis, A.N.; Damsky, W.E., Jr.; You, M.J.; DePinho, R.A.; McMahon, M.; Bosenberg, M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009, 41, 544–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, G.; Ombra, M.; Colombino, M.; Casula, M.; Sini, M.; Manca, A.; Paliogiannis, P.; Ascierto, P.A.; Cossu, A. Multiple Molecular Pathways in Melanomagenesis: Characterization of Therapeutic Targets. Front. Oncol. 2015, 5, 183. [Google Scholar] [CrossRef]
- Tengda, L.; Shuping, L.; Mingli, G.; Jie, G.; Yun, L.; Weiwei, Z.; Anmei, D. Serum exosomal microRNAs as potent circulating biomarkers for melanoma. Melanoma Res. 2018, 28, 295–303. [Google Scholar] [CrossRef]
- Shu, S.L.; Yang, Y.; Allen, C.L.; Maguire, O.; Minderman, H.; Sen, A.; Ciesielski, M.J.; Collins, K.A.; Bush, P.J.; Singh, P.; et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci. Rep. 2018, 8, 12905. [Google Scholar] [CrossRef] [Green Version]
- Gajos-Michniewicz, A.; Czyz, M. Role of miRNAs in Melanoma Metastasis. Cancers 2019, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, A.Y.; Fan, C.; Zheng, H.; Li, Y.; Zhang, C.; Wu, S.; Yu, D.; Huang, Z.; Liu, F.; et al. MicroRNA-33b Inhibits Breast Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci. Rep. 2015, 5, 9995. [Google Scholar] [CrossRef]
- Stark, M.S.; Bonazzi, V.F.; Boyle, G.M.; Palmer, J.M.; Symmons, J.; Lanagan, C.M.; Schmidt, C.W.; Herington, A.C.; Ballotti, R.; Pollock, P.M.; et al. miR-514a regulates the tumour suppressor NF1 and modulates BRAFi sensitivity in melanoma. Oncotarget 2015, 6, 17753–17763. [Google Scholar] [CrossRef] [Green Version]
- Raimo, M.; Orso, F.; Grassi, E.; Cimino, D.; Penna, E.; De Pitta, C.; Stadler, M.B.; Primo, L.; Calautti, E.; Quaglino, P.; et al. miR-146a Exerts Differential Effects on Melanoma Growth and Metastatization. Mol. Cancer Res. 2016, 14, 548–562. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.Z.; Buart, S.; Romero, P.; Ketari, S.; Janji, B.; Mari, B.; Mami-Chouaib, F.; Chouaib, S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012, 72, 4629–4641. [Google Scholar] [CrossRef] [Green Version]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.G.; Croce, C.M.; Negrini, M.; et al. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, H.; Dai, H.; Walsh, R.M.; Imakura, M.; Schelter, J.; Burchard, J.; Dai, X.; Chang, A.N.; Diaz, R.L.; et al. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle 2009, 8, 2756–2768. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Le, Q.T.; Giaccia, A.J. MiR-210--micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.L.; Mohamadzadeh, M. Macrophages and chemokines as mediators of angiogenesis. Front. Physiol. 2013, 4, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]





| KEGG ID | KEGG Pathways | % | p-Value | Benjamini |
|---|---|---|---|---|
| sa05200 | Pathways in cancer | 2.598 | 2.92 × 10−9 | 2.598 |
