Increased Plasma Levels of lncRNAs LINC01268, GAS5 and MALAT1 Correlate with Negative Prognostic Factors in Myelofibrosis
Abstract
Simple Summary
Abstract
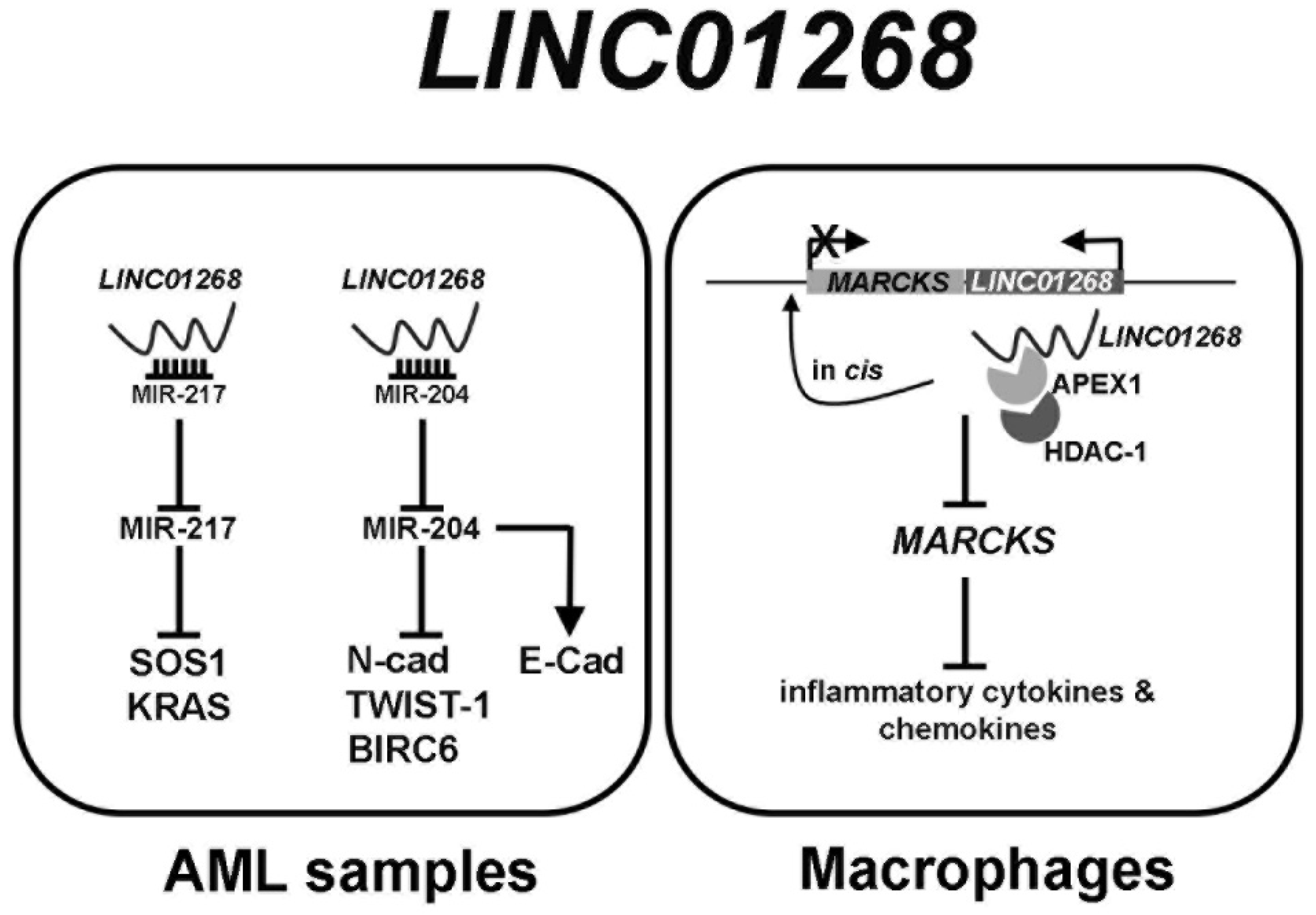
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Plasma Isolation
2.3. RNA Purification
2.4. Measuring lncRNAs Expression in CD34+ Cells
2.5. Measuring lncRNAs Levels in Plasma
2.6. Statistical Analysis
3. Results
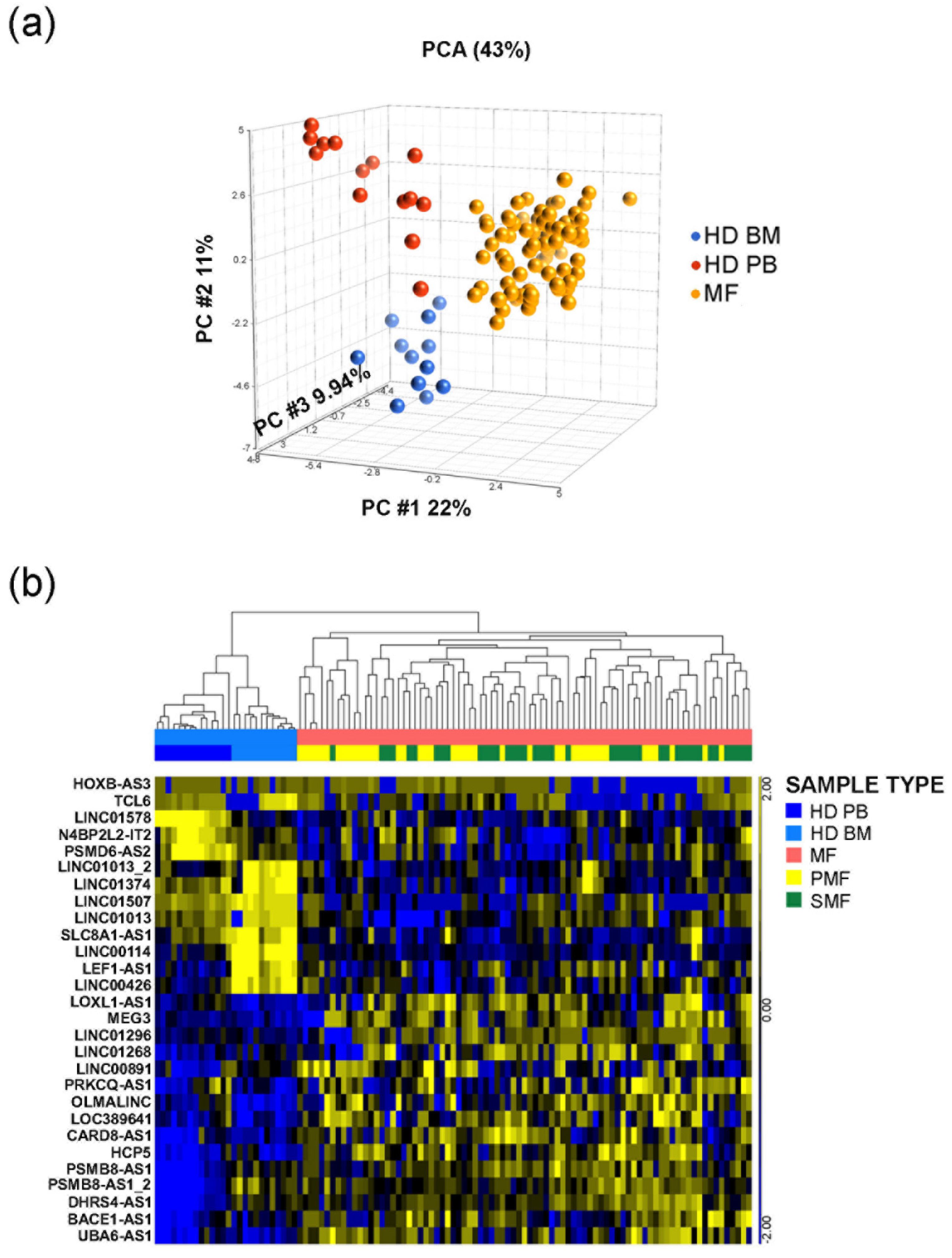
3.1. LncRNAs Expression Profile in CD34+ Cells from MF Patients
3.2. Seven Circulating lncRNAs Are Increased in MF Patients
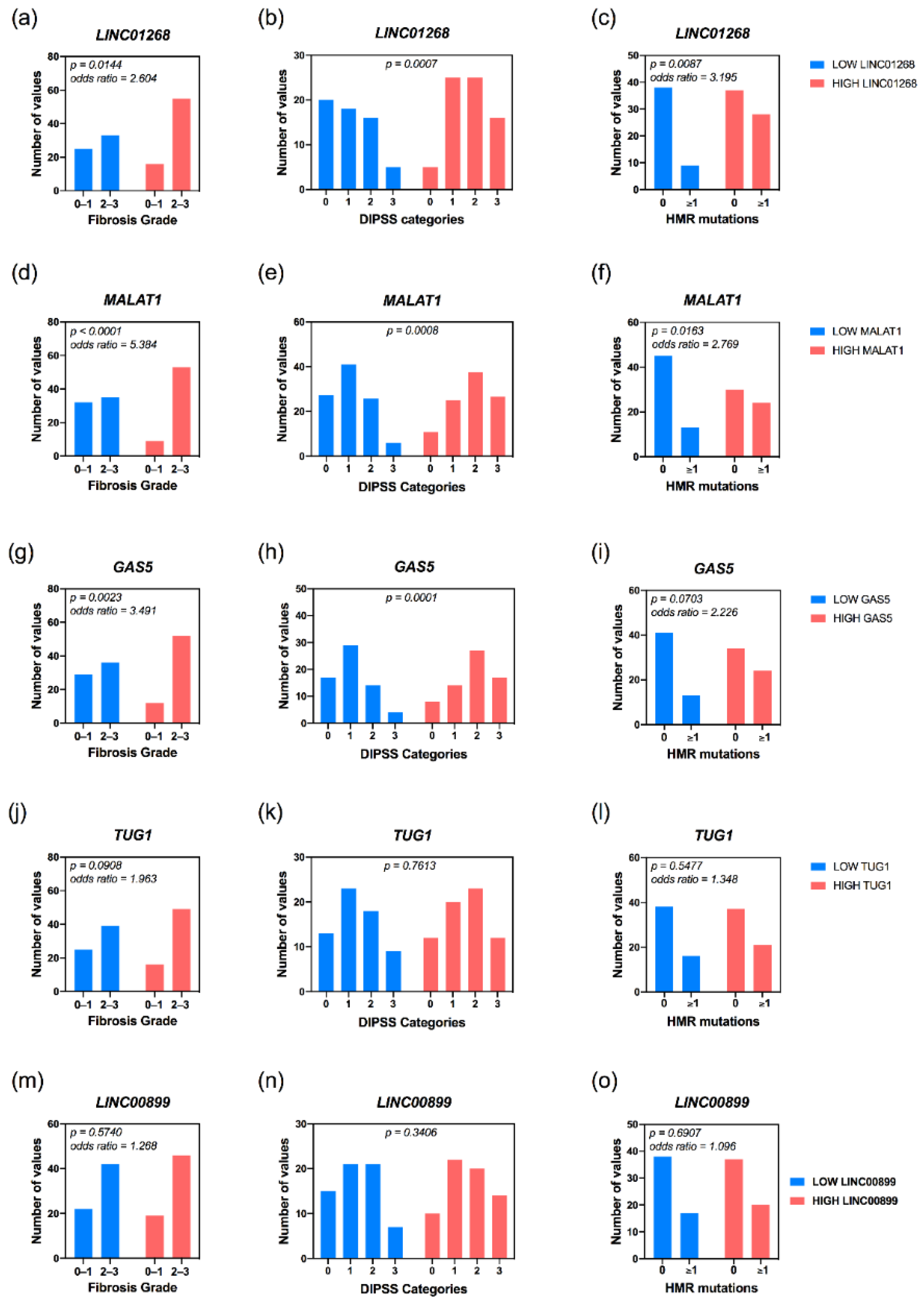
3.3. Increased Plasma Levels of LINC01268, MALAT1, GAS5, LINC00899 and TUG1 lncRNAs Correlate with Clinical Detrimental Features
3.4. High Plasma Levels of LINC01268, MALAT1, GAS5, TUG1 and NEAT1 Are Associated with MF Patients’ Molecular Features
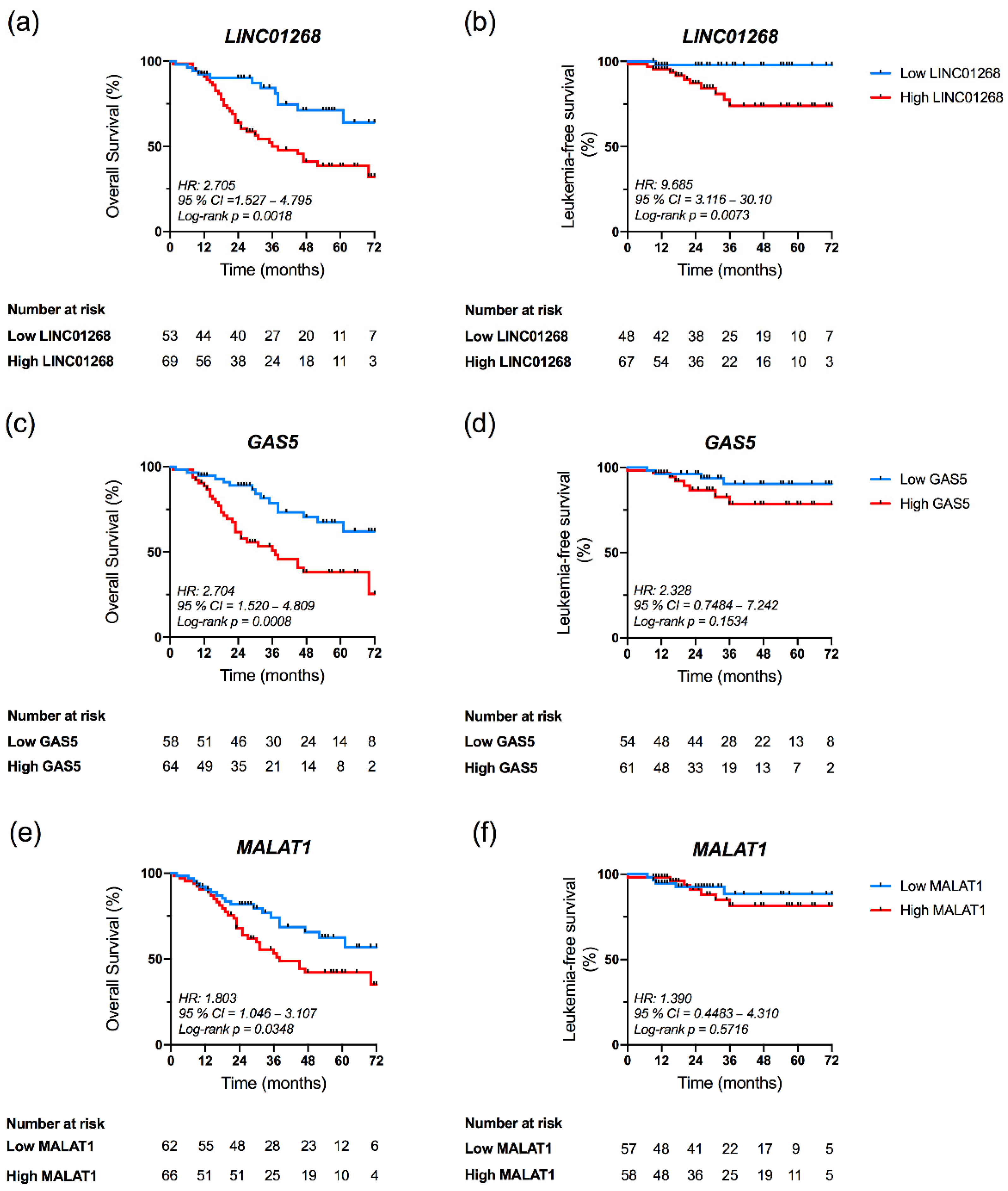
3.5. High Plasma Levels of LINC01268, GAS5 and MALAT1 Affect OS
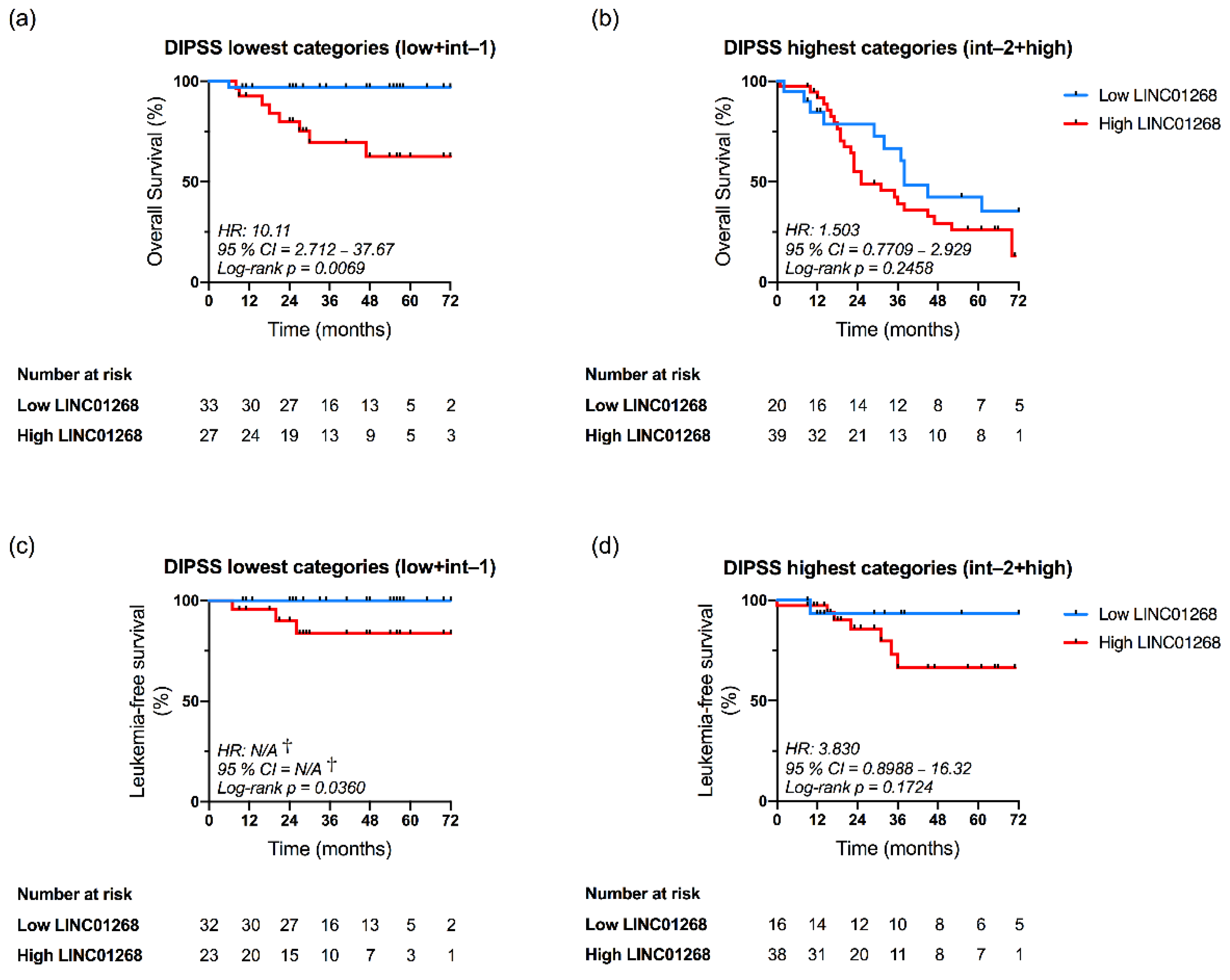
3.6. LINC01268 Plasma Level Is an Independent Prognostic Factor for OS and LFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- On behalf of the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT); Barosi, G.; Mesa, R.A.; Thiele, J.; Cervantes, F.; Campbell, P.J.; Verstovsek, S.; Dupriez, B.; Levine, R.L.; Passamonti, F.; et al. Proposed Criteria for the Diagnosis of Post-Polycythemia Vera and Post-Essential Thrombocythemia Myelofibrosis: A Consensus Statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008, 22, 437–438. [Google Scholar] [CrossRef]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.M. Genetic Risk Assessment in Myeloproliferative Neoplasms. Mayo Clin. Proc. 2017, 92, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Parenti, S.; Rontauroli, S.; Carretta, C.; Mallia, S.; Genovese, E.; Chiereghin, C.; Peano, C.; Tavernari, L.; Bianchi, E.; Fantini, S.; et al. Mutated Clones Driving Leukemic Transformation Are Already Detectable at the Single-Cell Level in CD34-Positive Cells in the Chronic Phase of Primary Myelofibrosis. npj Precis. Onc. 2021, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Hibbin, J.A.; Njoku, O.S.; Matutes, E.; Lewis, S.M.; Goldman, J.M. Myeloid Progenitor Cells in the Circulation of Patients with Myelofibrosis and Other Myeloproliferative Disorders. Br. J. Haematol. 1984, 57, 495–503. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.-L.; Barosi, G.; et al. New Prognostic Scoring System for Primary Myelofibrosis Based on a Study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A Dynamic Prognostic Model to Predict Survival in Primary Myelofibrosis: A Study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. JCO 2018, 36, 310–318. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Gangat, N.; Ketterling, R.P.; Pardanani, A.; Vannucchi, A.M. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis. JCO 2018, 36, 1769–1770. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; et al. A Clinical-Molecular Prognostic Model to Predict Survival in Patients with Post Polycythemia Vera and Post Essential Thrombocythemia Myelofibrosis. Leukemia 2017, 31, 2726–2731. [Google Scholar] [CrossRef]
