Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
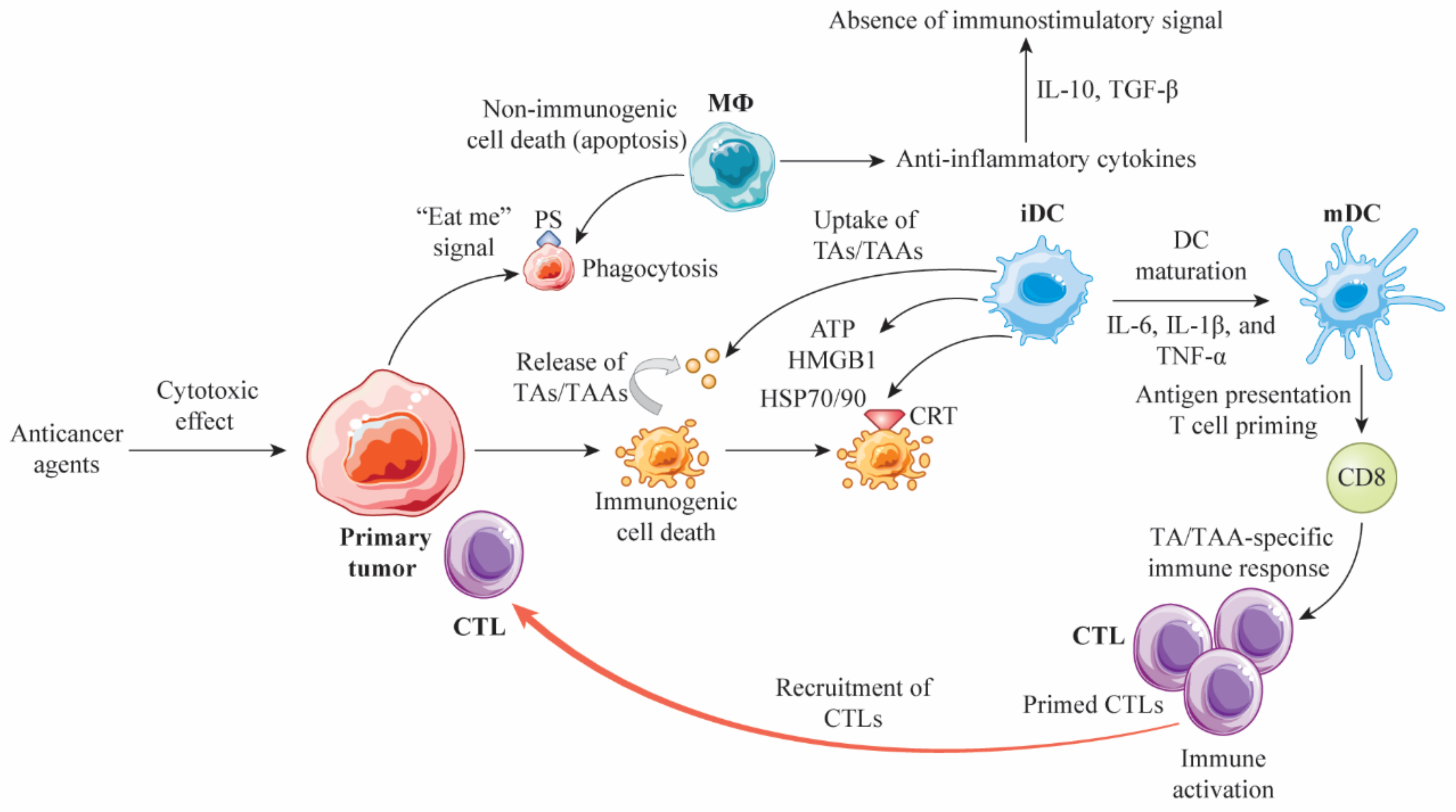
2. Cell Death Modalities and Immunogenicity
3. Immunomodulation by Conventional Anticancer Agents
3.1. Anthracyclines
3.2. Taxanes
3.3. Cyclophosphamide
3.4. Methotrexate
3.5. 5-Fluorouracil
3.6. Capecitabine
3.7. Trastuzumab
3.8. Pertuzumab
3.9. Trastuzumab Emtansine
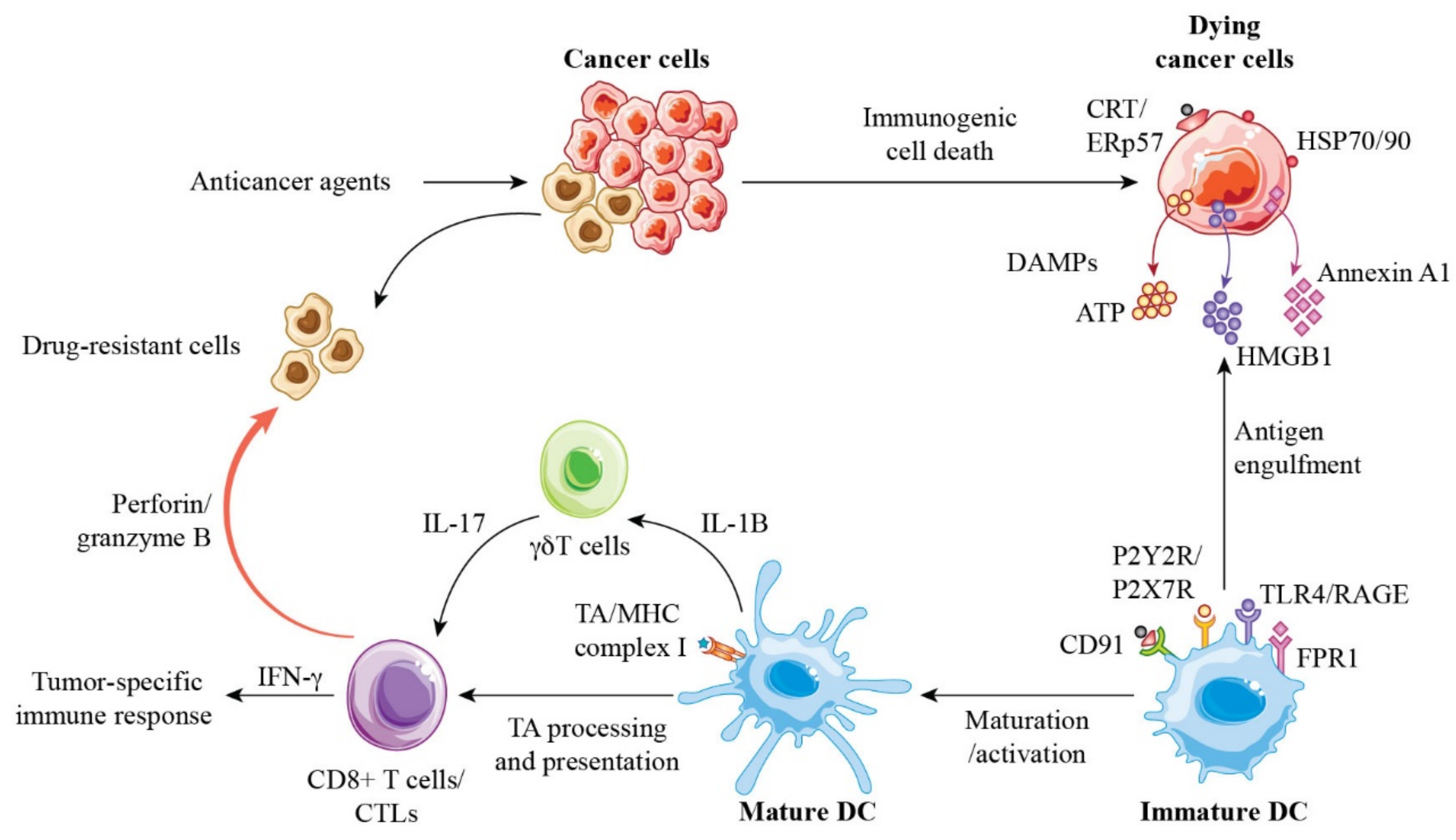
4. Molecular Characteristics of Anticancer Agent-Induced ICD
5. Immunogenic Modulation by Anticancer Agents as Non-Classical ICD
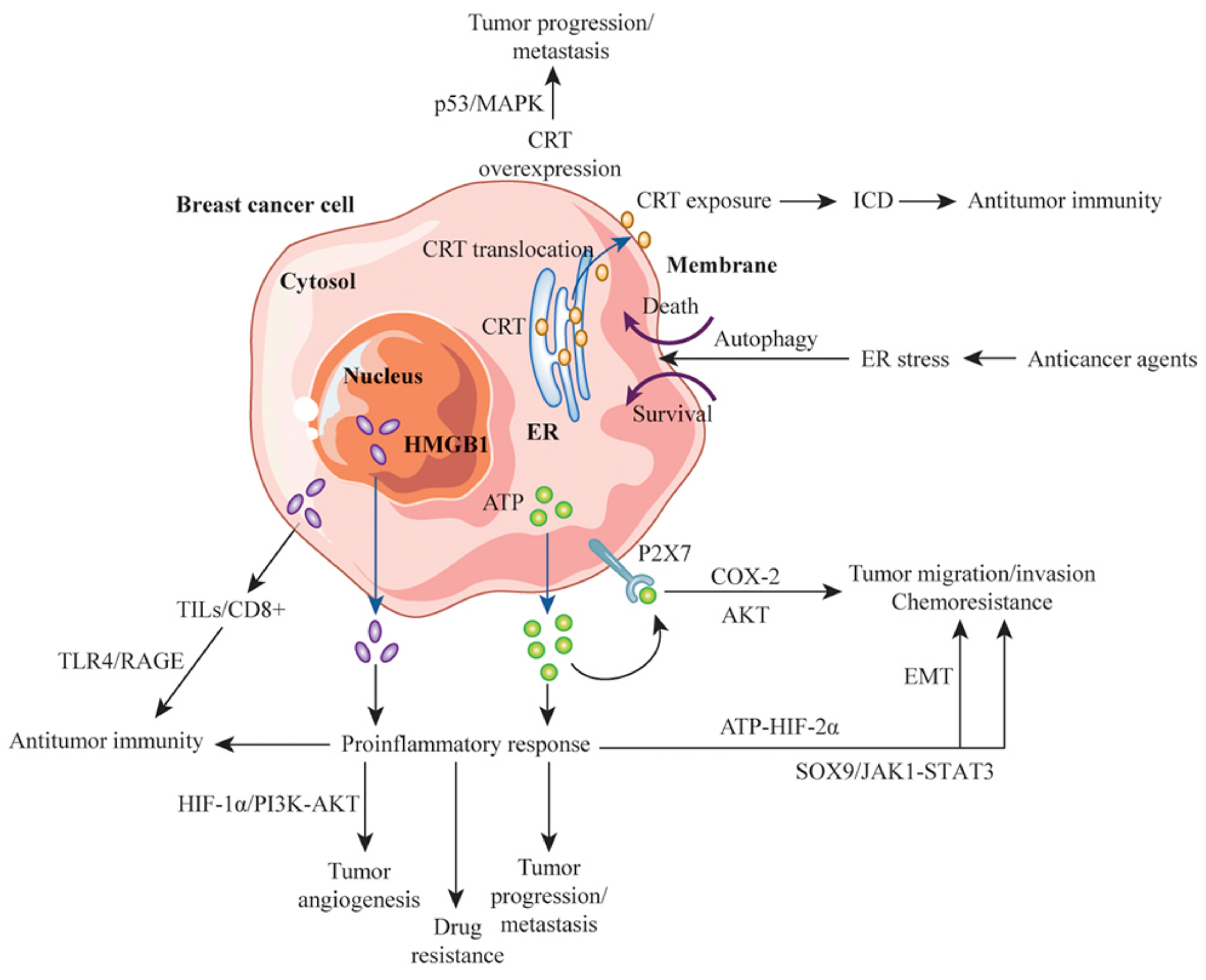
6. Roles of DAMPs and ICD in the Clinical Significance of Breast Cancer
7. Exploiting the Double-Edged Sword of DAMPs for Antitumor Immunity
8. Immunomodulatory Effects of Anticancer Agents and the Enhancement of Antitumor Immunity in Combination with Immune Checkpoint Inhibitors
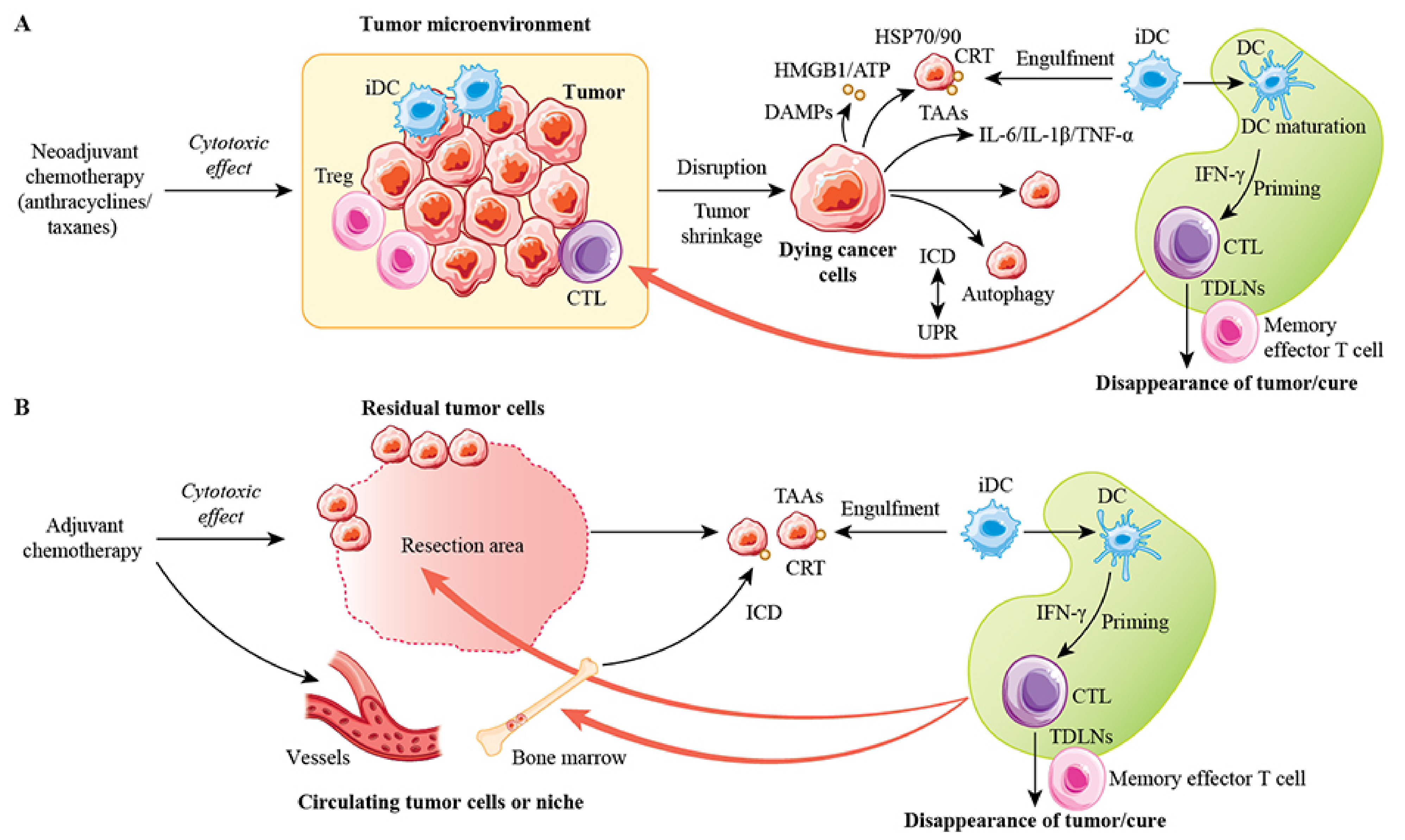
9. ICD and Long-Term Antitumor Immune Memory
10. Future Therapies
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Martin, S.; Golab, J.; Agostinis, P. Danger signalling during cancer cell death: Origins, plasticity and regulation. Cell Death Differ. 2014, 21, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current approaches for combination therapy of cancer: The role of immunogenic cell death. Cancers 2020, 12, 1047. [Google Scholar] [CrossRef]
- Cruickshank, B.; Giacomantonio, M.; Marcato, P.; McFarland, S.; Pol, J.; Gujar, S. Dying to Be Noticed: Epigenetic Regulation of Immunogenic Cell Death for Cancer Immunotherapy. Front. Immunol. 2018, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Løve Aaes, T.; Bachert, C.; Vandenabeele, P.; Krysko, D.V. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013, 4, e631. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Fu, Y.; Tian, D.; Yan, W. The contrasting roles of inflammasomes in cancer. Am. J. Cancer Res. 2018, 8, 566–583. [Google Scholar] [PubMed]
- Pandey, S.; Singh, S.; Anang, V.; Bhatt, A.N.; Natarajan, K.; Dwarakanath, B.S. Pattern Recognition Receptors in Cancer Progression and Metastasis. Cancer Growth Metastasis 2015, 8, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Chen, D.; Huang, J.; Wang, R.; Feng, B.; Song, H.; Chen, L. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma. Mol. Cancer 2014, 13, 165. [Google Scholar] [CrossRef]
- Pennisi, A.; Kieber-Emmons, T.; Makhoul, I.; Hutchins, L. Relevance of pathological complete response after neoadjuvant therapy for breast cancer. Breast Cancer 2016, 10, 103–106. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Wu, Q.; Song, Y.; Ma, X.; Zhang, B.; Wang, H.; Huang, Y. Association between levels of tumor-infiltrating lymphocytes in different subtypes of primary breast tumors and prognostic outcomes: A meta-analysis. BMC Womens Health. 2020, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Badr, N.M.; Berditchevski, F.; Shaaban, A.M. The Immune Microenvironment in Breast Carcinoma: Predictive and Prognostic Role in the Neoadjuvant Setting. Pathobiology 2020, 87, 61–74. [Google Scholar] [CrossRef]
- Kim, R.; Kawai, A.; Wakisaka, M.; Sawada, S.; Shimoyama, M.; Yasuda, N.; Hidaka, M.; Morita, Y.; Ohtani, S.; Arihiro, K. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 2020, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. Regulated necrotic cell death in alternative tumor therapeutic strategies. Cells 2020, 9, 2709. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, É.; Köröskényi, K. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef] [PubMed]