| hsa04810 | Regulation of actin cytoskeleton | 1.520 | 1.04 × 10−8 | 1.520 |
| hsa05231 | Choline metabolism in cancer | 0.846 | 1.09 × 10−8 | 0.846 |
| hsa04713 | Ras signaling pathway | 1.569 | 1.72 × 10−7 | 1.569 |
| hsa04151 | Circadian entrainment | 0.772 | 2.75 × 10−7 | 0.772 |
| hsa04152 | PI3K-Akt signaling pathway | 2.206 | 1.09 × 10−6 | 2.206 |
| hsa04010 | AMPK signaling pathway | 0.907 | 5.42 × 10−6 | 0.907 |
| hsa04070 | Endocytosis | 1.581 | 8.61 × 10−6 | 1.581 |
| hsa04015 | Phosphatidylinositol signaling system | 0.748 | 9.53 × 10−5 | 0.748 |
| hsa04010 | MAPK signaling pathway | 1.65 | 1.19 × 10−5 | 1.65 |
| hsa04012 | Rap1 signaling pathway | 1.397 | 1.34 × 10−5 | 1.397 |
| hsa04071 | ErbB signaling pathway | 0.674 | 1.50 × 10−5 | 0.674 |
| hsa04120 | Sphingolipid signaling pathway | 0.870 | 1.92 × 10−5 | 0.870 |
| hsa04921 | Ubiquitin mediated proteolysis | 0.968 | 2.20 × 10−5 | 0.968 |
| hsa04919 | Oxytocin signaling pathway | 1.042 | 2.52 × 10−5 | 1.042 |
| hsa04068 | Thyroid hormone signaling pathway | 0.833 | 3.04 × 10−5 | 0.833 |
| hsa04150 | FoxO signaling pathway | 0.944 | 3.36 × 10−5 | 0.944 |
| hsa04540 | mTOR signaling pathway | 0.478 | 5.00 × 10−5 | 0.478 |
| hsa04024 | Gap junction | 0.662 | 5.93 × 10−5 | 0.662 |
| hsa04261 | cAMP signaling pathway | 1.299 | 5.99 × 10−5 | 1.299 |
| hsa05218 | Adrenergic signaling | 0.956 | 6.41 × 10−5 | 0.956 |
| hsa05205 | Melanoma | 0.551 | 9.65 × 10−5 | 0.551 |
| hsa04020 | Proteoglycans in cancer | 1.299 | 1.02 × 10−5 | 1.299 |
| hsa04022 | Calcium signaling pathway | 1.176 | 1.28 × 10−5 | 1.176 |
| hsa04666 | cGMP-PKG signaling pathway | 1.042 | 2.86 × 10−5 | 1.042 |
| hsa04930 | Fc gamma R-mediated phagocytosis | 0.613 | 3.32 × 10−5 | 0.613 |
| hsa03018 | Type II diabetes mellitus | 0.392 | 3.62 × 10−5 | 0.392 |
| hsa04722 | Prostate cancer | 0.625 | 6.8 × 10−5 | 0.625 |
| hsa04510 | Neurotrophin signaling pathway | 0.809 | 7.02 × 10−4 | 0.809 |
| hsa05220 | Focal adhesion | 1.287 | 7.25 × 10−4 | 1.287 |
| hsa05221 | Chronic myeloid leukemia | 0.527 | 8.68 × 10−4 | 0.527 |
| hsa04520 | Acute myeloid leukemia | 0.429 | 1.13 × 10−3 | 0.429 |
| hsa05212 | Adherens junction | 0.515 | 1.13 × 10−3 | 0.515 |
| hsa04310 | Pancreatic cancer | 0.478 | 1.42 × 10−3 | 0.478 |
| hsa00564 | Wnt signaling pathway | 0.895 | 1.49 × 10−3 | 0.895 |
| hsa04931 | Glycerophospholipid metabolism | 0.650 | 1.75 × 10−3 | 0.650 |
| hsa04931 | Insulin resistance | 0.723 | 1.74 × 10−3 | 0.723 |
| KEGG ID | KEGG Pathways | % | p-Value | Benjamini |
|---|---|---|---|---|
| hsa05205 | Proteoglycans in cancer | 1.833 | 1.46 × 10−4 | 0.03 |
| hsa05202 | Transcriptional mis-regulation in cancer | 1.586 | 2.37 × 10−4 | 0.03 |
| hsa04024 | cAMP signaling pathway | 1.784 | 2.60 × 10−4 | 0.02 |
| hsa04012 | ErbB signaling pathway | 0.991 | 4.87 × 10−4 | 0.03 |
| hsa04010 | MAPK signaling pathway | 2.081 | 5.37 × 10−4 | 0.02 |