- Tefferi, A. Primary Myelofibrosis: 2021 Update on Diagnosis, Risk-stratification and Management. Am. J. Hematol. 2021, 96, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Rontauroli, S.; Castellano, S.; Guglielmelli, P.; Zini, R.; Bianchi, E.; Genovese, E.; Carretta, C.; Parenti, S.; Fantini, S.; Mallia, S.; et al. Gene Expression Profile Correlates with Molecular and Clinical Features in Patients with Myelofibrosis. Blood Adv. 2021, 5, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative Annotation of Human Large Intergenic Noncoding RNAs Reveals Global Properties and Specific Subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.A.; Christensen, C.J.; Dunn, D.M.; Spangrude, G.J.; Georgelas, A.; Kelley, L.; Esplin, M.S.; Weiss, R.B.; Gleich, G.J. EGO, a Novel, Noncoding RNA Gene, Regulates Eosinophil Granule Protein Transcript Expression. Blood 2007, 109, 5191–5198. [Google Scholar] [CrossRef]
- Zhang, X.; Weissman, S.M.; Newburger, P.E. Long Intergenic Non-Coding RNA HOTAIRM1 Regulates Cell Cycle Progression during Myeloid Maturation in NB4 Human Promyelocytic Leukemia Cells. RNA Biol. 2014, 11, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, J.R.; Hu, W.; Gromatzky, A.A.; Lodish, H.F. Long Noncoding RNAs during Normal and Malignant Hematopoiesis. Int. J. Hematol. 2014, 99, 531–541. [Google Scholar] [CrossRef]
- Pennucci, V.; Zini, R.; Norfo, R.; Guglielmelli, P.; Bianchi, E.; Salati, S.; Sacchi, G.; Prudente, Z.; Tenedini, E.; Ruberti, S.; et al. Abnormal Expression Patterns of WT1-as, MEG3 and ANRIL Long Non-Coding RNAs in CD34+ Cells from Patients with Primary Myelofibrosis and Their Clinical Correlations. Leuk. Lymphoma 2015, 56, 492–496. [Google Scholar] [CrossRef]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical Utility of Circulating Non-Coding RNAs—an Update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Gruner, H.N.; McManus, M.T. Examining the Evidence for Extracellular RNA Function in Mammals. Nat. Rev. Genet. 2021, 22, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Characteristics and Applications. Eur. J. Hum. Genet. 2018, 26, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum MicroRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Roy, N.V.; Paepe, A.D. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, J.R.; Lodish, H.F. Emerging Mechanisms of Long Noncoding RNA Function during Normal and Malignant Hematopoiesis. Blood 2017, 130, 1965–1975. [Google Scholar] [CrossRef]