- Sachet, M.; Liang, Y.Y.; Oehler, R. The immune response to secondary necrotic cells. Apoptosis 2017, 22, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Metur, S.P.; Klionsky, D.J. Adaptive immunity at the crossroads of autophagy and metabolism. Cell Mol. Immunol. 2021, 18, 1096–1105. [Google Scholar] [CrossRef]
- Ma, Y.; Aymeric, L.; Locher, C.; Mattarollo, S.R.; Delahaye, N.F.; Pereira, P.; Boucontet, L.; Apetoh, L.; Ghiringhelli, F.; Casares, N.; et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011, 208, 491–503. [Google Scholar] [CrossRef]
- Mattarollo, S.R.; Loi, S.; Duret, H.; Ma, Y.; Zitvogel, L.; Smyth, M.J. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011, 71, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, D.; Trad, M.; Hanke, N.T.; Larmonier, C.B.; Janikashvili, N.; Bonnotte, B.; Katsanis, E.; Larmonier, N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014, 74, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Domschke, C.; Stoiber, N.; Schott, S.; Heil, J.; Rom, J.; Blumenstein, M.; Thum, J.; Sohn, C.; Schneeweiss, A.; et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 2012, 61, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, R.; Duggan, M.C.; Stiff, A.; Markowitz, J.; Trikha, P.; Levine, K.M.; Schoenfield, L.; Abdel-Rasoul, M.; Layman, R.; Ramaswamy, B.; et al. Circulating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Immunol. Immunother. 2017, 66, 1437–1447. [Google Scholar] [CrossRef]
- Ding, Z.C.; Lu, X.; Yu, M.; Lemos, H.; Huang, L.; Chandler, P.; Liu, K.; Walters, M.; Krasinski, A.; Mack, M.; et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1-PD-L1 axis. Cancer Res. 2014, 74, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Wehner, R.; Bitterlich, A.; Meyer, N.; Kloß, A.; Schäkel, K.; Bachmann, M.; Schmitz, M. Impact of chemotherapeutic agents on the immunostimulatory properties of human 6-sulfo LacNAc+ (slan) dendritic cells. Int. J. Cancer 2013, 132, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Dobrzanski, M.J.; Reome, J.B.; Hylind, J.C.; Rewers-Felkins, K.A.; Abulsamad, K.; Adams, S.L. Ag-specific type 1 CD8 effector cells enhance methotrexate-mediated antitumor responses by modulating differentiated T cell localization, activation and chemokine production in established breast cancer. Clin. Immunol. 2008, 128, 205–218. [Google Scholar] [CrossRef]
- Galetto, A.; Buttiglieri, S.; Forno, S.; Moro, F.; Mussa, A.; Matera, L. Drug- and cell-mediated antitumor cytotoxicities modulate cross-presentation of tumor antigens by myeloid dendritic cells. Anticancer Drugs 2003, 14, 833–843. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Asleh, K.; Brauer, H.A.; Sullivan, A.; Lauttia, S.; Lindman, H.; Nielsen, T.O.; Joensuu, H.; Thompson, E.A.; Chumsri, S. Predictive Biomarkers for Adjuvant Capecitabine Benefit in Early-Stage Triple-Negative Breast Cancer in the FinXX Clinical Trial. Clin. Cancer Res. 2020, 26, 2603–2614. [Google Scholar] [CrossRef]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Kanelopoulos, P.; Boulamatsis, D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer 2002, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, N.; Xiong, S.D.; Zheng, Y.J.; Chu, Y.W. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scand. J. Immunol. 2011, 73, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Assudani, D.; Nagaraj, S.; Hunter, T.; Cho, H.I.; Antonia, S.; Altiok, S.; Celis, E.; Gabrilovich, D.I. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest. 2010, 120, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Kodumudi, K.N.; Woan, K.; Gilvary, D.L.; Sahakian, E.; Wei, S.; Djeu, J.Y. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 2010, 16, 4583–4594. [Google Scholar] [CrossRef]
