Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. ccRCC Cell Line and Immunocytochemistry (ICC)
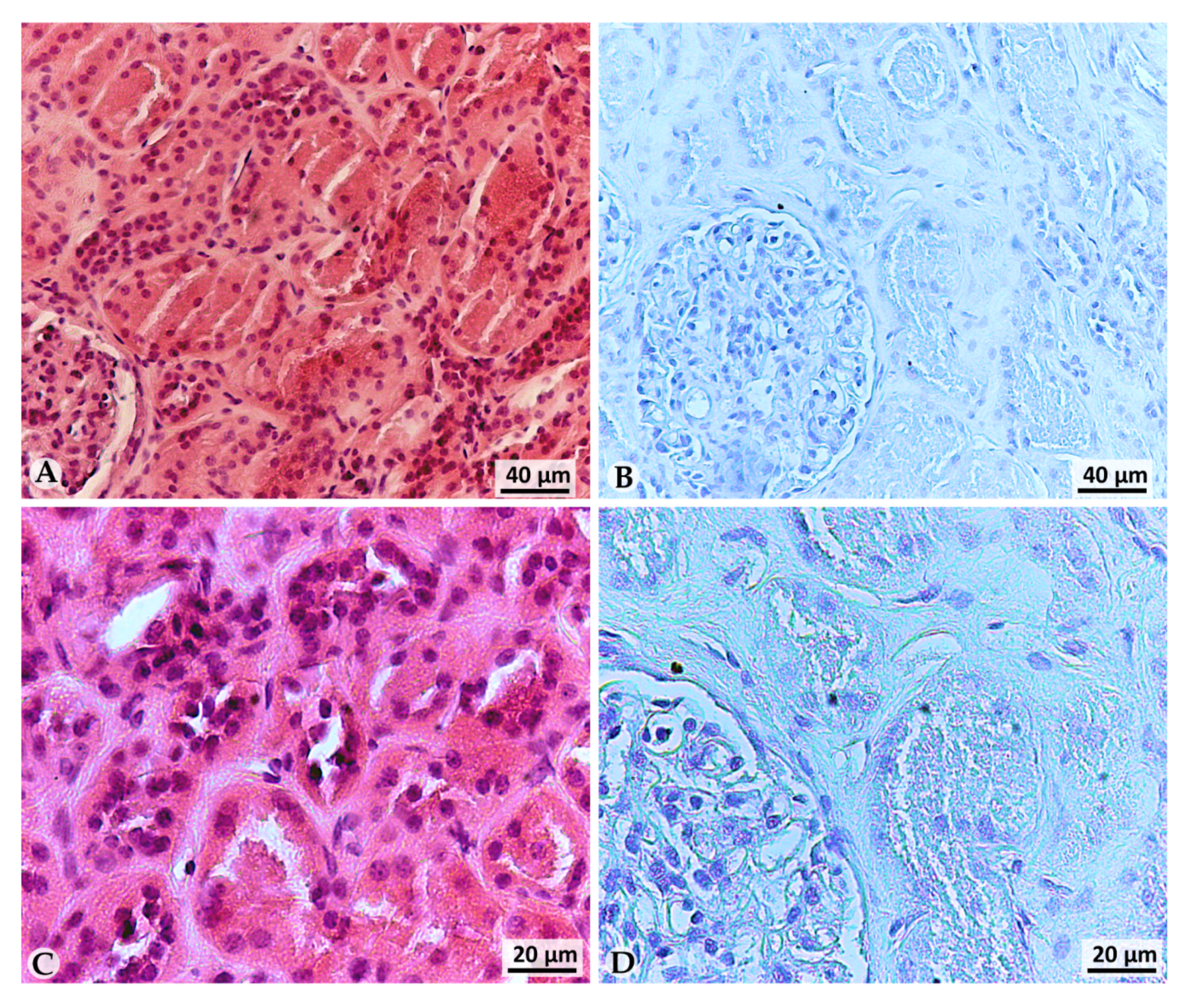
2.3. Immunohistochemistry (IHC)
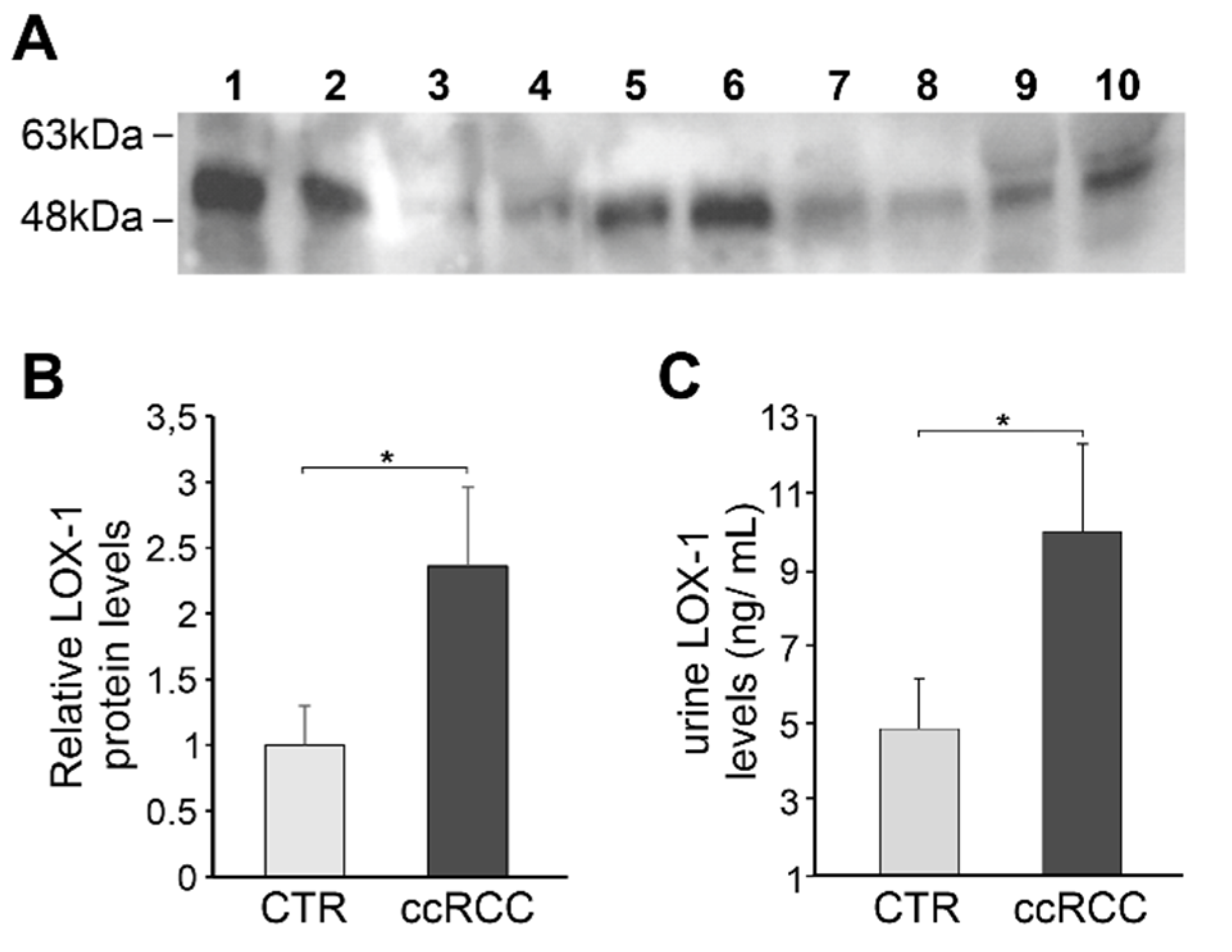
2.4. Quantitative Measurement of Urine LOX-1 Levels
2.5. Western Blot of LOX-1 in Urine
2.6. Gas Chromatography Mass Spectrometry (GC/MS)
2.7. Gas Sensor Array Analysis
2.8. Statistical Analysis
3. Results
3.1. LOX-1 Is Expressed in a Representative ccRCC Cell Line
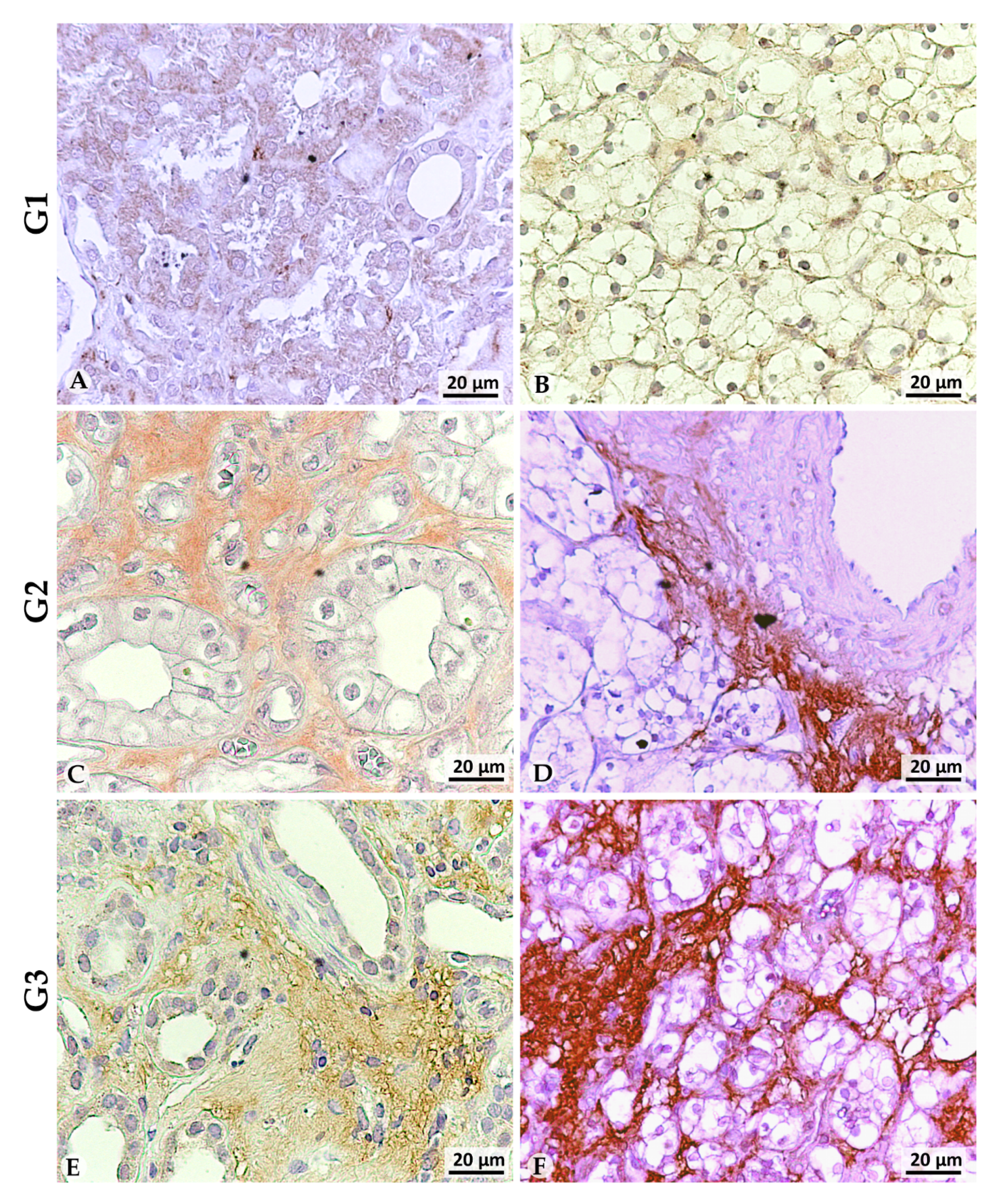
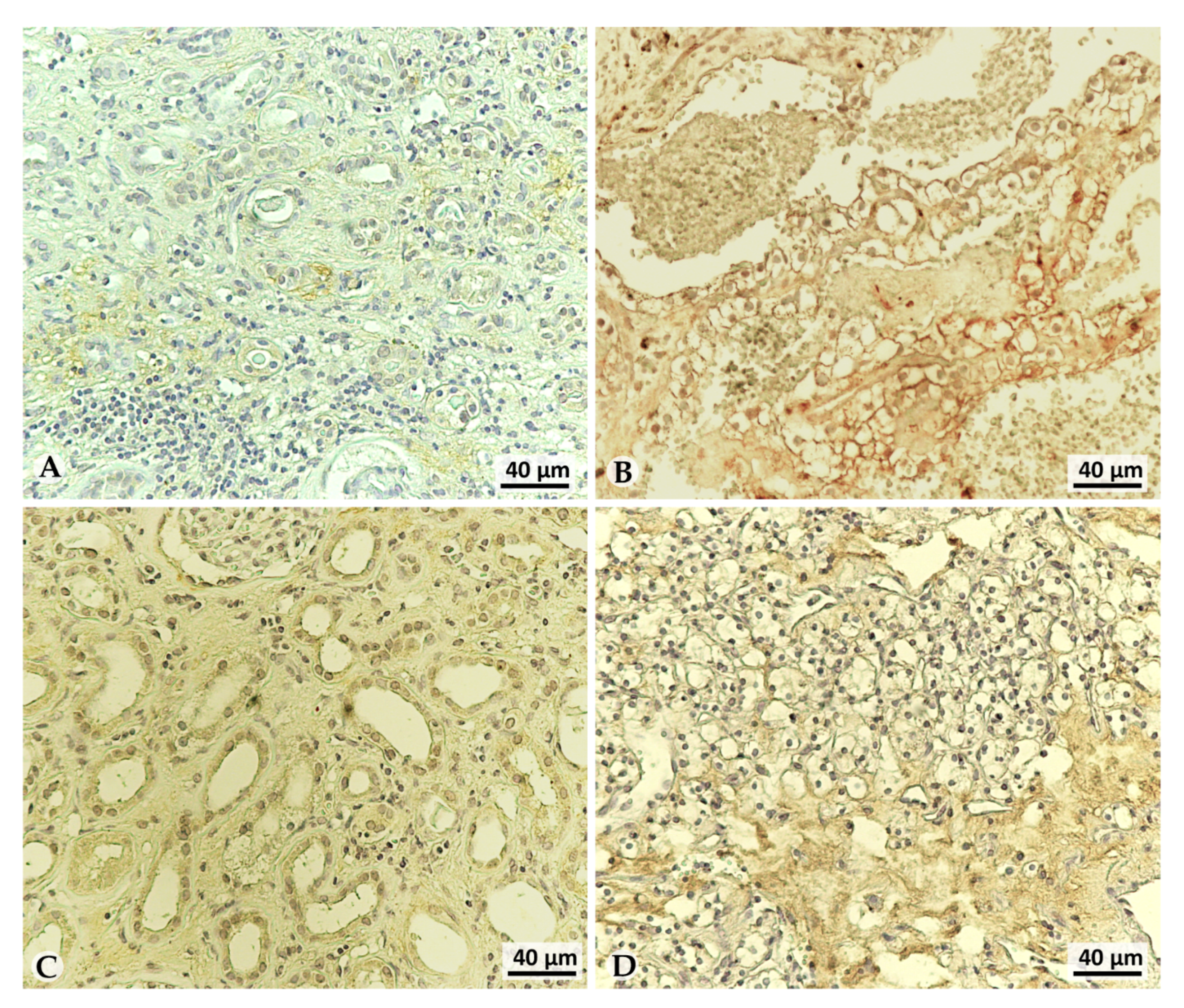
3.2. LOX-1 Is Overexpressed in the Extracellular Space of G2 and G3 Tumors
3.3. LOX-1 Levels Are Impaired in Clear Cell Renal Cancer (ccRC) Urine Patients
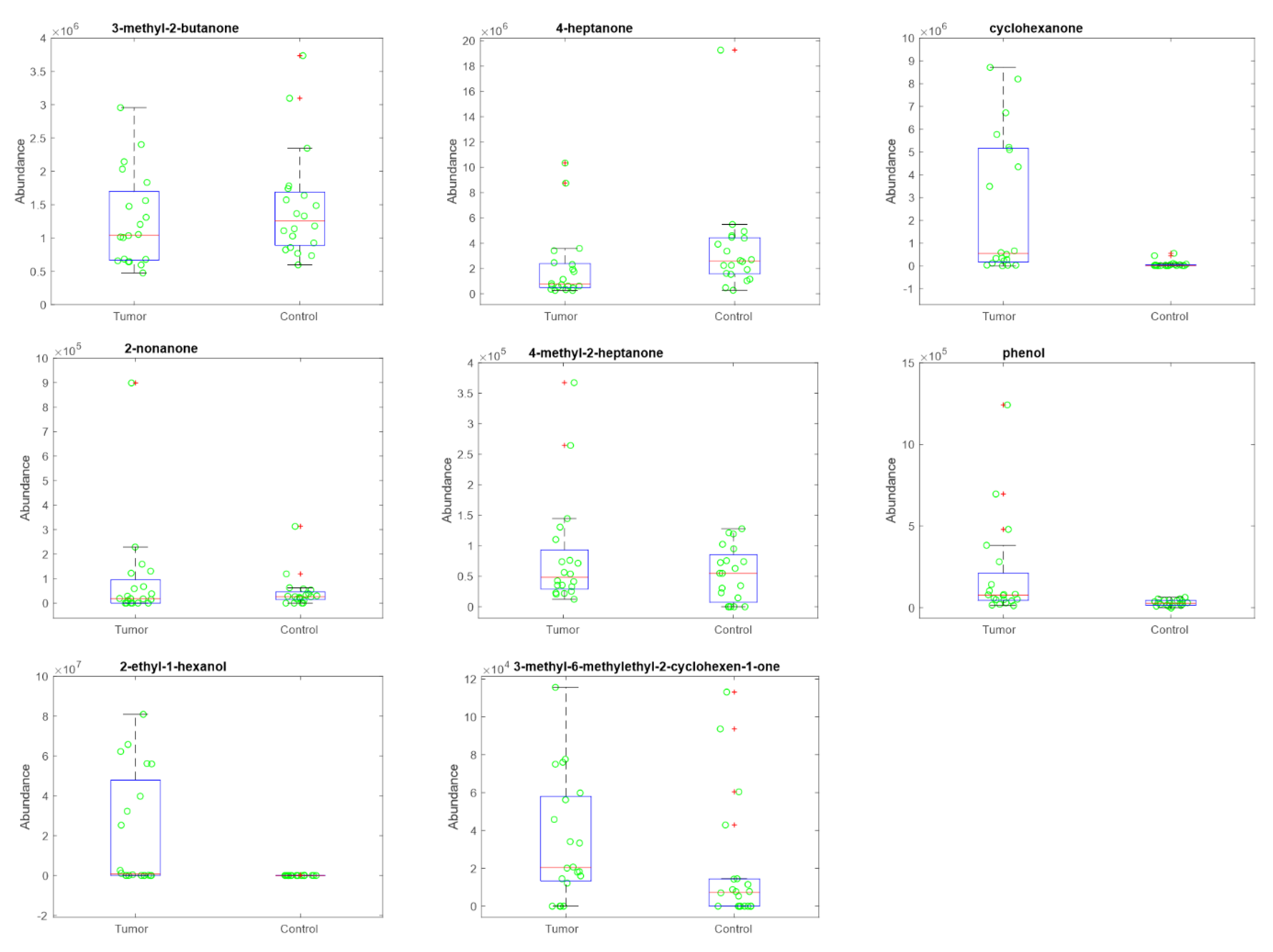
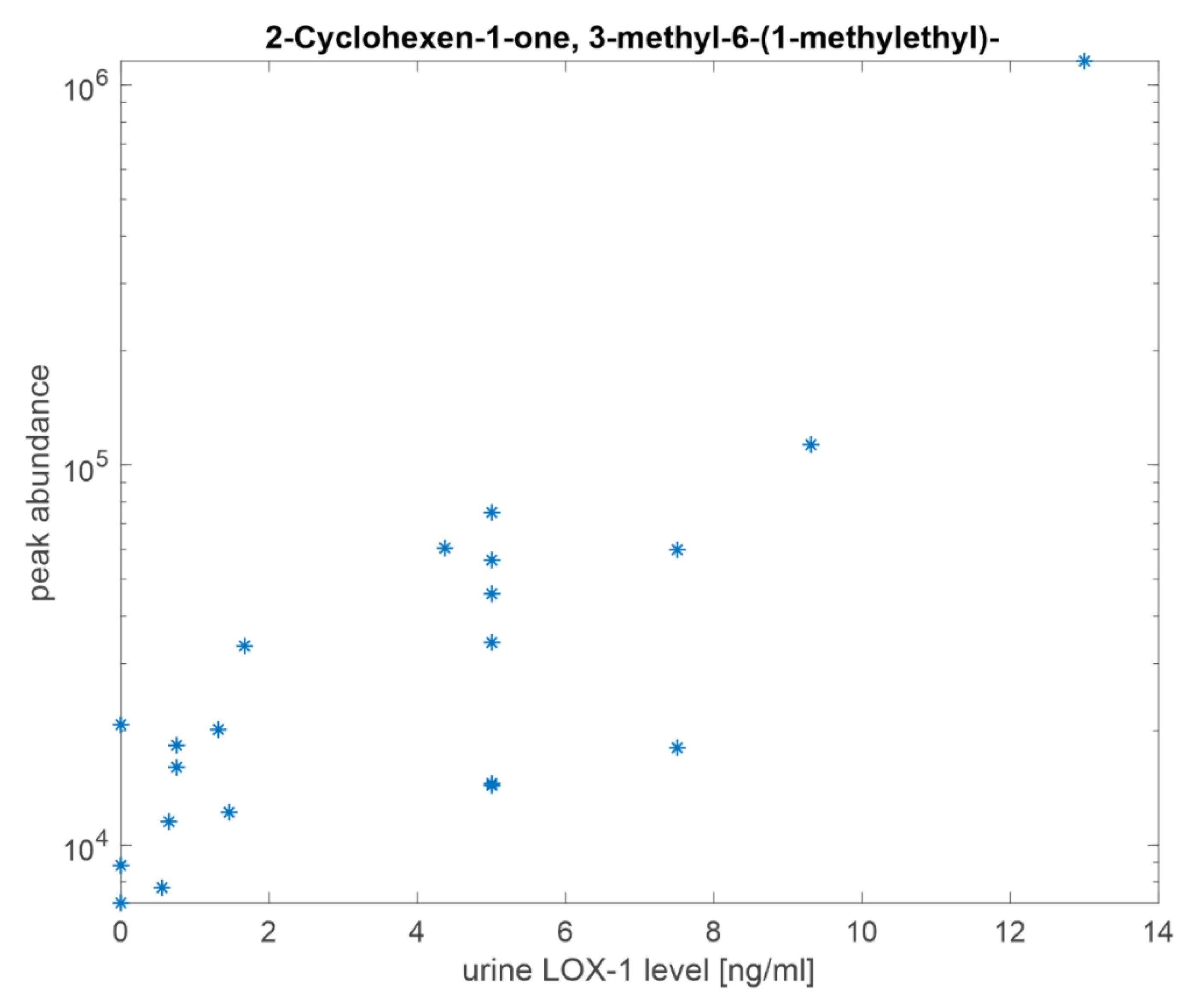
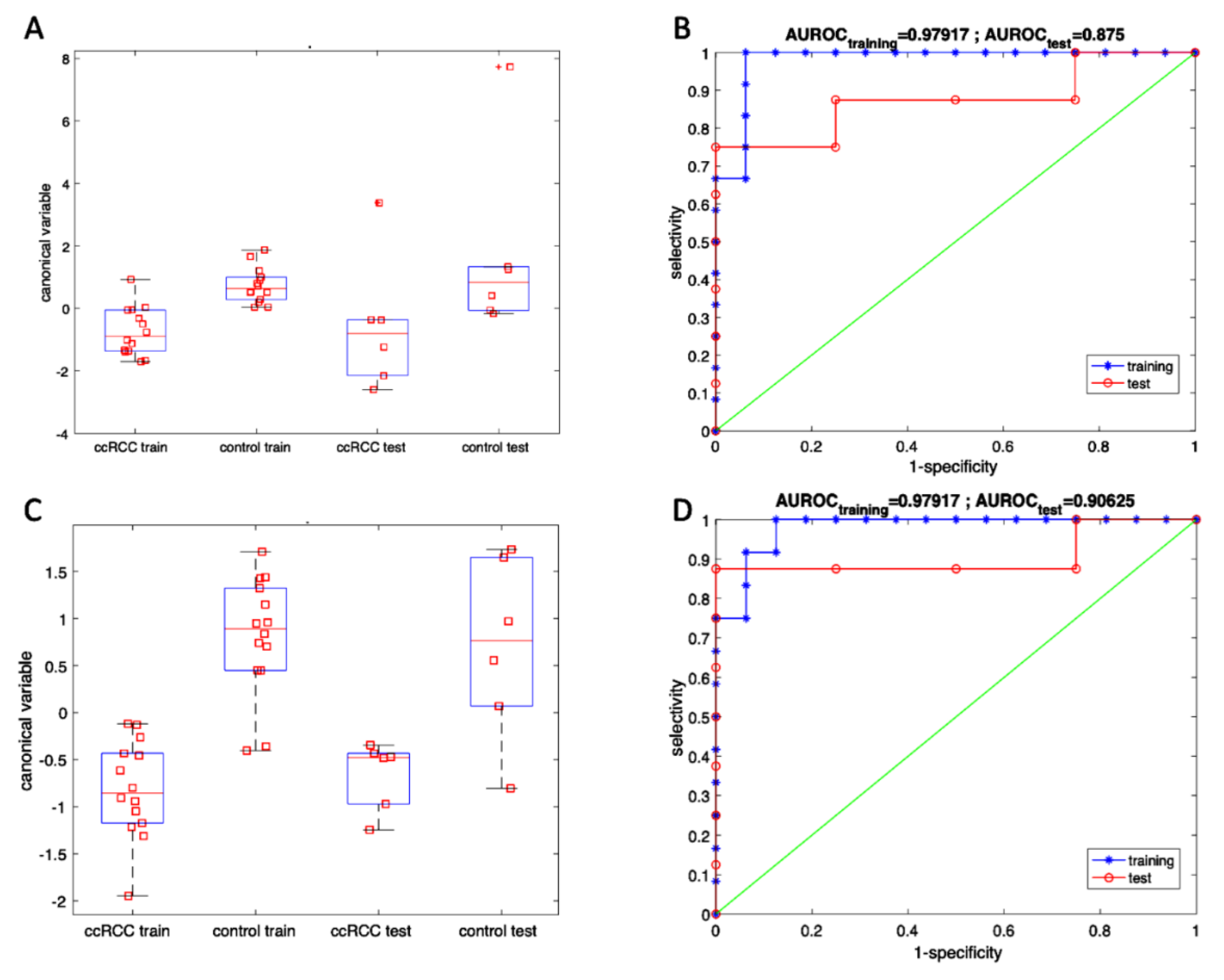
3.4. Volatile Compounds Analysis in Urine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Thorstenson, A.; Bergman, M.; Scherman-Plogell, A.H.; Hosseinnia, S.; Ljungberg, B.; Adolfsson, J.; Lundstam, S. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005–2010: A population-based study from the national Swedish kidney cancer register. Scand. J. Urol. 2014, 48, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.S.; Linehan, W.M. Genetic predisposition to kidney cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Levi, F.; Ferlay, J.; Galeone, C.; Lucchini, F.; Negri, E.; Boyle, P.; La Vecchia, C. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008, 101, 949–958. [Google Scholar] [CrossRef]
- Pastore, A.L.; Palleschi, G.; Silvestri, L.; Moschese, D.; Ricci, S.; Petrozza, V.; Carbone, A.; Di Carlo, A. Serum and urine biomarkers for human renal cell carcinoma. Dis. Markers 2015, 2015, 251403. [Google Scholar] [CrossRef]