- Li, W.; Ren, Y.; Si, Y.; Wang, F.; Yu, J. Long Non-Coding RNAs in Hematopoietic Regulation. Cell Regen. 2018, 7, 27–32. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Song, H.-Q.; Sun, G.-W. Long Non-Coding RNA LINC00899 as a Novel Serum Biomarker for Diagnosis and Prognosis Prediction of Acute Myeloid Leukemia. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7364–7370. [Google Scholar] [CrossRef]
- Cho, S.-F.; Chang, Y.C.; Chang, C.-S.; Lin, S.-F.; Liu, Y.-C.; Hsiao, H.-H.; Chang, J.-G.; Liu, T.-C. MALAT1 Long Non-Coding RNA Is Overexpressed in Multiple Myeloma and May Serve as a Marker to Predict Disease Progression. BMC Cancer 2014, 14, 809. [Google Scholar] [CrossRef]
- Nobili, L.; Lionetti, M.; Neri, A. Long Non-Coding RNAs in Normal and Malignant Hematopoiesis. Oncotarget 2016, 7, 50666–50681. [Google Scholar] [CrossRef] [PubMed]
- Kotzin, J.J.; Spencer, S.P.; McCright, S.J.; Kumar, D.B.U.; Collet, M.A.; Mowel, W.K.; Elliott, E.N.; Uyar, A.; Makiya, M.A.; Dunagin, M.C.; et al. The Long Non-Coding RNA Morrbid Regulates Bim and Short-Lived Myeloid Cell Lifespan. Nature 2016, 537, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Fallik, N.; Bar-Lavan, Y.; Greenshpan, Y.; Goldstein, O.; Grosch, M.; Drukker, M.; Gazit, R. Neat1 in Hematopoietic Stem Cells. Oncotarget 2017, 8, 109575–109586. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, M.; Andrades, Á.; Coira, I.F.; Baliñas, C.; Rodríguez, M.I.; Álvarez-Pérez, J.C.; Peinado, P.; Arenas, A.M.; García, D.J.; Jiménez, P.; et al. Expression of the Long Non-Coding RNA TCL6 Is Associated with Clinical Outcome in Pediatric B-Cell Acute Lymphoblastic Leukemia. Blood Cancer J. 2019, 9, 93. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; et al. Mutations and Prognosis in Primary Myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef]
- Bao, T.; Davidson, N.E. Gene Expression Profiling of Breast Cancer. Adv. Surg. 2008, 42, 249–260. [Google Scholar] [CrossRef][Green Version]
- Oliveira, D.V.N.P.; Prahm, K.P.; Christensen, I.J.; Hansen, A.; Høgdall, C.K.; Høgdall, E.V. Gene Expression Profile Association with Poor Prognosis in Epithelial Ovarian Cancer Patients. Sci. Rep. 2021, 11, 5438. [Google Scholar] [CrossRef]
- Grossman, D.; Kim, C.C.; Hartman, R.I.; Berry, E.; Nelson, K.C.; Okwundu, N.; Curiel-Lewandrowski, C.; Leachman, S.A.; Swetter, S.M. Prognostic Gene Expression Profiling in Melanoma: Necessary Steps to Incorporate into Clinical Practice. Melanoma Manag. 2019, 6, MMT32. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-Gene Stemness Score for Rapid Determination of Risk in Acute Leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Le Bousse-Kerdilès, M.-C. Primary Myelofibrosis and the “Bad Seeds in Bad Soil” Concept. Fibrogenes. Tissue Repair 2012, 5, S20. [Google Scholar] [CrossRef]
