Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
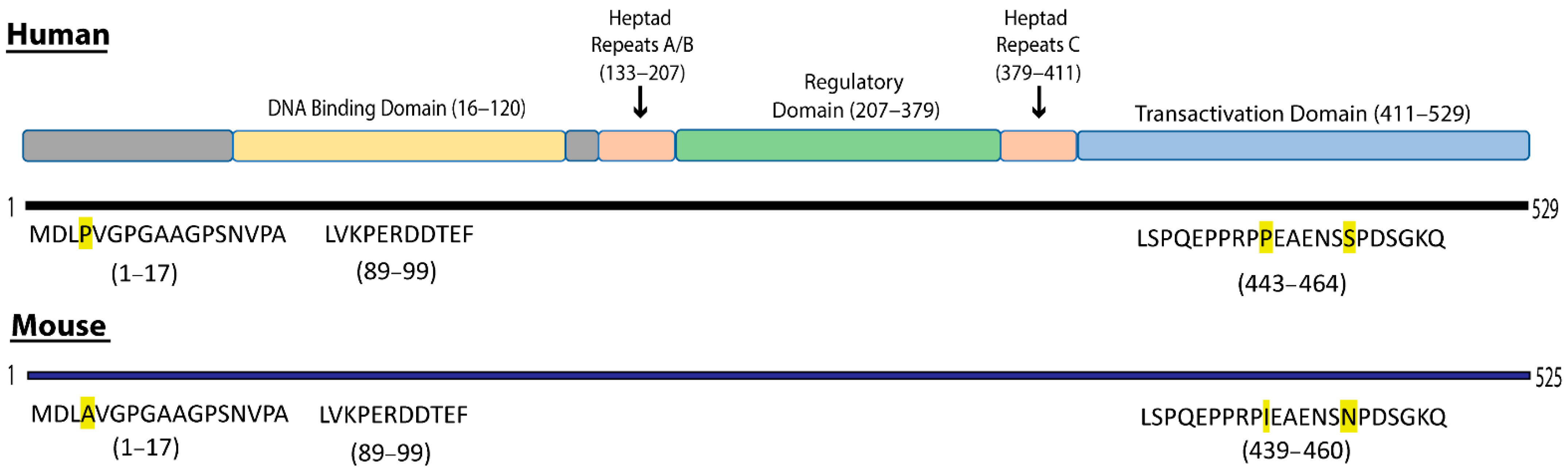
2.2. Peptide Design and Synthesis
2.3. Enzyme-Linked Immunosorbent (ELISA) Assay
2.4. Statistical Analysis
3. Results
3.1. Definition of Selected B-Cell Epitopes in Heat Shock Factor 1
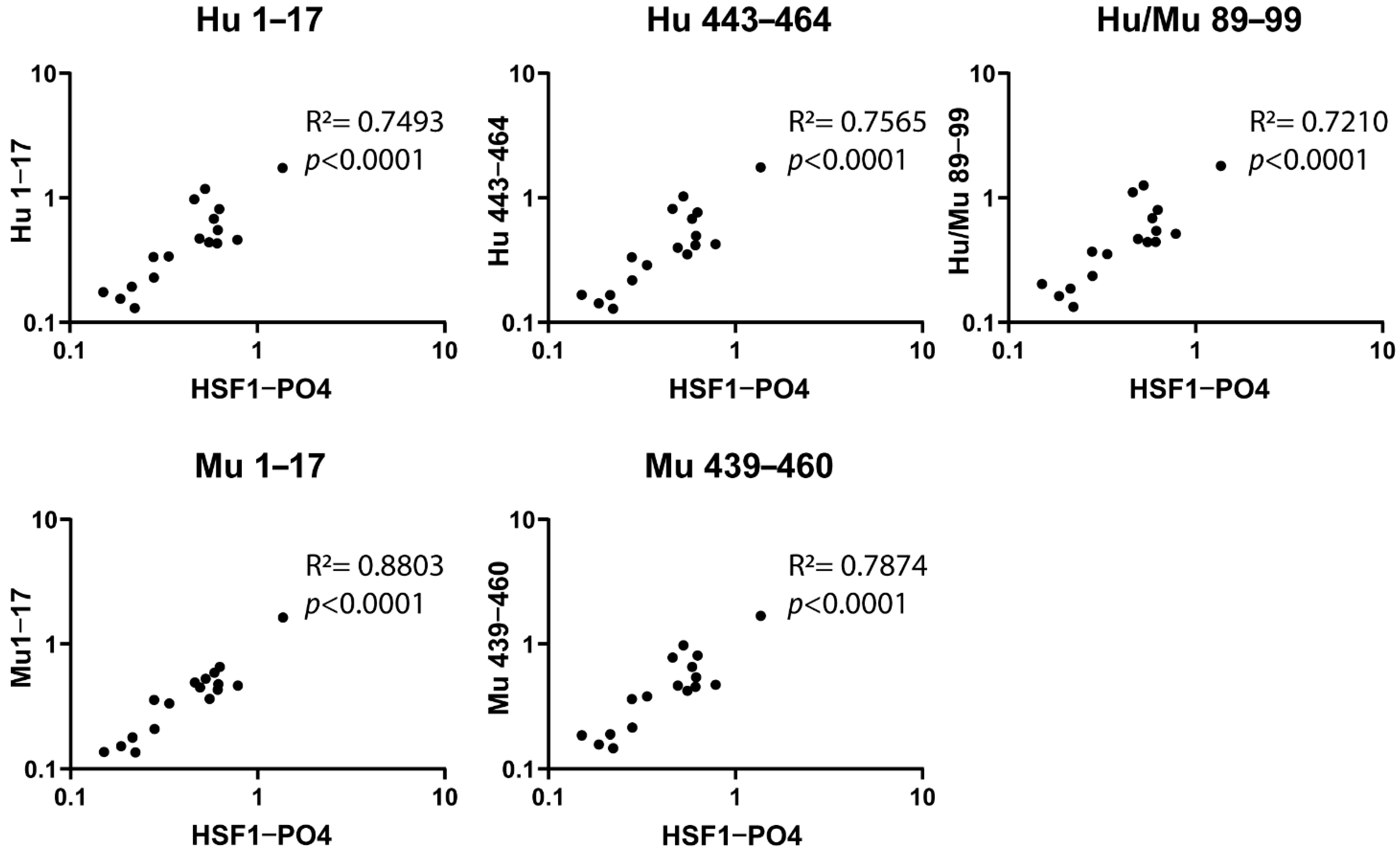
3.2. Designed Synthetic Peptides Have a Positive Correlation to HSF1-PO4 Antibody Responses
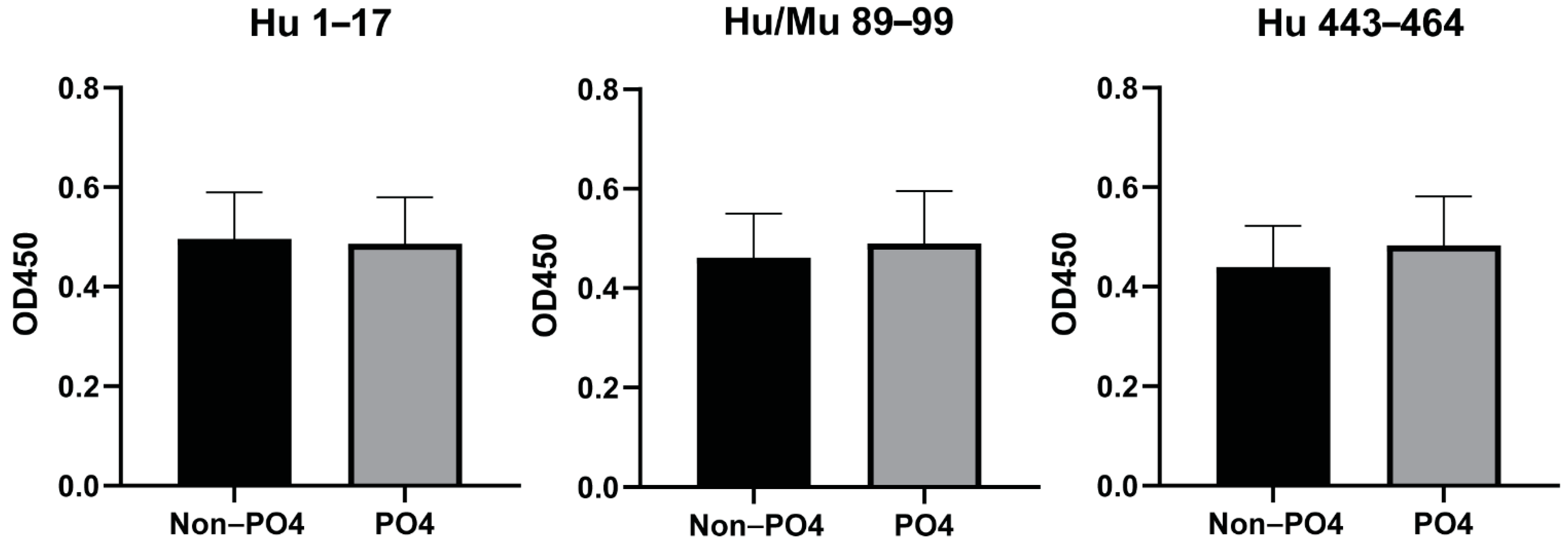
3.3. Phosphorylation of Peptides Does Not Affect the Antibody Responses to the Predicted Epitopes
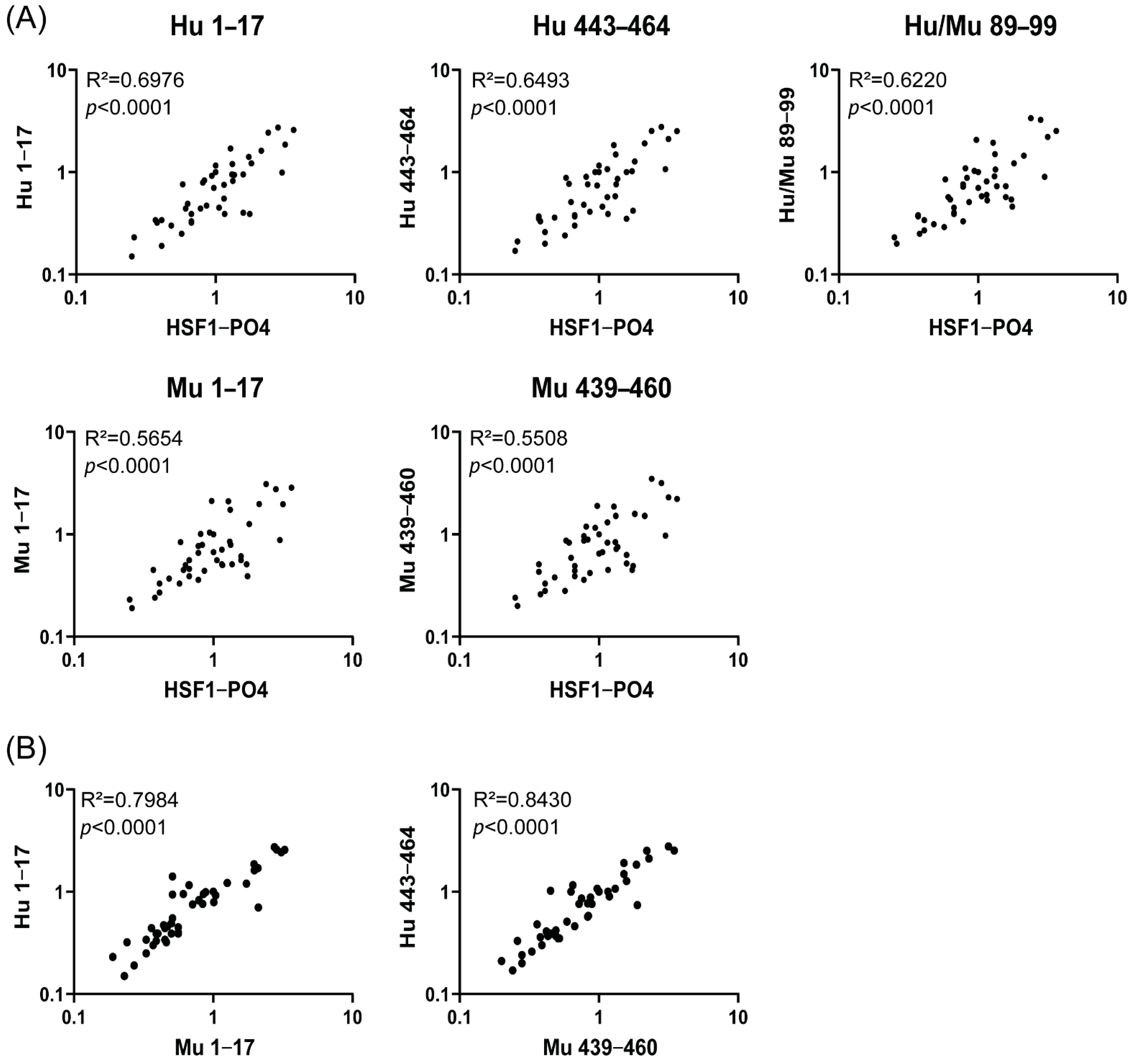
3.4. Correlation Validation between Responses to HSF1-PO4 and Predicted Peptide Epitope and the Assessment of Species Cross-Reactivity
3.5. Sensitivity Assays Show Predicted Epitopes with Equal or Higher Measurements in Comparison to HSF1-PO4
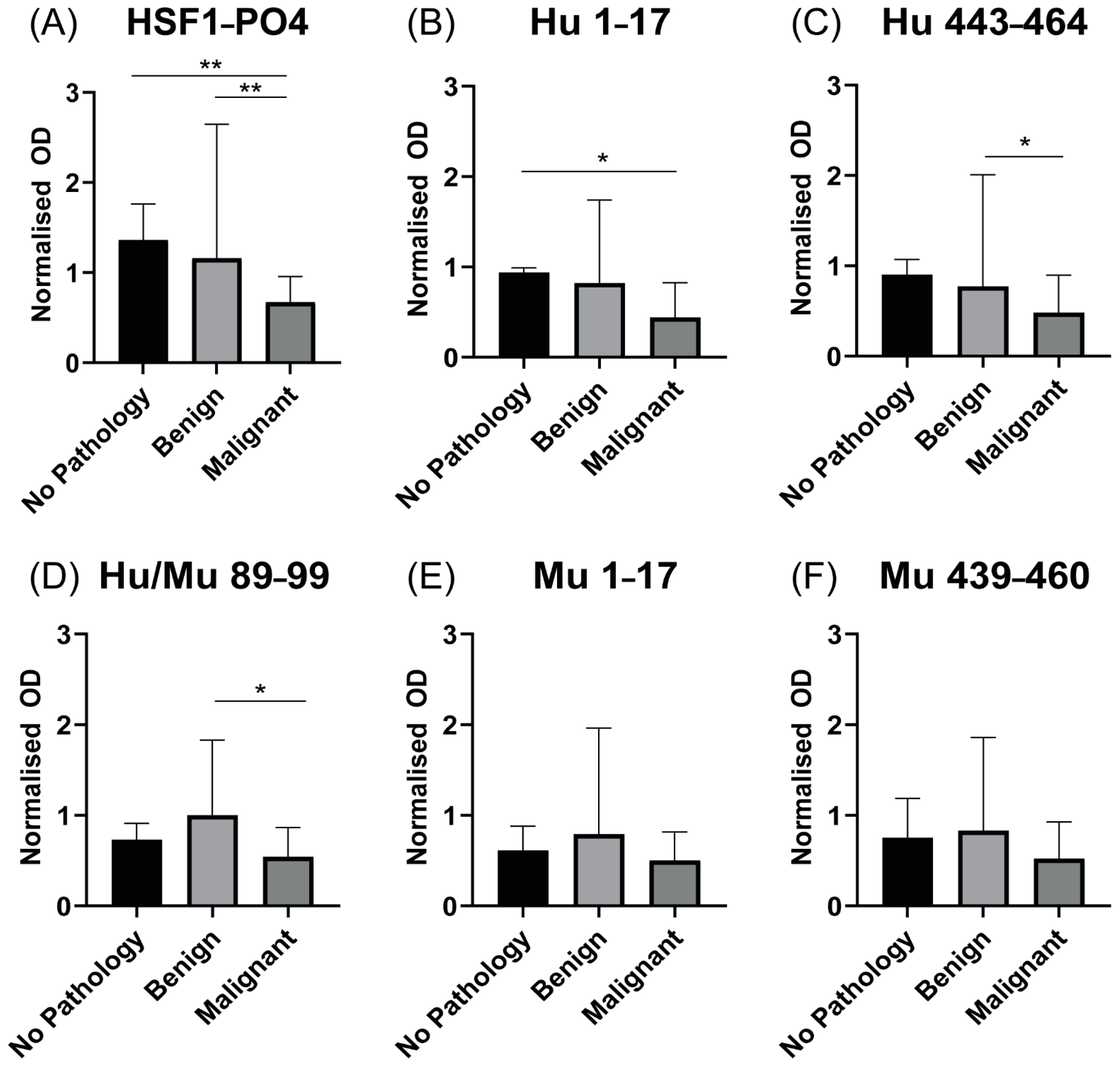
3.6. Patients with Advanced HGSOC Have Decreased Anti HSF1-PO4-Specific AAb Responses
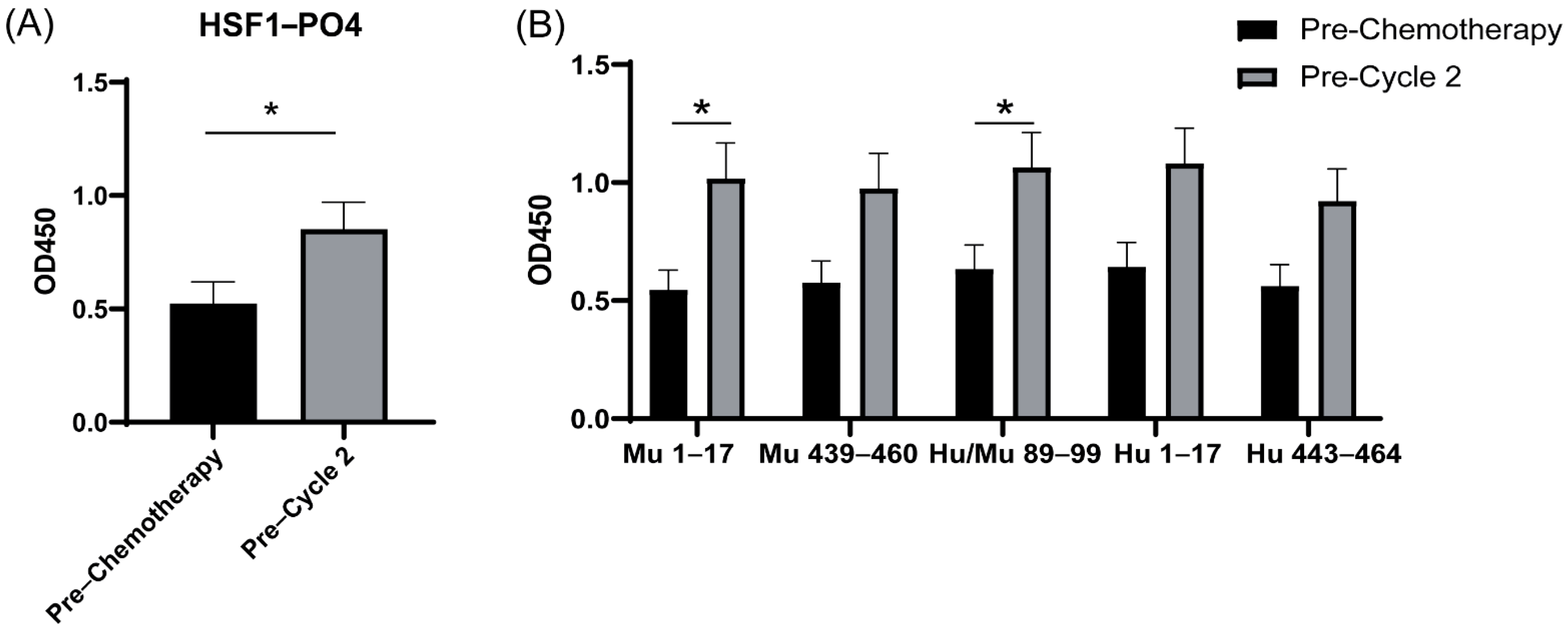
3.7. Increased IgA-Specific AAb Responses to HSF1 following a Cycle of Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Lanneau, D.; De Thonel, A.; Berthenet, K.; Hazoumé, A.; Droin, N.; Hamman, A.; Girodon, F.; Bellaye, P.-S.; Wettstein, G.; et al. Dual regulation of SPI1/PU.1 transcription factor by heat shock factor 1 (HSF1) during macrophage differentiation of monocytes. Leukemia 2014, 28, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Liangliang, X.; Yonghui, H.; Shunmei, E.; Shoufang, G.; Wei, Z.; Jiangying, Z. Dominant-positive HSF1 decreases alpha-synuclein level and alpha-synuclein-induced toxicity. Mol. Biol. Rep. 2010, 37, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-N.; Huang, L.; Zimonjic, D.B.; Moskophidis, D.; Mivechi, N.F. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene 2007, 26, 5086–5097. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat Shock Factor Hsf1 Cooperates with ErbB2 (Her2/Neu) Protein to Promote Mammary Tumorigenesis and Metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef]
- O’Callaghan-Sunol, C.; Sherman, M.Y. Heat Shock Transcription Factor HSF1 Plays a Critical Role in Cell Migration via Maintaining MAP Kinase Signaling. Cell Cycle 2006, 5, 1431–1437. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujimoto, M.; Hayashida, N.; Takii, R.; Nakai, A.; Muto, M. Silencing HSF1 by short hairpin RNA decreases cell proliferation and enhances sensitivity to hyperthermia in human melanoma cell lines. J. Dermatol. Sci. 2010, 60, 187–192. [Google Scholar] [CrossRef]
- Santagata, S.; Hu, R.; Lin, N.U.; Mendillo, M.; Collins, L.C.; Hankinson, S.E.; Schnitt, S.J.; Whitesell, L.; Tamimi, R.M.; Lindquist, S.; et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 18378–18383. [Google Scholar] [CrossRef]
- Desai, S.; Liu, Z.; Yao, J.; Patel, N.; Chen, J.; Wu, Y.; Ahn, E.E.-Y.; Fodstad, O.; Tan, M. Heat Shock Factor 1 (HSF1) Controls Chemoresistance and Autophagy through Transcriptional Regulation of Autophagy-related Protein 7 (ATG7). J. Biol. Chem. 2013, 288, 9165–9176. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tian, H.; Chen, G. Upregulation of Nuclear Heat Shock Factor 1 Contributes to Tumor Angiogenesis and Poor Survival in Patients with Non–Small Cell Lung Cancer. Ann. Thorac. Surg. 2015, 100, 465–472. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, S.-C.; Kang, H.-G.; Gim, J.; Lee, K.-H.; Lee, S.-H.; Chun, K.-H. Heat Shock Factor 1 Predicts Poor Prognosis of Gastric Cancer. Yonsei Med. J. 2018, 59, 1041–1048. [Google Scholar] [CrossRef]
- Tsukao, Y.; Yamasaki, M.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Kurokawa, Y.; Miyata, H.; Nakajima, K.; Takiguchi, S.; Mimori, K.; et al. Overexpression of heat-shock factor 1 is associated with a poor prognosis in esophageal squamous cell carcinoma. Oncol. Lett. 2017, 13, 1819–1825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasuda, K.; Hirohashi, Y.; Mariya, T.; Murai, A.; Tabuchi, Y.; Kuroda, T.; Kusumoto, H.; Takaya, A.; Yamamoto, E.; Kubo, T.; et al. Phosphorylation of HSF1 at serine 326 residue is related to the maintenance of gynecologic cancer stem cells through expression of HSP. Oncotarget 2017, 8, 31540–31553. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Figo’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Cadron, I.; Despierre, E.; Daemen, M.; Leunen, K.; Amant, F.; Timmerman, D.; De Moor, B.; Vergote, I. HE4 and CA125 as a diagnostic test in ovarian cancer: Prospective validation of the Risk of Ovarian Malignancy Algorithm. Br. J. Cancer 2011, 104, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cao, Y.; Liu, X.; Zeng, X.-T.; Li, Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget 2017, 8, 63963–63970. [Google Scholar] [CrossRef]