- Zum Büschenfelde, C.M.; Hermann, C.; Schmidt, B.; Peschel, C.; Bernhard, H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res. 2002, 62, 2244–2247. [Google Scholar]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef]
- Tóth, G.; Szöőr, Á.; Simon, L.; Yarden, Y.; Szöllősi, J.; Vereb, G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. MAbs 2016, 8, 1361–1370. [Google Scholar] [CrossRef]
- Mamidi, S.; Cinci, M.; Hasmann, M.; Fehring, V.; Kirschfink, M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol. Oncol. 2013, 7, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Müller, P.; Schreiner, J.; Prince, S.S.; Lardinois, D.; Heinzelmann-Schwarz, V.A.; Thommen, D.S.; Zippelius, A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol. Immunother. 2014, 63, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Ugel, S.; Delpozzo, F.; Desantis, G.; Papalini, F.; Simonato, F.; Sonda, N.; Zilio, S.; Bronte, V. Therapeutic targeting of myeloid-derived suppressor cells. Curr. Opin. Pharmacol. 2009, 9, 470–481. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L. Enhancing the anticancer effects of 5-fluorouracil: Current challenges and future perspectives. Biomed. J. 2015, 38, 111–116. [Google Scholar]
- Zhang, P.; Su, D.M.; Liang, M.; Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 2008, 45, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Toi, M.; Atiqur Rahman, M.; Bando, H.; Chow, L.W. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005, 6, 158–166. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; van Endert, P.; et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009, 28, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Panaretakis, T.; Tesniere, A.; Joza, N.; Tufi, R.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Leveraging the immune system during chemotherapy: Moving calreticulin to the cell surface converts apoptotic death from ”silent” to immunogenic. Cancer Res. 2007, 67, 7941–7944. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.A.; Lin, J.K.; Lin, S.Y. Mechanisms of immunogenic cell death and immune checkpoint blockade therapy. Kaohsiung J. Med. Sci. 2021, 37, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Vandenberk, L.; Fang, S.; Fasche, T.; Van Eygen, S.; Maes, J.; Van Woensel, M.; Koks, C.; Vanthillo, N.; Graf, N.; et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017, 24, 832–843. [Google Scholar] [CrossRef]
- Hodge, J.W.; Garnett, C.T.; Farsaci, B.; Palena, C.; Tsang, K.Y.; Ferrone, S.; Gameiro, S.R. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int. J. Cancer 2013, 133, 624–636. [Google Scholar] [CrossRef]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef]
- Martins, I.; Tesniere, A.; Kepp, O.; Michaud, M.; Schlemmer, F.; Senovilla, L.; Séror, C.; Métivier, D.; Perfettini, J.L.; Zitvogel, L.; et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle 2009, 8, 3723–3728. [Google Scholar] [CrossRef]
- Fumet, J.D.; Limagne, E.; Thibaudin, M.; Ghiringhelli, F. Immunogenic Cell Death and Elimination of Immunosuppressive Cells: A Double-Edged Sword of Chemotherapy. Cancers 2020, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- Sohun, M.; Shen, H. The implication and potential applications of high-mobility group box 1 protein in breast cancer. Ann. Transl. Med. 2016, 4, 217. [Google Scholar] [CrossRef] [PubMed]