- Schodel, J.; Grampp, S.; Maher, E.R.; Moch, H.; Ratcliffe, P.J.; Russo, P.; Mole, D.R. Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur. Urol. 2016, 69, 646–657. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Aboud, O.A.; Lara, P.N., Jr.; Weiss, R.H. Metabolic reprogramming in clear cell renal cell carcinoma. Nat. Rev. Nephrol. 2017, 13, 410. [Google Scholar] [CrossRef]
- Linehan, W.M.; Srinivasan, R.; Schmidt, L.S. The genetic basis of kidney cancer: A metabolic disease. Nat. Rev. Urol. 2010, 7, 277–285. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Pham, C.G.; Hsieh, J.J. A clear picture of renal cell carcinoma. Nat. Genet. 2013, 45, 849–850. [Google Scholar] [CrossRef]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.J.; Simon, M.C. Genetic and metabolic hallmarks of clear cell renal cell carcinoma. BBA Rev. Cancer. 2018, 1870, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Tew, K.D. Redox metabolism and malignancy. Curr. Opin. Pharmacol. 2010, 10, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Rizzacasa, B.; Morini, E.; Pucci, S.; Murdocca, M.; Novelli, G.; Amati, F. LOX-1 and its splice variants: A new challenge for atherosclerosis and cancer-targeted therapies. Int. J. Mol. Sci. 2017, 18, 290. [Google Scholar] [CrossRef]
- Balzan, S.; Lubrano, V. LOX-1 receptor: A potential link in atherosclerosis and cancer. Life Sci. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Liu, X.S.; Struhl, K. Transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Francesco, R.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Deshmukh, R. VEGF: A critical driver for angiogenesis and subsequent tumor growth: An IHC study. J. Oral Maxillofac. Pathol. 2012, 16, 330–337. [Google Scholar] [CrossRef] [PubMed]