- Santulli, P.; Streuli, I.; Melonio, I.; Marcellin, L.; M’Baye, M.; Bititi, A.; Borghese, B.; Pillet, M.-C.L.; Chapron, C. Increased Serum Cancer Antigen-125 Is a Marker for Severity of Deep Endometriosis. J. Minim. Invasive Gynecol. 2015, 22, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Moley, K.H.; Massad, L.S.; Mutch, D.G. Pelvic inflammatory disease. Correlation of severity with CA-125 levels. J. Reprod. Med. 1996, 41, 341–346. [Google Scholar] [PubMed]
- Chapman, C.J.; Thorpe, A.J.; Murray, A.; Parsy-Kowalska, C.B.; Allen, J.; Stafford, K.M.; Chauhan, A.S.; Kite, T.A.; Maddison, P.; Robertson, J.F. Immunobiomarkers in small cell lung cancer: Potential early cancer signals. Clin. Cancer Res. 2011, 17, 1474–1480. [Google Scholar] [CrossRef]
- Anderson, K.S.; Sibani, S.; Wallstrom, G.; Qiu, J.; Mendoza, E.A.; Raphael, J.; Hainsworth, E.; Montor, W.R.; Wong, J.; Park, J.G.; et al. Protein Microarray Signature of Autoantibody Biomarkers for the Early Detection of Breast Cancer. J. Proteome Res. 2011, 10, 85–96. [Google Scholar] [CrossRef]
- Zayakin, P.; Ancāns, G.; Silina, K.; Meistere, I.; Kalniņa, Z.; Andrējeva, D.; Endzeliņš, E.; Ivanova, L.; Pismennaja, A.; Ruskule, A.; et al. Tumor-associated autoantibody signature for the early detection of gastric cancer. Int. J. Cancer 2013, 132, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Cramer, D.W.; Sibani, S.; Wallstrom, G.; Wong, J.; Park, J.; Qiu, J.; Vitonis, A.; LaBaer, J. Autoantibody Signature for the Serologic Detection of Ovarian Cancer. J. Proteome Res. 2015, 14, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Moffitt, L.R.; Duffield, N.; Rainczuk, A.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Autoantibodies against HSF1 and CCDC155 as Biomarkers of Early-Stage, High-Grade Serous Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 183–192. [Google Scholar] [CrossRef]
