Difference in Computed Tomography Image Quality between Central Vein and Peripheral Vein Enhancement in Treatment Naive Esophageal Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Enrollment
2.2. Image Settings
2.2.1. Chest CT via Central Vein Enhancement
2.2.2. Chest CT via Peripheral Vein Enhancement
2.3. Blind Independent Central Review
2.4. Statistical Analysis
3. Results
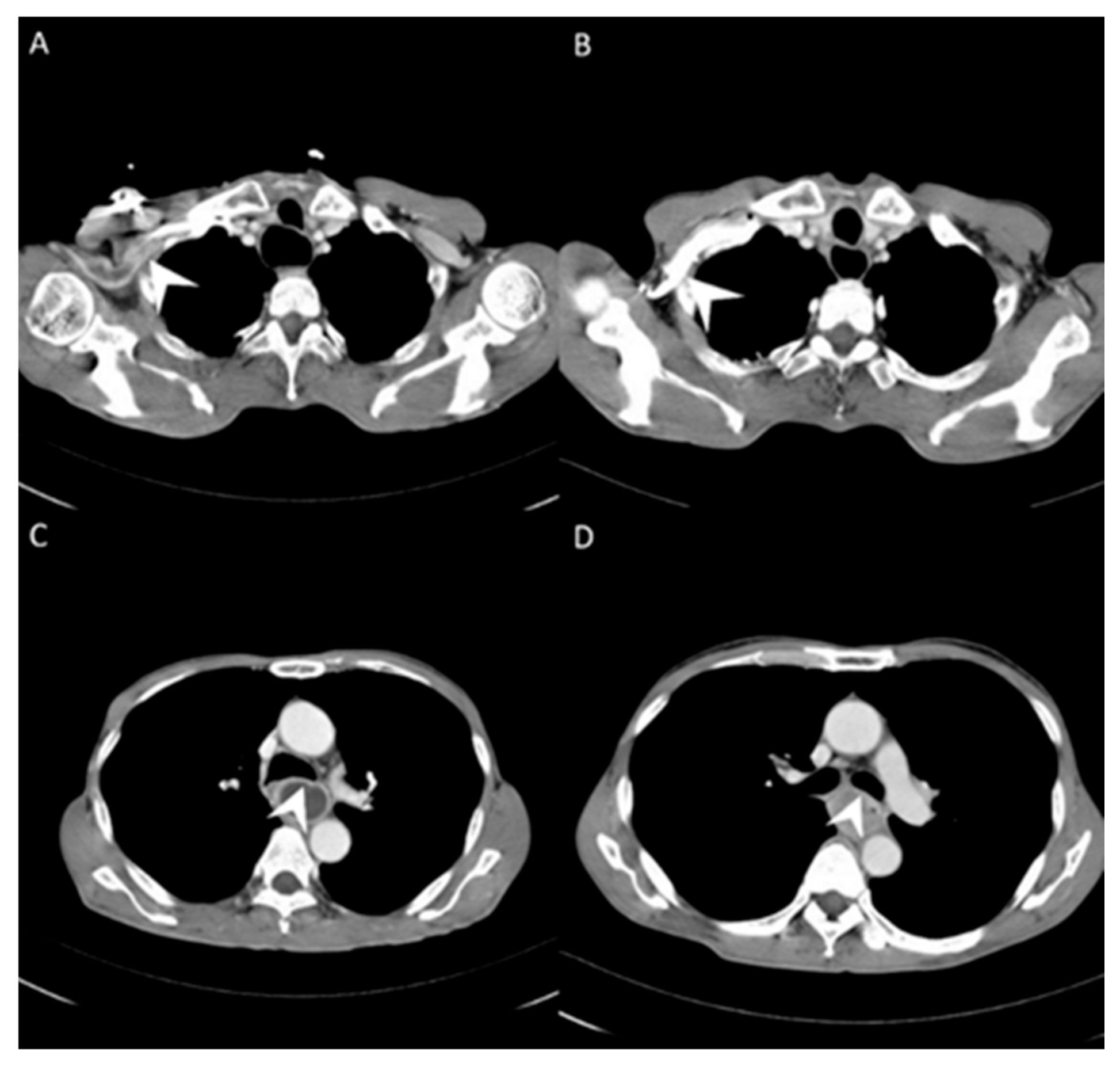
3.1. Image Differences between Central Vein Enhanced and Peripheral Vein Enhanced CT
3.2. Tumor Invasion and Lymph Node Extension in Central Vein and Peripheral Vein Enhanced CT
3.2.1. Image Difference in Tumor Invasion Status
3.2.2. Image Difference in Lymph Node Extension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charvát, J.; Linke, Z.; Horáèková, M.; Prausová, J. Implantation of central venous ports with catheter nsertion via the right internal jugular vein in oncology patients: Single center experience. Support. Care Cancer 2006, 14, 1162–1165. [Google Scholar] [CrossRef]
- Copper, C.M.; Pacanowski, J.P.; Bell, J.L. The trapezius port: A novel approach for port access. Am. Surg. 2005, 71, 106–109. [Google Scholar] [CrossRef]
- Broviac, J.W.; Cole, J.J.; Scribner, B.H. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg. Gynecol. Obstet. 1973, 136, 602–606. [Google Scholar]
- Hickman, R.O.; Buckner, C.D.; Clift, R.A.; Sanders, J.E.; Stewart, P.; Thomas, E.D. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg. Gynecol. Obstet. 1979, 148, 871–875. [Google Scholar] [PubMed]
- Niederhuber, J.E.; Ensminger, W.; Gyves, J.W.; Liepman, M.; Doan, K.; Cozzi, E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982, 92, 706–712. [Google Scholar] [PubMed]
- Teichgräber, U.K.; Gebauer, B.; Benter, T.; Wagner, H.J. Central venous access catheters: Radiological management of complications. Cardiovasc. Interv. Radiol. 2003, 26, 321–333. [Google Scholar]
- Belzunegui, T.; Louis, C.J.; Torrededia, L.; Oteiza, J. Extravasation of radiographic contrast material and compartment syndrome in the hand: A case report. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 9. [Google Scholar] [CrossRef]
- Azaïs, H.; Bresson, L.; Bassil, A.; Katdare, N.; Merlot, B.; Houpeau, J.L.; El Bedoui, S.; Meurant, J.P.; Tresch, E.; Narducci, F. Chemotherapy drug extravasation in totally implantable venous access port systems: How effective is early surgical lavage? J. Vasc. Access 2014, 16, 31–37. [Google Scholar] [CrossRef]
- Rice, T.W. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg. Clin. N. Am. 2000, 10, 471–485. [Google Scholar] [PubMed]