- González-Chavarría, I.; Cerro, R.P.; Parra, N.P.; Sandoval, F.A.; Zuñiga, F.A.; Omazábal, V.A.; Lamperti, L.I.; Jiménez, S.P.; Fernandez, E.A.; Gutiérrez, N.A.; et al. Lectin-like oxidized LDL receptor-1 is an enhancer of tumor angiogenesis in human prostate cancer cells. PLoS ONE 2014, 9, e106219. [Google Scholar] [CrossRef] [PubMed]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of OxLDL in cancer. BBA -Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef] [PubMed]
- Zabirnyk, O.; Liu, W.; Khalil, S.; Sharma, A.; Phang, J.M. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis 2010, 31, 446–454. [Google Scholar] [CrossRef]
- Scoles, D.R.; Xu, X.; Wang, H.; Tran, H.; Taylor-Harding, B.; Li, A.; Karlan, B.Y. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol. Oncol. 2010, 116, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Murdocca, M.; De Masi, C.; Pucci, S.; Mango, R.; Novelli, G.; Di Natale, C.; Sangiuolo, F. LOX-1 and cancer: An indissoluble liaison. Cancer Gene Ther. 2020. [Google Scholar] [CrossRef]
- Khaidakov, M.; Mitra, S.; Kang, B.Y.; Wang, X.; Kadlubar, S.; Novelli, G.; Raj, V.; Winters, M.; Carter, W.C.; Mehta, J.L. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS ONE 2011, 6, e20277. [Google Scholar] [CrossRef]
- Yong, C.; Stewart, G.D.; Frezza, C. Oncometabolites in renal cancer: Warburg’s hypothesis re-examined. Nat. Rev. Nephrol. 2020, 16, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Serasanambati, M.; Broza, Y.; Marmur, A.; Haick, H. Profiling single cancer cells with volatolomics approach. iScience 2019, 11, 178–188. [Google Scholar] [CrossRef]