- Anderton, S.M.; van der Zee, R.; Prakken, B.; Noordzij, A.; Van Eden, W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J. Exp. Med. 1995, 181, 943–952. [Google Scholar] [CrossRef]
- Van Herwijnen, M.; Wieten, L.; van der Zee, R.; van Kooten, P.J.; Wagenaar-Hilbers, J.P.; Hoek, A.; Braber, I.D.; Anderton, S.M.; Singh, M.; Meiring, H.D.; et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 14134–14139. [Google Scholar] [CrossRef]
- Elfaitouri, A.; Herrmann, B.; Bölin-Wiener, A.; Wang, Y.; Gottfries, C.-G.; Zachrisson, O.; Pipkorn, R.; Rönnblom, L.; Blomberg, J. Epitopes of Microbial and Human Heat Shock Protein 60 and Their Recognition in Myalgic Encephalomyelitis. PLoS ONE 2013, 8, e81155. [Google Scholar] [CrossRef]
- Perschinka, H.; Mayr, M.; Millonig, G.; Mayerl, C.; van der Zee, R.; Morrison Sandra, G.; Morrison Richard, P.; Xu, Q.; Wick, G. Cross-reactive b-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1060–1065. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci. Rep. 2020, 10, 2213. [Google Scholar] [CrossRef]
- Neudegger, T.; Verghese, J.; Hayer-Hartl, M.; Hartl, F.U.; Bracher, A. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat. Struct. Mol. Biol. 2016, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bridges, T.M.; Scheraga, R.; Tulapurkar, M.E.; Suffredini, D.; Liggett, S.B.; Ramarathnam, A.; Potla, R.; Singh, I.S.; Hasday, J.D. Polymorphisms in human heat shock factor-1 and analysis of potential biological consequences. Cell Stress Chaperones 2015, 20, 47–59. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Brady, M.F.; McGuire, W.P.; Harper, P.G.; Alberts, D.S.; Friedlander, M.; Colombo, N.; Fowler, J.M.; Argenta, P.A.; De Geest, K.; et al. Evaluation of New Platinum-Based Treatment Regimens in Advanced-Stage Ovarian Cancer: A Phase III Trial of the Gynecologic Cancer InterGroup. J. Clin. Oncol. 2009, 27, 1419–1425. [Google Scholar] [CrossRef]
- Grimmig, T.; Moll, E.-M.; Kloos, K.; Thumm, R.; Moench, R.; Callies, S.; Kreckel, J.; Vetterlein, M.; Pelz, J.; Polat, B.; et al. Upregulated Heat Shock Proteins after Hyperthermic Chemotherapy Point to Induced Cell Survival Mechanisms in Affected Tumor Cells From Peritoneal Carcinomatosis. Cancer Growth Metastasis 2017, 10, 1179064417730559. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kashaninejad, N.; Masud, M.K.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J. Autoantibodies as diagnostic and prognostic cancer biomarker: Detection techniques and approaches. Biosens. Bioelectron. 2019, 139, 111315. [Google Scholar] [CrossRef] [PubMed]
- Karabudak, A.A.; Hafner, J.; Shetty, V.; Chen, S.; Secord, A.A.; Morse, M.A.; Philip, R. Autoantibody biomarkers identified by proteomics methods distinguish ovarian cancer from non-ovarian cancer with various CA-125 levels. J. Cancer Res. Clin. Oncol. 2013, 139, 1757–1770. [Google Scholar] [CrossRef]
- Katchman, B.A.; Chowell, D.; Wallstrom, G.; Vitonis, A.F.; LaBaer, J.; Cramer, D.W.; Anderson, K.S. Autoantibody biomarkers for the detection of serous ovarian cancer. Gynecol. Oncol. 2017, 146, 129–136. [Google Scholar] [CrossRef] [PubMed]