- Orvain, C.; Luque Paz, D.; Dobo, I.; Cottin, L.; Le Calvez, G.; Chauveau, A.; Mercier, M.; Farhi, J.; Boyer, F.; Ianotto, J.C.; et al. Circulating Cd34+ Cell Count Differentiates Primary Myelofibrosis from Other Philadelphia-Negative Myeloproliferative Neoplasms: A Pragmatic Study. Ann. Hematol. 2016, 95, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-W.; Lo, Y.D.; Leung, S.-F.; Tsang, Y.-S.; Chan, L.Y.; Johnson, P.J.; Hjelm, N.M.; Lee, J.C.; Huang, D.P. Analysis of Cell-Free Epstein-Barr Virus-Associated RNA in the Plasma of Patients with Nasopharyngeal Carcinoma. Clin. Chem. 1999, 45, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Kopreski, M.S.; Benko, F.A.; Kwak, L.W.; Gocke, C.D. Detection of Tumor Messenger RNA in the Serum of Patients with Malignant Melanoma. Clin. Cancer Res. 1999, 5, 1961–1965. [Google Scholar] [PubMed]
- Reis, P.P.; Drigo, S.A.; Carvalho, R.F.; Lopez Lapa, R.M.; Felix, T.F.; Patel, D.; Cheng, D.; Pintilie, M.; Liu, G.; Tsao, M.-S. Circulating MiR-16-5p, MiR-92a-3p, and MiR-451a in Plasma from Lung Cancer Patients: Potential Application in Early Detection and a Regulatory Role in Tumorigenesis Pathways. Cancers 2020, 12, 2071. [Google Scholar] [CrossRef] [PubMed]
- Penna, D.; Nicolosi, M.; Mudireddy, M.; Szuber, N.; Vallapureddy, R.; Lasho, T.L.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; et al. 20+ Years and Alive with Primary Myelofibrosis: Phenotypic Signature of Very Long-Lived Patients. Blood 2018, 132, 4301. [Google Scholar] [CrossRef]
- Tefferi, A. Primary Myelofibrosis: 2017 Update on Diagnosis, Risk-stratification, and Management. Am. J. Hematol. 2016, 91, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Dai, X.; Yeung, S.-C.J.; He, X. The Role of Long Non-Coding RNA GAS5 in Cancers. CMAR 2019, 11, 2729–2737. [Google Scholar] [CrossRef]
- The MoMar Study Group; Weber, D.G.; Casjens, S.; Brik, A.; Raiko, I.; Lehnert, M.; Taeger, D.; Gleichenhagen, J.; Kollmeier, J.; Bauer, T.T.; et al. Circulating Long Non-Coding RNA GAS5 (Growth Arrest-Specific Transcript 5) as a Complement Marker for the Detection of Malignant Mesothelioma Using Liquid Biopsies. Biomark. Res. 2020, 8, 15. [Google Scholar] [CrossRef]
- Visconti, V.V.; Fittipaldi, S.; Ciuffi, S.; Marini, F.; Isaia, G.; D’Amelio, P.; Migliaccio, S.; Marcocci, C.; Minisola, S.; Nuti, R.; et al. Circulating Long Non-Coding RNA GAS5 Is Overexpressed in Serum from Osteoporotic Patients and Is Associated with Increased Risk of Bone Fragility. Int. J. Mol. Sci. 2020, 21, 6930. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, L.; Liu, X.; Zhou, L.; Wang, W.; Han, Z.; Sui, H.; Tang, Y.; Wang, Y.; Liu, N.; et al. Long Non-Coding RNA MALAT1 Promotes Tumour Growth and Metastasis in Colorectal Cancer through Binding to SFPQ and Releasing Oncogene PTBP2 from SFPQ/PTBP2 Complex. Br. J. Cancer 2014, 111, 736–748. [Google Scholar] [CrossRef]