- Dummer, J.; Storer, M.; Swanney, M.; McEwan, M.; Scott-Thomas, A.; Bhandari, S.; Chambers, S.; Dweik, R.; Epton, M. Analysis of biogenic volatile organic compounds in human health and disease. TrAC Trends Anal. Chem. 2011, 30, 960–967. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Martino, R.; Anikst, V.; Xu, Z.; Mix, S.; Benjamin, R.; Schub, H.; Eiden, M.; Paul, A.; Rhodes, P.-A.; et al. Rapid diagnosis of tuberculosis from analysis of urine volatile organic compounds. ACS Sens. 2016, 1, 852–856. [Google Scholar] [CrossRef]
- D’Amico, A.; Bono, R.; Pennazza, G.; Santonico, M.; Mantini, G.; Bernabei, M.; Zarlenga, M.; Roscioni, C.; Martinelli, E.; Paolesse, R.; et al. Identification of melanoma with a gas sensor array. Ski. Res. Technol. 2008, 14, 226–236. [Google Scholar] [CrossRef]
- Murdocca, M.; Mango, R.; Pucci, S.; Biocca, S.; Testa, B.; Capuano, R.; Paolesse, R.; Sanchez, M.; Orlandi, A.; Di Natale, C.; et al. The lectin-like oxidized LDL receptor-1: A new potential molecular target in colorectal cancer. Oncotarget 2016, 7, 14765–14780. [Google Scholar] [CrossRef]
- Murdocca, M.; Capuano, R.; Pucci, S.; Cicconi, R.; Polidoro, C.; Catini, A.; Martinelli, E.; Paolesse, R.; Orlandi, A.; Mango, R.; et al. Targeting LOX-1 Inhibits Colorectal Cancer Metastasis in an Animal Model. Front. Oncol. 2019, 9, 927. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Pi, X.; Guo, L.; Wang, Y.; Li, M.; Feng, Y.; Lin, Z.; Hou, W.; Li, E. Urinary volatile comounds as potential biomarkers for renal cell carcinoma. Biomed. Rep. 2016, 5, 68–71. [Google Scholar] [CrossRef]