| hsa04071 | Sphingolipid signaling pathway | 1.189 | 9.34 × 10−4 | 0.02 |
| Cell Type | Genes | M1 Markers | Genes | M2 Markers | ||||
|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | p-Value | Normoxia | Hypoxia | p-Value | |||
| Exosomes from 1424 cells on monocytes | CCR7 | 1 | 0.90 ± 0.06 | 0.541 | CD206 | 1 | 0.83 ± 0.05 | 0.542 |
| CXCL10 | 1 | 1.28 ± 0.014 | 0.023 | CD163 | 1 | 1.17 ± 0.016 | 0.421 | |
| IL6 | 1 | 1.21 ± 0.013 | 0.014 | IL10 | 1 | 1.11 ± 0.011 | 0.621 | |
| Cell Type | Genes | M1 Markers | Genes | M2 Markers | ||||
| Normoxia | Hypoxia | p-Value | Normoxia | Hypoxia | p-Value | |||
| Exosomes from 1675 cells on monocytes | CCR7 | 1 | 1.75 ± 0.013 | 0.012 | CD206 | 1 | 1.21 ± 0.014 | 0.023 |
| CXCL10 | 1 | 1.52 ± 0.014 | 0.024 | CD163 | 1 | 1.86 ± 0.016 | 0.045 | |
| IL6 | 1 | 1.77 ± 0.015 | 0.004 | IL10 | 1 | 1.44 ± 0.014 | 0.041 | |
| Cell Type | Genes | M1 Markers | Genes | M2 Markers | ||||
|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | p-Value | Normoxia | Hypoxia | p-Value | |||
| Exosomes from 1424 cells on macrophages | CCR7 | 1 | 1.11 ± 0.012 | 0.121 | CD206 | 1 | 1.02 ± 0.01 | 0.091 |
| CXCL10 | 1 | 1.53 ± 0.016 | 0.001 | CD163 | 1 | 0.98 ± 0.07 | 0.131 | |
| IL6 | 1 | 0.93 ± 0.06 | 0.071 | IL10 | 1 | 0.91 ± 0.08 | 0.082 | |
| Cell Type | Genes | M1 Markers | Genes | M2 Markers | ||||
| Normoxia | Hypoxia | p-Value | RA | IH | p-Value | |||
| Exosomes from 1675 cells on macrophages | CCR7 | 1 | 1.26 ± 0.016 | 0.042 | CD206 | 1 | 1.01 ± 0.00 | 0.122 |
| CXCL10 | 1 | 1.35± 0.014 | 0.022 | CD163 | 1 | 1.06 ± 0.00 | 0.092 | |
| IL6 | 1 | 1.12 ± 0.011 | 0.061 | IL10 | 1 | 1.00 ± 0.00 | 0.211 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Qiao, Z.; Sanz-Rubio, D.; Marin, J.M.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Almendros, I.; Farre, R.; et al. Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia. Cancers 2021, 13, 4781. https://doi.org/10.3390/cancers13194781
Khalyfa A, Trzepizur W, Gileles-Hillel A, Qiao Z, Sanz-Rubio D, Marin JM, Martinez-Garcia MA, Campos-Rodriguez F, Almendros I, Farre R, et al. Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia. Cancers. 2021; 13(19):4781. https://doi.org/10.3390/cancers13194781
Chicago/Turabian StyleKhalyfa, Abdelnaby, Wojciech Trzepizur, Alex Gileles-Hillel, Zhuanhong Qiao, David Sanz-Rubio, José M. Marin, Miguel A. Martinez-Garcia, Francisco Campos-Rodriguez, Isaac Almendros, Ramon Farre, and et al. 2021. "Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia" Cancers 13, no. 19: 4781. https://doi.org/10.3390/cancers13194781
APA StyleKhalyfa, A., Trzepizur, W., Gileles-Hillel, A., Qiao, Z., Sanz-Rubio, D., Marin, J. M., Martinez-Garcia, M. A., Campos-Rodriguez, F., Almendros, I., Farre, R., Sanchez-de-la-Torre, M., García-Río, F., & Gozal, D. (2021). Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia. Cancers, 13(19), 4781. https://doi.org/10.3390/cancers13194781