- Kim, J.; Piao, H.-L.; Kim, B.-J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N.; et al. Long Noncoding RNA MALAT1 Suppresses Breast Cancer Metastasis. Nat. Genet. 2018, 50, 1705–1715. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Nie, Y.; Tang, A.; Zhou, Q. Long Non-Coding RNA LINC01268 Promotes Cell Growth and Inhibits Cell Apoptosis by Modulating MiR-217/SOS1 Axis in Acute Myeloid Leukemia. Braz. J. Med. Biol. Res. 2020, 53, e9299. [Google Scholar] [CrossRef]
- Xue, F.; Che, H. The Long Non-coding RNA LOC285758 Promotes Invasion of Acute Myeloid Leukemia Cells by Down-regulating MiR-204-5p. FEBS Open Bio 2020, 10, 734–743. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, T.; Su, C.; Shang, Z. MicroRNA 217 Inhibits Cell Proliferation and Enhances Chemosensitivity to Doxorubicin in Acute Myeloid Leukemia by Targeting KRAS. Oncol. Lett. 2017, 13, 4986–4994. [Google Scholar] [CrossRef] [PubMed]
- Butrym, A.; Rybka, J.; Baczyńska, D.; Tukiendorf, A.; Kuliczkowski, K.; Mazur, G. Low Expression of MicroRNA-204 (MiR-204) Is Associated with Poor Clinical Outcome of Acute Myeloid Leukemia (AML) Patients. J. Exp. Clin. Cancer Res. 2015, 34, 68. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, H.; Fang, Z.; Fan, Y.; Liu, X.; Zhang, Y.; Rui, S.; Chen, Y.; Hong, L.; Gao, J.; et al. MiR-204 Acts as a Potential Therapeutic Target in Acute Myeloid Leukemia by Increasing BIRC6-Mediated Apoptosis. BMB Rep. 2018, 51, 444–449. [Google Scholar] [CrossRef]
- Zhang, Q.; Chao, T.; Patil, V.S.; Qin, Y.; Tiwari, S.K.; Chiou, J.; Dobin, A.; Tsai, C.; Li, Z.; Dang, J.; et al. The Long Noncoding RNAROCKI Regulates Inflammatory Gene Expression. EMBO J. 2019, 38, e100041. [Google Scholar] [CrossRef] [PubMed]


| LINC01268 | MALAT1 | GAS5 | TUG1 | LINC00899 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Low | High | p | Low | High | p | Low | High | p | Low | High | p | Low | High | p |
| Males (n evaluable, total = 134) | 34 (56.67%) | 41 (55.41%) | >0.9999 | 37 (53.62%) | 38 (58.46%) | 0.6048 | 38 (57.58%) | 37 (54.41%) | 0.7310 | 39 (59.09%) | 36 (52.94%) | 0.4916 | 39 (59.09%) | 36 (52.94%) | 0.4916 |
| Age. Median, y (n evaluable, total = 135) | 62.5 | 66.0 | 0.0208 | 65.0 | 65.5 | 0.2762 | 64.5 | 66.0 | 0.1084 | 65.0 | 65.0 | 0.537 | 65.0 | 65.0 | 0.5366 |
| Hemoglobin (Hb) (n evaluable, total = 126) | |||||||||||||||
| Median, g/L | 11.75 | 11.00 | 0.1475 | 11.90 | 10.90 | 0.0139 | 11.95 | 10.75 | 0.0072 | 11.30 | 11.20 | 0.9622 | 11.20 | 11.20 | 0.5025 |
| <10 g/L | 12 (21.43%) | 19 (27.14%) | 0.5349 | 13 (20.31%) | 18 (29.03%) | 0.4058 | 12 (20.00%) | 19 (28.79%) | 0.3030 | 14 (22.58%) | 17 (26.56%) | 0.6811 | 12 (19.67%) | 19 (29.23%) | 0.2236 |