- Stoetzer, O.J.; Fersching, D.M.; Salat, C.; Steinkohl, O.; Gabka, C.J.; Hamann, U.; Braun, M.; Feller, A.M.; Heinemann, V.; Siegele, B.; et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol. 2013, 34, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Exner, R.; Sachet, M.; Arnold, T.; Zinn-Zinnenburg, M.; Michlmayr, A.; Dubsky, P.; Bartsch, R.; Steger, G.; Gnant, M.; Bergmann, M.; et al. Prognostic value of HMGB1 in early breast cancer patients under neoadjuvant chemotherapy. Cancer Med. 2016, 5, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Aoto, K.; Mimura, K.; Okayama, H.; Saito, M.; Chida, S.; Noda, M.; Nakajima, T.; Saito, K.; Abe, N.; Ohki, S.; et al. Immunogenic tumor cell death induced by chemotherapy in patients with breast cancer and esophageal squamous cell carcinoma. Oncol. Rep. 2018, 39, 151–159. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, L.; Guo, X.; Li, Z.; Liao, X.; Zhang, X.; Huang, L.; He, J. HMGB1-activated fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma 2021, 68, 71–78. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Li, L.; Jin, X.; Zhang, Z.; Zheng, H.; Pan, J.; Shi, L.; Jiang, Z.; Su, K.; et al. Tumor-derived HMGB1 induces CD62L dim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis 2020, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, X.; Chen, J.; Sun, L.; Sun, H.; Xie, K. High-Mobility Group Box 1 (HMGB1) Promotes Angiogenesis and Tumor Migration by Regulating Hypoxia-Inducible Factor 1 (HIF-1α) Expression via the Phosphatidylinositol 3-Kinase (PI3K)/AKT Signaling Pathway in Breast Cancer Cells. Med. Sci. Monit. 2019, 25, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Steel, H.C.; Theron, A.J.; Heyman, L.; Smit, T.; Ramdas, Y.; Anderson, R. High Mobility Group Box 1 in Human Cancer. Cells 2020, 9, 1664. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, H.; Fu, Y.; Zhu, Y.; Kong, L.; Chen, L.; Zhao, F.; Yu, L.; Chen, X. TLR4/MyD88 signaling determines the metastatic potential of breast cancer cells. Mol. Med. Rep. 2018, 18, 3411–3420. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Lin, Y.; Jiang, J.; Tang, Z.; Yang, S.; Lu, L.; Liang, Y.; Liu, X.; Tan, J.; Hu, X.G.; et al. High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. J. Pathol. 2017, 243, 376–389. [Google Scholar] [CrossRef]
- Ai, H.; Zhou, W.; Wang, Z.; Qiong, G.; Chen, Z.; Deng, S. microRNAs-107 inhibited autophagy, proliferation, and migration of breast cancer cells by targeting HMGB1. J. Cell Biochem. 2018, 120, 8696–8705. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Wang, J.; Yang, C.; He, D.W. Downregulation of miR-205 contributes to epithelial-mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1-RAGE signaling pathway. Anticancer Drugs 2019, 30, 225–232. [Google Scholar] [CrossRef]
- Liang, L.; Fu, J.; Wang, S.; Cen, H.; Zhang, L.; Mandukhail, S.R.; Du, L.; Wu, Q.; Zhang, P.; Yu, X. MiR-142-3p enhances chemosensitivity of breast cancer cells and inhibits autophagy by targeting HMGB1. Acta Pharm. Sin. B 2020, 10, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gong, W.; Lu, L.; Wang, Y.; Ren, J. Upregulation of miR-129-5p increases the sensitivity to Taxol through inhibiting HMGB1-mediated cell autophagy in breast cancer MCF-7 cells. Braz. J. Med. Biol. Res. 2019, 52, e8657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Li, J.; Zhou, X.; Yin, L.; Wang, Y.; Gu, Y.; Niu, X.; Yang, Y.; Ji, H.; et al. HMGB1 is a key factor for tamoxifen resistance and has the potential to predict the efficacy of CDK4/6 inhibitors in breast cancer. Cancer Sci. 2021, 112, 1603–1613. [Google Scholar] [CrossRef]