- OCRF. OCRF Annual Report 2019–2020; OCRF: Toorak, VIC, Australia, 2020; pp. 1–31. [Google Scholar]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Herzog, T.J. Recurrent ovarian cancer: How important is it to treat to disease progression? Clin. Cancer Res. 2004, 10, 7439–7449. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. Platinum resistant ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mandal, G.; Payne, K.K.; Anadon, C.M.; Gatenbee, C.D.; Chaurio, R.A.; Costich, T.L.; Moran, C.; Harro, C.M.; Rigolizzo, K.E.; et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nat. Cell Biol. 2021, 591, 464–470. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, S.-Y.; Yang, Y.-H.; Zheng, J.; Liu, T.; Wang, L. Targeting HSF1 leads to an antitumor effect in human epithelial ovarian cancer. Int. J. Mol. Med. 2017, 39, 1564–1570. [Google Scholar] [CrossRef]
- Powell, C.D.; Paullin, T.R.; Aoisa, C.; Menzie, C.J.; Ubaldini, A.; Westerheide, S.D. The Heat Shock Transcription Factor HSF1 Induces Ovarian Cancer Epithelial-Mesenchymal Transition in a 3D Spheroid Growth Model. PLoS ONE 2016, 11, e0168389. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Rocchi, P.; Wang, M.; Fan, Y.; Qu, F.; Iovanna, J.L.; Peng, L. Targeting heat shock factor 1 with a triazole nucleoside analog to elicit potent anticancer activity on drug-resistant pancreatic cancer. Cancer Lett. 2012, 318, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Dong, Z.; Xia, Y.; Tang, J.; Peng, L.; Wang, S.; Lai, D. Nucleoside analog inhibits microRNA-214 through targeting heat-shock factor 1 in human epithelial ovarian cancer. Cancer Sci. 2013, 104, 1683–1689. [Google Scholar] [CrossRef] [PubMed]