| Hematocrit (HCT) (n evaluable, total = 99) | 36.00 | 33.80 | 0.1051 | 38 | 34.95 | 0.0386 | 37.75 | 34.90 | 0.1026 | 34.95 | 35.9 | 0.9819 | 35.9 | 34.95 | 0.6925 |
| Leukocytes (n evaluable, total = 120) | |||||||||||||||
| Median, × 109/L | 7.60 | 11.00 | 0.0012 | 7.48 | 11.50 | <0.0001 | 7.42 | 12.21 | <0.0001 | 8.00 | 10.90 | 0.0011 | 8.05 | 9.29 | 0.0390 |
| >25 × 109/L | 4 (7.14%) | 13 (18.57%) | 0.0621 | 7 (10.94%) | 10 (16.13%) | 0.3938 | 4 (6.67%) | 13 (19.70%) | 0.0325 | 4 (6.45%) | 13 (20.31%) | 0.0228 | 5 (8.20%) | 12 (18.46%) | 0.1123 |
| Platelets (n evaluable, total = 120) | |||||||||||||||
| Median, × 109/L | 439.00 | 246.00 | 0.0021 | 402.00 | 267.00 | 0.1364 | 492.00 | 246.00 | <0.0001 | 406.00 | 267.00 | 0.0886 | 354.00 | 267.00 | 0.0835 |
| <100 × 109/L | 7 (12.50%) | 9 (12.86%) | >0.9999 | 10 (15.63%) | 6 (9.68%) | 0.4241 | 5 (8.33%) | 11 (16.67%) | 0.1887 | 8 (12.90%) | 8 (12.50%) | >0.9999 | 6 (9.84%) | 10 (15.38%) | 0.4273 |
| Circulating CD34 × 106/L (n evaluable, total = 80) | 2.00 | 35.00 | <0.0001 | 1.30 | 63.30 | <0.0001 | 1.20 | 68.30 | <0.0001 | 2.00 | 29.00 | 0.0002 | 3.13 | 22.40 | 0.0026 |
| Constitutional symptoms (n evaluable, total = 134) | 18 (30.00%) | 29 (39.19%) | 0.2815 | 18 (26.09%) | 29 (44.62%) | 0.0302 | 17 (25.76%) | 30 (44.12%) | 0.0306 | 24 (36.36%) | 23 (33.82%) | 0.8567 | 22 (33.33%) | 25 (36.76%) | 0.7197 |
| Splenomegaly (n evaluable, total = 128) | 30 (51.72%) | 56 (80.00%) | 0.0012 | 31 (46.97%) | 55 (88.71%) | <0.0001 | 33 (52.38%) | 53 (81.54%) | 0.0006 | 38 (61.29%) | 48 (72.73%) | 0.1907 | 39 (63.93%) | 47 (70.15%) | 0.5721 |
| LDH (n evaluable, total = 104) | 317 | 604 | 0.0002 | 338.00 | 716.00 | <0.0001 | 362.00 | 604.00 | 0.0004 | 382 | 592 | 0.0008 | 382 | 701 | 0.0001 |
| Thrombosis (n evaluable, total = 133) | 13 (22.03%) | 13 (17.57%) | 0.6604 | 15 (22.06%) | 11 (16.92%) | 0.5159 | 13 (20.00%) | 13 (19.12%) | >0.9999 | 15 (23.08%) | 11 (16.18%) | 0.3838 | 11 (16.92%) | 15 (22.06%) | 0.5159 |
| Bleeding (n evaluable, total = 132) | 6 (10.17%) | 10 (13.70%) | 0.6005 | 7 (10.45%) | 9 (13.85%) | 0.6020 | 7 (10.77%) | 9 (13.43%) | 0.7910 | 6 (9.38%) | 10 (14.71%) | 0.4284 | 5 (7.81%) | 11 (16.18%) | 0.1848 |
| Disease (n evaluable, total = 119) | |||||||||||||||
| Pre-PMF | 24 (42.11%) | 15 (20.83%) | 32 (47.06%) | 7 (11.48%) | 28 (42.42%) | 11 (17.46%) | 24 (36.92%) | 15 (23.44%) | 21 (32.31%) | 18 (28.13%) | |||||
| Overt PMF | 16 (28.07%) | 29 (40.28%) | 15 (22.06%) | 30 (49.18%) | 19 (28.79%) | 26 (41.27%) | 21 (32.31%) | 24 (37.50%) | 25 (38.46%) | 20 (31.25%) | |||||
| PET-MF | 9 (15.79%) | 15 (20.83%) | 11 (16.18%) | 13 (21.31%) | 11 (16.67%) | 13 (20.63%) | 10 (15.38%) | 14 (21.88%) | 10 (15.38%) | 14 (21.88%) | |||||