- Wang, N.; Yang, B.; Muhetaer, G.; Wang, S.; Zheng, Y.; Lu, J.; Li, M.; Zhang, F.; Situ, H.; Lin, Y.; et al. XIAOPI formula promotes breast cancer chemosensitivity via inhibiting CXCL1/HMGB1-mediated autophagy. Biomed. Pharmacother. 2019, 120, 109519. [Google Scholar] [CrossRef]
- Liu, B.; Qi, X.; Zhang, X.; Gao, D.; Fang, K.; Guo, Z.; Li, L. Med19 is involved in chemoresistance by mediating autophagy through HMGB1 in breast cancer. J. Cell Biochem. 2019, 120, 507–518. [Google Scholar] [CrossRef]
- Folkerts, H.; Hilgendorf, S.; Vellenga, E.; Bremer, E.; Wiersma, V.R. The multifaceted role of autophagy in cancer and the microenvironment. Med. Res. Rev. 2019, 39, 517–560. [Google Scholar] [CrossRef]
- Park, I.A.; Heo, S.H.; Song, I.H.; Kim, Y.A.; Park, H.S.; Bang, W.S.; Park, S.Y.; Jo, J.H.; Lee, H.J.; Gong, G. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget 2016, 7, 59957–59964. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Kim, A.; Song, I.H.; Park, I.A.; Yu, J.H.; Ahn, J.H.; Gong, G. Cytoplasmic expression of high mobility group B1 (HMGB1) is associated with tumor-infiltrating lymphocytes (TILs) in breast cancer. Pathol. Int. 2016, 66, 202–209. [Google Scholar] [CrossRef]
- Vacchelli, E.; Enot, D.P.; Pietrocola, F.; Zitvogel, L.; Kroemer, G. Impact of Pattern Recognition Receptors on the Prognosis of Breast Cancer Patients Undergoing Adjuvant Chemotherapy. Cancer Res. 2016, 76, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Ladoire, S.; Enot, D.; Andre, F.; Zitvogel, L.; Kroemer, G. Immunogenic cell death-related biomarkers: Impact on the survival of breast cancer patients after adjuvant chemotherapy. Oncoimmunology 2015, 5, e1082706. [Google Scholar] [CrossRef] [PubMed]
- Ladoire, S.; Enot, D.; Senovilla, L.; Ghiringhelli, F.; Poirier-Colame, V.; Chaba, K.; Semeraro, M.; Chaix, M.; Penault-Llorca, F.; Arnould, L.; et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 2016, 12, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Qader Hamadneh, L.A.; Veerakumarasivam, A.; Abdul Rahman, S.; Shohaimi, S.; Rosli, R. Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell Int. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Kabbage, M.; Trimeche, M.; Bergaoui, S.; Hammann, P.; Kuhn, L.; Hamrita, B.; ben Nasr, H.; Chaieb, A.; Chouchane, L.; Chahed, K. Calreticulin expression in infiltrating ductal breast carcinomas: Relationships with disease progression and humoral immune responses. Tumour Biol. 2013, 34, 1177–1188. [Google Scholar] [CrossRef]
- Lwin, Z.M.; Guo, C.; Salim, A.; Yip, G.W.; Chew, F.T.; Nan, J.; Thike, A.A.; Tan, P.H.; Bay, B.H. Clinicopathological significance of calreticulin in breast invasive ductal carcinoma. Mod. Pathol. 2010, 23, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Z.P.; Qin, S.; Liu, C.B.; Zou, L.L. Calreticulin is an effective immunologic adjuvant to tumor-associated antigens. Exp. Ther. Med. 2017, 14, 3399–3406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prathyuman, S.; Sellappa, S.; Joseph, S.; Keyan, K.S. Enhanced calreticulin expression triggers apoptosis in the MCF-7 cell line. Asian Pac. J. Cancer Prev. 2010, 11, 1133–1136. [Google Scholar] [PubMed]