- Pinto, J.; Amaro, F.; Lima, A.; Carvalho, C.; Jeronimo, C.; Henrique, R.; Bastos, M.; Carvalho, M.; De Pinho, P. Urinary volatolomics unveils a candidate biomarkerpanel for non invasive detection of clear cell renal cell carcinoma. J. Proteome Res. 2021, 20, 3068–3077. [Google Scholar] [CrossRef]
- Mochalski, P.; Krapf, K.; Ager, C.; Wiesenhofer, H.; Agapiou, A.; Statheropoulos, M.; Fuchs, D.; Ellmerer, E.; Buszewski, B.; Amann, A. Temporal profiling of human urine VOCs and its potential role under the ruins of collapsed buildings. Toxicol. Mech. Methods 2012, 22, 502–511. [Google Scholar] [CrossRef]
- Santos, P.M.; Sánchez, M.; Crisolino Pozas, A.P.; Pérez Pavón, J.L.; Moreno Cordero, B. Determination of ketones and ethyl acetate—A preliminary study for the discrimination of patients with lung cancer. Anal. Bioanal. Chem. 2017, 409, 5689–5696. [Google Scholar] [CrossRef]
- Da Rocha, E.L.; Finoto Caramori, G.; Rambo, C.R. Nanoparticle translocation through a lipid bilayer tuned by surface chemistry. Phys. Chem. Chem. Phys. 2012, 15, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Eichelberg, C.; Junker, K.; Ljungberg, B.; Moch, H. Diagnostic and prognostic molecular marker for renal cell carcinoma: A critical appraisal of the current state of research and clinical applicability. Eur. Urol. 2009, 55, 851–863. [Google Scholar] [CrossRef]
- Lam, J.S.; Pantuck, A.J.; Belldegrun, A.S.; Figlin, R.A. Protein expression profiles in renal cell carcinoma: Staging, prognosis, and patient selection for clinical trial. Clin. Cancer Res. 2007, 13, 703s–708s. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinges, S.S.; Hohm, A.; Vandergrift, L.A.; Nowak, J.; Habbel, P.; Kaltashov, I.A.; Cheng, L.L. Cancer metabolomic markers in urine: Evidence, techniques and recommendations. Nat. Rev. Urol. 2019, 16, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T. Inconvenient truth: Cancer biomarker development by using proteomics. Biochim. Biophys Acta BBA Proteins Proteom. 2014, 1844, 861–865. [Google Scholar] [CrossRef]
- Gupta, S.; Venkatesh, A.; Ray, S.; Srivastava, S. Challenges and prospects for biomarker research: A current perspective from the developing world. Biochim. Biophys Acta BBA Proteins Proteom. 2014, 1844, 899–908. [Google Scholar] [CrossRef]
- Rodrıguez-Suarez, E.; Siwy, J.; Zurbig, P.; Mischak, H. Urine as a source for clinical proteome analysis: From discovery to clinical application. Biochim. Biophys Acta BBA Proteins Proteom. 2014, 1844, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Bird, D.; Welti, J.C.; Gourlaouen, M.; Lang, G.; Murray, G.I.; Reynolds, A.R.; Cox, T.R.; Erler, J.T. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 2013, 73, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.C.; Rini, B.I. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Stefanius, K.; Servage, K.; Orth, K. Exosomes in cancer development. Curr. Opin. Genet. Dev. 2021, 66, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; Vindigni, G.; Testa, B.; Raniolo, S.; Fasciglione, G.F.; Coletta, M.; Biocca, S. Membrane Cholesterol Modulates LOX-1 Shedding in Endothelial Cells. PLoS ONE 2015, 10, e0141270. [Google Scholar] [CrossRef] [PubMed]