| PPV-MF | 8 (14.04%) | 13 (18.06%) | 0.0761 | 10 (14.71%) | 11 (18.03%) | 0.0001 | 8 (12.12%) | 13 (20.63%) | 0.0204 | 10 (15.38%) | 11 (17.19%) | 0.3942 | 9 (13.85%) | 12 (18.75%) | 0.5990 |
| Fibrosis grade ≥ 2 (n evaluable, total = 129) | 33 (56.90%) | 55 (77.46%) | 0.0144 | 35 (52.24%) | 53 (85.48%) | <0.0001 | 36 (55.38%) | 52 (81.25%) | 0.0023 | 39 (75.38%) | 49 (60.94%) | 0.0908 | 42 (65.63%) | 46 (70.77%) | 0.5740 |
| Driver mutation (n evaluable, total = 133) | |||||||||||||||
| JAK2 | 42 (70.00%) | 39 (53.42%) | 0.0737 | 46 (66.67%) | 35 (54.69%) | 0.2131 | 40 (61.54%) | 41 (60.29%) | >0.9999 | 41 (63.08%) | 40 (58.82%) | 0.7225 | 41 (63.08%) | 40 (58.82%) | 0.7225 |
| MPL | 4 (6.67%) | 4 (5.48%) | >0.9999 | 4 (5.80%) | 4 (6.25%) | >0.9999 | 4 (6.15%) | 4 (5.88%) | >0.9999 | 6 (9.23%) | 2 (2.94%) | 0.1587 | 4 (6.15%) | 4 (5.88%) | >0.9999 |
| CALR | 10 (16.67%) | 26 (35.62%) | 0.0184 | 14 (20.29%) | 22 (34.38%) | 0.0804 | 17 (26.15%) | 19 (27.94%) | 0.8474 | 12 (18.46%) | 24 (35.29%) | 0.0331 | 17 (26.15%) | 19 (27.94%) | 0.8474 |
| TN | 4 (6.67%) | 4 (5.48%) | >0.9999 | 5 (7.25%) | 3 (4.69%) | 0.7196 | 4 (6.15%) | 4 (5.88%) | >0.9999 | 6 (9.23%) | 2 (2.94%) | 0.1587 | 3 (4.62%) | 5 (7.35%) | 0.7186 |
| High risk mutations (n evaluable, total = 112) | |||||||||||||||
| ≥ 1 | 9 (19.15%) | 28 (47.08%) | 0.0087 | 13 (22.41%) | 24 (44.44%) | 0.0163 | 13 (24.07%) | 24 (41.34%) | 0.0703 | 16 (29.63%) | 21 (36.21%) | 0.5477 | 17 (30.91%) | 20 (35.09%) | 0.6907 |
| ≥ 2 | 0 (0.00%) | 9 (13.85%) | — | 2 (3.45%) | 7 (12.96%) | 0.0860 | 2 (3.70%) | 7 (12.07%) | 0.1643 | 3 (5.56%) | 6 (10.34%) | 0.4921 | 4 (7.27%) | 5 (8.77%) | >0.9999 |
| High-risk mutations (n evaluable, total = 105) | |||||||||||||||
| ASXL1 | 6 (13.64%) | 25 (40.98%) | 0.0025 | 10 (18.18%) | 21 (42.00%) | 0.0101 | 9 (18.00%) | 22 (40.00%) | 0.0183 | 11 (21.57%) | 20 (37.04%) | 0.0917 | 14 (28.00%) | 17 (30.91%) | 0.8316 |
| EZH2 | 1 (2.17%) | 6 (9.52%) | 0.2349 | 2 (3.45%) | 5 (9.80%) | 0.2486 | 2 (3.70%) | 5 (9.09%) | 0.4376 | 3 (5.56%) | 4 (7.27%) | >0.9999 | 2 (3.70%) | 5 (9.09%) | 0.4376 |
| IDH1/2 | 0 (0.00%) | 4 (6.35%) | 0.1364 | 1 (1.72%) | 3 (5.88%) | 0.3383 | 2 (3.64%) | 2 (3.70%) | >0.9999 | 2 (3.70%) | 2 (3.64%) | >0.9999 | 3 (5.56%) | 1 (1.82%) | 0.3634 |
| SRSF2 | 2 (4.35%) | 4 (6.35%) | >0.9999 | 3 (5.17%) | 3 (5.88%) | >0.9999 | 3 (5.56%) | 3 (5.45%) | >0.9999 | 4 (7.41%) | 2 (3.64%) | 0.4376 | 3 (5.56%) | 3 (5.45%) | >0.9999 |
| DIPSS (n evaluable, total = 130) | |||||||||||||||
| Low | 20 (33.90%) | 5 (7.04%) | 18 (27.27%) | 7 (10.94%) | 17 (26.56%) | 8 (12.12%) | 13 (20.63%) | 12 (17.91%) | 15 (23.44%) | 10 (15.15%) | |||||
| Intermediate-1 | 18 (30.51%) | 25 (35.21%) | 27 (40.90%) | 16 (25.00%) | 29 (45.31%) | 14 (21.21%) | 23 (36.51%) | 20 (29.85%) | 21 (32.81%) | 22 (33.33%) | |||||