- Kopecka, J.; Godel, M.; Dei, S.; Giampietro, R.; Belisario, D.C.; Akman, M.; Contino, M.; Teodori, E.; Riganti, C. Insights into P-Glycoprotein Inhibitors: New Inducers of Immunogenic Cell Death. Cells 2020, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Deng, F.; Jia, W. Inhibition of Indoleamine 2,3-Dioxygenase Enhances the Therapeutic Efficacy of Immunogenic Chemotherapeutics in Breast Cancer. J. Breast Cancer 2019, 22, 196–209. [Google Scholar] [CrossRef]
- Iribarren, K.; Buque, A.; Mondragon, L.; Xie, W.; Lévesque, S.; Pol, J.; Zitvogel, L.; Kepp, O.; Kroemer, G. Anticancer effects of anti-CD47 immunotherapy in vivo. Oncoimmunology 2018, 8, 1550619. [Google Scholar] [CrossRef]
- Sharma, S.; Kalra, H.; Akundi, R.S. Extracellular ATP Mediates Cancer Cell Migration and Invasion Through Increased Expression of Cyclooxygenase 2. Front. Pharmacol. 2021, 11, 617211. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Song, W.; Peng, X.; Zhao, R. P2X7 receptor: A critical regulator and potential target for breast cancer. J. Mol. Med. 2021, 99, 349–358. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.H.; Wang, P.; Yang, H.; Zhou, Y.T.; Zhang, H.Q.; He, H.Y.; Fang, W.G.; Tian, X.X. Extracellular ATP promotes breast cancer invasion and chemoresistance via SOX9 signaling. Oncogene 2020, 39, 5795–5810. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.H.; Wang, P.; Zhou, Y.T.; Yang, H.; Huo, Y.F.; Zhang, H.Q.; Li, Y.; He, H.Y.; Tian, X.X.; et al. Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2α signaling. Cancer Sci. 2019, 110, 2456–2470. [Google Scholar] [CrossRef]
- Kim, D.C.; Jin, H.; Lee, J.S.; Son, E.; Lee, G.W.; Kim, H.J. P2Y 2 R has a significant correlation with Notch-4 in patients with breast cancer. Oncol. Lett. 2020, 20, 647–654. [Google Scholar] [CrossRef]
- Gale, M.; Li, Y.; Cao, J.; Liu, Z.Z.; Holmbeck, M.A.; Zhang, M.; Lang, S.M.; Wu, L.; Do Carmo, M.; Gupta, S.; et al. Acquired Resistance to HER2-Targeted Therapies Creates Vulnerability to ATP Synthase Inhibition. Cancer Res. 2020, 80, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nikolos, F.; Lee, Y.C.; Jain, A.; Tsouko, E.; Gao, H.; Kasabyan, A.; Leung, H.E.; Osipov, A.; Jung, S.Y.; et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat. Commun. 2020, 11, 6299. [Google Scholar] [CrossRef]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, 1. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.-A.; Wright, G.S.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; Michiels, S.; Le Quesne, J.; Nielsen, T.O.; Laenkholm, A.V.; et al. The path to a better biomarker: Application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers into breast cancer clinical trials and daily practice. J. Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment for high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized ISPY2 trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Campbell, M.J.; Yau, C.; Bolen, J.; Vandenberg, S.; Hoyt, C.; Brown-Swigart, L.; Hirst, G.; Nanda, R.; Liu, M.; Asare, S.; et al. Analysis of immune cell infiltrates as predictors of response to the checkpoint inhibitor pembrolizumab in the neoadjuvant I-SPY 2 TRIAL. Cancer Res. 2019, 79 (Suppl. S13), CT003. [Google Scholar]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Heinhuis, K.M.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.H.; Schellens, J.H.M. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019, 30, 219–235. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Beebe, S.J.; Lassiter, B.P.; Guo, S. Nanopulse Stimulation (NPS) Induces Tumor Ablation and Immunity in Orthotopic 4T1 Mouse Breast Cancer: A Review. Cancers 2018, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kin, T. Clinical Perspectives in Addressing Unsolved Issues in (Neo)Adjuvant Therapy for Primary Breast Cancer. Cancers 2021, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Park, G.Y.; Bae, M.J.; Kim, J.S.; Jo, W.S.; Lee, C.G. Hypoxia induces immunogenic cell death of cancer cells by enhancing the exposure of cell surface calreticulin in an endoplasmic reticulum stress-dependent manner. Oncol. Lett. 2019, 18, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Cerda, E.E.; Franco-Molina, M.A.; Mendoza-Gamboa, E.; Prado-García, H.; Rivera-Morales, L.G.; Zapata-Benavides, P.; Rodríguez-Salazar Mdel, C.; Caballero-Hernandez, D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. In Vivo Chemoprotective Activity of Bovine Dialyzable Leukocyte Extract in Mouse Bone Marrow Cells against Damage Induced by 5-Fluorouracil. J. Immunol. Res. 2016, 2016, 6942321. [Google Scholar] [CrossRef] [PubMed]
- Santana-Krímskaya, S.E.; Franco-Molina, M.A.; Zárate-Triviño, D.G.; Prado-García, H.; Zapata-Benavides, P.; Torres-Del-Muro, F.; Rodríguez-Padilla, C. IMMUNEPOTENT CRP plus doxorubicin/cyclophosphamide chemotherapy remodel the tumor microenvironment in an air pouch triple-negative breast cancer murine model. Biomed. Pharmacother. 2020, 126, 110062. [Google Scholar] [CrossRef]
- Reyes-Ruiz, A.; Calvillo-Rodriguez, K.M.; Martínez-Torres, A.C.; Rodríguez-Padilla, C. The bovine dialysable leukocyte extract IMMUNEPOTENT CRP induces immunogenic cell death in breast cancer cells leading to long-term antitumour memory. Br. J. Cancer. 2021, 124, 1398–1410. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Song, Y.; Wang, Z.; Wang, Y.; Luo, F.; Xu, Y.; Zhao, Y.; Wu, Z.; Xu, Y. Mechanism of immune evasion in breast cancer. Onco. Targets Ther. 2017, 10, 1561–1573. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Tari, G.; Bagirova, M.; Abamor, E.S. Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer. J. Breast Cancer 2018, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Türeci, Ö.; Vormehr, M.; Diken, M.; Kreiter, S.; Huber, C.; Sahin, U. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin. Cancer Res. 2016, 22, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, R.; Dell’Aversana, C.; Giorgio, C.; Astorri, R.; Altucci, L. Breast Cancer Vaccines: New Insights. Front. Endocrinol. 2017, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Conte, F.; Pane, K.; Fiscon, G.; Mirabelli, P.; Baselice, S.; Giannatiempo, R.; Messina, F.; Franzese, M.; Salvatore, M.; et al. The New Paradigm of Network Medicine to Analyze Breast Cancer Phenotypes. Int. J. Mol. Sci. 2020, 21, 6690. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; McIntyre, K.; Wilks, S.; Ma, L.; Block, M.; Andorsky, D.; Danso, M.; Locke, T.; Scales, A.; Wang, Y. Efficacy and Safety of Weekly Paclitaxel with or Without Oral Alisertib in Patients With Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e214103. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Zhao, Z.B.; Guo, J.; Wang, T.; Yang, J.B.; Wang, C.; Long, J.; Ma, S.; Huang, Q.; Zhang, K.; et al. Aurora a Inhibition Eliminates Myeloid Cell-Mediated Immunosuppression and Enhances the Efficacy of Anti-PD-L1 Therapy in Breast Cancer. Cancer Res. 2019, 79, 3431–3444. [Google Scholar] [CrossRef]
| Anticancer Agents | Mechanism of Action | Immunomodulatory Effect |
|---|---|---|
| Anthracyclines | ||