- Ganti, S.; Weiss, R.H. Urine metabolomics for kidney cancer detection and biomarker discovery. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 551–557. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Alaiwi, S.A.; Chakravarthy, A.; Shen, S.Y.; Bakouny, Z.; Boccardo, F.; et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef]


| Clinical Features | ccRCC Patients = 40 |
|---|---|
| Age | 62.3 yrs. (range 26–81) |
| Gender | |
| Male | 24 (60%) |
| Female | 16 (40%) |
| Surgery | |
| Partial nephrectomy | 20 (50%) |
| Radical nephrectomy | 20 (50%) |
| Tumor Site | |
| Left kidney | 19 (47.5%) |
| Right kidney | 21 (52.5%) |
| Tumor size | 4.55 cm (range 1–11) |
| TNM Tumor Staging | |
| T1a | 18 (45%) |
| T1b | 11 (27.5%) |
| T2a | 4 (10%) |
| T2b | 1 (2.5%) |
| T3a | 6 (15%) |
| N0-Nx | 40 (100%) |
| N1 | |
| M0 | 39 (97.5%) |
| M1 | 1 (2.5%) |
| ISUP Tumor Grading | |
| G1 | 8 (20%) |
| G2 | 24 (60%) |
| G3 | 8 (20%) |
| Tumor Necrosis | |
| Presence | 5 (12.5%) |
| Absence | 35 (87.5%) |
| Comorbidities | |
| Hypertension | 28/40 (70%) |
| Diabetes | 6/40 (15%) |
| Dyslipidemia | 17/40 (42.5%) |
| Smoking habit | 10/40 (25%) |
| BMI | |
| Weight | 82.2 kg (range 56–134) |
| Height | 170 cm (range 160–184) |
| Patients | Grade | Normal Peritumoral Tissues (NpT) | Tumoral Tissues | |||
|---|---|---|---|---|---|---|
| Nucleus | Cytoplasm | Nucleus | Cytoplasm | Extracellular Space | ||
| 8 | G1 | − | ++ | + | +/− | 50% ++ |
| 23 | G2 | − | + | 10% cells +/− | − | ++ |
| 9 | G3 | − | + | − | − | ++ |
| VOC | p-Value |
|---|---|
| 2-Butanone, 3-methyl- | 0.26 |
| 4-Heptanone | 0.01 |
| Cyclohexanone | <0.001 |
| 2-Nonanone | 0.79 |
| 2-Heptanone, 4-methyl- | 0.55 |
| Phenol | <0.001 |
| 1-Hexanol, 2-ethyl- | <0.001 |