| Intermediate-2 | 16 (27.12%) | 25 (35.21%) | 17 (25.76%) | 24 (37.50%) | 14 (21.88%) | 27 (40.90%) | 18 (28.57%) | 23 (34.33%) | 21 (31.81%) | 20 (30.30%) | |||||
| High | 5 (8.47%) | 16 (22.54%) | 0.0007 | 4 (6.06%) | 17 (26.56%) | 0.0008 | 4 (6.25%) | 17 (25.76%) | 0.0001 | 9 (14.29%) | 12 (17.91%) | 0.7613 | 7 (10.94%) | 14 (21.21%) | 0.3406 |
| Death (n evaluable, total = 133) | 14 (25.45%) | 36 (51.43%) | 0.0035 | 20 (32.26%) | 32 (48.48%) | 0.0436 | 17 (28.33%) | 33 (50.77%) | 0.0115 | 22 (44.00%) | 28 (42.42%) | 0.5876 | 27 (44.26%) | 23 (35.94%) | 0.3663 |
| AML transformation (n evaluable, total = 133) | 1 (1.92%) | 10 (14.71%) | 0.0225 | 5 (8.20%) | 6 (10.17%) | 0.7605 | 4 (6.90%) | 7 (11.29%) | 0.5317 | 3 (5.36%) | 8 (12.50%) | 0.2164 | 3 (5.00%) | 8 (13.33%) | 0.2043 |
| Overall Survival | Transformation into Acute Leukemia | |||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p | Hazard Ratio (95%CI) | p | |
| High LINC01268 | 2.104 (1.08—4.12) | 0.0297 | 8.190 (1.02—65.78) | 0.0479 |
| High MALAT1 | 1.213 (0.66—2.23) | 0.5343 | 1.792 (0.41—7.81) | 0.4374 |
| High GAS5 | 1.742 (0.91—3.33) | 0.09348 | 1.492 (0.40—5.56) | 0.5507 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantini, S.; Rontauroli, S.; Sartini, S.; Mirabile, M.; Bianchi, E.; Badii, F.; Maccaferri, M.; Guglielmelli, P.; Ottone, T.; Palmieri, R.; et al. Increased Plasma Levels of lncRNAs LINC01268, GAS5 and MALAT1 Correlate with Negative Prognostic Factors in Myelofibrosis. Cancers 2021, 13, 4744. https://doi.org/10.3390/cancers13194744
Fantini S, Rontauroli S, Sartini S, Mirabile M, Bianchi E, Badii F, Maccaferri M, Guglielmelli P, Ottone T, Palmieri R, et al. Increased Plasma Levels of lncRNAs LINC01268, GAS5 and MALAT1 Correlate with Negative Prognostic Factors in Myelofibrosis. Cancers. 2021; 13(19):4744. https://doi.org/10.3390/cancers13194744
Chicago/Turabian StyleFantini, Sebastian, Sebastiano Rontauroli, Stefano Sartini, Margherita Mirabile, Elisa Bianchi, Filippo Badii, Monica Maccaferri, Paola Guglielmelli, Tiziana Ottone, Raffaele Palmieri, and et al. 2021. "Increased Plasma Levels of lncRNAs LINC01268, GAS5 and MALAT1 Correlate with Negative Prognostic Factors in Myelofibrosis" Cancers 13, no. 19: 4744. https://doi.org/10.3390/cancers13194744
APA StyleFantini, S., Rontauroli, S., Sartini, S., Mirabile, M., Bianchi, E., Badii, F., Maccaferri, M., Guglielmelli, P., Ottone, T., Palmieri, R., Genovese, E., Carretta, C., Parenti, S., Mallia, S., Tavernari, L., Salvadori, C., Gesullo, F., Maccari, C., Zizza, M., ... Manfredini, R., on behalf of Mynerva (MYeloid NEoplasms Research Venture AIRC) Investigators. (2021). Increased Plasma Levels of lncRNAs LINC01268, GAS5 and MALAT1 Correlate with Negative Prognostic Factors in Myelofibrosis. Cancers, 13(19), 4744. https://doi.org/10.3390/cancers13194744