| Group/Disease Status | Stage of Disease | #Patient | Median Age (Range) |
|---|---|---|---|
| Healthy Controls | n/a | n = 10 | 59.5 (56–65.5) |
| Early Stage | I (a–c) | n = 8 | 63.0 (53–86) |
| Group/Disease Status | Stage of Disease | #Patient | Median Age (Range) |
|---|---|---|---|
| Malignant | III (n = 23), IV (n = 2) | n = 25 | 60 (49–83) |
| Benign serous cystadenoma | n/a | n = 9 | 52 (35–70) |
| No pathology | n/a | n = 11 | 45 (42–59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moody, R.; Wilson, K.; Kampan, N.C.; McNally, O.M.; Jobling, T.W.; Jaworowski, A.; Stephens, A.N.; Plebanski, M. Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy. Cancers 2021, 13, 4201. https://doi.org/10.3390/cancers13164201
Moody R, Wilson K, Kampan NC, McNally OM, Jobling TW, Jaworowski A, Stephens AN, Plebanski M. Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy. Cancers. 2021; 13(16):4201. https://doi.org/10.3390/cancers13164201
Chicago/Turabian StyleMoody, Rhiane, Kirsty Wilson, Nirmala Chandralega Kampan, Orla M. McNally, Thomas W. Jobling, Anthony Jaworowski, Andrew N. Stephens, and Magdalena Plebanski. 2021. "Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy" Cancers 13, no. 16: 4201. https://doi.org/10.3390/cancers13164201
APA StyleMoody, R., Wilson, K., Kampan, N. C., McNally, O. M., Jobling, T. W., Jaworowski, A., Stephens, A. N., & Plebanski, M. (2021). Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy. Cancers, 13(16), 4201. https://doi.org/10.3390/cancers13164201