| 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)- | 0.02 |
| Analytical Instrument | Accuracy | Sensitivity | Selectivity | |||
|---|---|---|---|---|---|---|
| Training | Test | Training | Test | Training | Test | |
| GC/MS | 92.9% | 91.7% | 85.7% | 83.3% | 100% | 100% |
| Sensor array | 92.9% | 91.7% | 100% | 100% | 85.7% | 83.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdocca, M.; Torino, F.; Pucci, S.; Costantini, M.; Capuano, R.; Greggi, C.; Polidoro, C.; Somma, G.; Pasqualetti, V.; Ketchanji Mougang, Y.; et al. Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer. Cancers 2021, 13, 4213. https://doi.org/10.3390/cancers13164213
Murdocca M, Torino F, Pucci S, Costantini M, Capuano R, Greggi C, Polidoro C, Somma G, Pasqualetti V, Ketchanji Mougang Y, et al. Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer. Cancers. 2021; 13(16):4213. https://doi.org/10.3390/cancers13164213
Chicago/Turabian StyleMurdocca, Michela, Francesco Torino, Sabina Pucci, Manuela Costantini, Rosamaria Capuano, Chiara Greggi, Chiara Polidoro, Giuseppina Somma, Valentina Pasqualetti, Yolande Ketchanji Mougang, and et al. 2021. "Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer" Cancers 13, no. 16: 4213. https://doi.org/10.3390/cancers13164213
APA StyleMurdocca, M., Torino, F., Pucci, S., Costantini, M., Capuano, R., Greggi, C., Polidoro, C., Somma, G., Pasqualetti, V., Ketchanji Mougang, Y., Catini, A., Simone, G., Paolesse, R., Orlandi, A., Mauriello, A., Roselli, M., Magrini, A., Novelli, G., Di Natale, C., & Sangiuolo, F. C. (2021). Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer. Cancers, 13(16), 4213. https://doi.org/10.3390/cancers13164213