| Doxorubicin | Interferes with the correct unwinding of DNA during replication and transcription | Production of IFN-γ by CTLs, production of IL-17 by γδ T cells and promotion of CTL accumulation by γδ T cells, and IL-1β induction [20,21]. Reduction of MDSCs [22]. Increase in CD4, CD8, and NK cells and expression of IFN-γ, perforin, and granzyme B [22]. |
| Epirubicin | Inhibits the synthesis of nucleic acids and proteins, and forms complexes with DNA by base pair intercalation, thereby inhibiting topoisomerase II activity Promotes ICD | |
| Alkylating agent | ||
| Cyclophosphamide | Suppresses protein synthesis by inhibiting the transcription of DNA to RNA Promotes ICD | Suppression of Tregs at low dose and Increase in tumor-specific T cells [23]. Increase in MDSCs due to AC as a paradoxical effect [24,25]. |
| Antimetabolites | ||
| Methotrexate | Inhibits dihydrofolate reductase | Maintenance of the ability of DCs to produce pro-inflammatory cytokines and activate NK and T cells [26]. Combination with methotrexate and Tc1 cell transfer increases TILs and decreases Tregs [27]. |
| 5-Fluorouracil | Inhibits DNA and RNA synthesis | Promotion of antigen uptake by DCs and enhanced cytotoxicity of NK and CD8+ T cells [28]. Elimination of MDSCs [29]. |
| Capecitabine | Prodrug that converts 5′-deoxy-5-fluorouridine to 5-fluorouracil Inhibits DNA and RNA synthesis | Release of antigens by tumor cell death, activation of DCs, presentation of tumor antigens to T cells [30]. Depletion of MDSCs, alleviation of NK and T cell suppression [31] |
| Taxanes | ||
| Paclitaxel | Changes tubulin polymerization or depolymerization | Increased levels of IFN-γ, IL-2, IL-6, GM-CSF, and pNK cell activity [32]. Decreased Tregs, independently of TLR4 [33]. Increased permeability of granzyme B by perforin independent CTLs [34]. |
| Nab-paclitaxel | Paclitaxel bound to albumin nanoparticles. Transport of albumin by transcytosis increases its delivery to tumors | |
| Docetaxel | Promotes immunogenic modulation | Leads MDSCs to transform from the M2 to the M1 phenotype via STAT3 [35]. |
| Targeting agents | ||
| Trastuzumab | Inhibits signals transmitted by HER-2 and degrades HER-2 | Enhanced cytotoxicity of HER-2-specific CD8+CTLs [36]. Increase in tumor-associated NK cells and lymphocytes expressing granzyme B and TiA1 [37]. ADCC mediated by FcγRIII receptor [38]. |
| Pertuzumab | Inhibits dimerization between HER-2 and HER family receptors | Activation of ADCC [39]. Mobilization of NK cells by ADCC [40]. Complement- and macrophage-mediated cytotoxicity [41]. |
| Trastuzumab-emtansine | Taken up by endosomes and degraded, releasing T-DM1 Blocks the HER-2 signaling pathway and mediates ADCC | DC maturation and production of proinflammatory cytokines by ansamitocin P3. Enhanced antigen uptake and activation of tumor-specific T cells in tumor-draining lymph nodes [42,43]. Massive infiltration of T cells, polarization of Th1 cells, and increases in Tregs in combination with anti-CTLA-4/PD-1 agents [43]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Kin, T. Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers 2021, 13, 4756. https://doi.org/10.3390/cancers13194756
Kim R, Kin T. Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers. 2021; 13(19):4756. https://doi.org/10.3390/cancers13194756
Chicago/Turabian StyleKim, Ryungsa, and Takanori Kin. 2021. "Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer" Cancers 13, no. 19: 4756. https://doi.org/10.3390/cancers13194756
APA StyleKim, R., & Kin, T. (2021). Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers, 13(19), 4756. https://doi.org/10.3390/cancers13194756