- Wani, S.; Das, A.; Rastogi, A.; Drahos, J.; Ricker, W.; Parsons, R.; Bansal, A.; Yen, R.; Hosford, L.; Jankowski, M.; et al. Endoscopic ultrasonography in esophageal cancer leads to improved survival rates: Results from a population-based study. Cancer 2015, 121, 194–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yen, T.J.; Chung, C.S.; Wu, Y.W.; Yen, R.F.; Cheng, M.F.; Lee, J.M.; Hsu, C.H.; Chang, Y.L.; Wang, H.P. Comparative study between endoscopic ultrasonography and positron emission tomography-computed tomography in staging patients with esophageal squamous cell carcinoma. Dis. Esophagus 2012, 25, 40–47. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Ohja, B.; Bartolucci, A.A.; Eloubeidi, M.A. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J. Thorac. Cardiovasc. Surg. 2005, 129, 1232–1241. [Google Scholar] [CrossRef]
- Sandha, G.S.; Severin, D.; Postema, E.; McEwan, A.; Stewart, K. Is positron emission tomography useful in locoregional staging of esophageal cancer? Results of a multidisciplinary initiative comparing CT, positron emission tomography, and EUS. Gastrointest. Endosc. 2008, 67, 402–409. [Google Scholar] [CrossRef]
- Pfau, P.R.; Perlman, S.B.; Stanko, P.; Frick, T.J.; Gopal, D.V.; Said, A.; Zhang, Z.; Weigel, T. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest. Endosc. 2007, 65, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Sloof, G.W. Response monitoring of neoadjuvant therapy using CT, EUS, and FDG-PET. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Yegin, E.G.; Duman, D.G. Staging of esophageal and gastric cancer in 2014. Minerva Med. 2014, 105, 391–411. [Google Scholar] [PubMed]
- Isenberg, G.; Chak, A.; Canto, M.I.; Levitan, N.; Clayman, J.; Pollack, B.J.; Sivak, M.V., Jr. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest. Endosc. 1998, 48, 158–163. [Google Scholar] [CrossRef]
- Kalha, I.; Kaw, M.; Fukami, N.; Patel, M.; Singh, S.; Gagneja, H.; Cohen, D.; Morris, J. The accuracy of endoscopic ultrasound for restaging esophageal carcinoma after chemoradiation therapy. Cancer 2004, 101, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoed, R.D.; Feldberg, M.A.; van Leeuwen, M.S.; van Dalen, T.; Obertop, H.; Kooyman, C.D.; van der Schouw, Y.T.; de Graaf, P.W. CT prediction of irresectability in esophageal carcinoma: Value of additional patient positions and relation to patient outcome. Abdom. Imaging 1997, 22, 132–137. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Ichikura, T.; Aiko, S.; Yaguchi, Y.; Kumano, I.; Takahata, R.; Matsumoto, Y.; Yoshida, K.; Ono, S.; Yamamoto, J.; et al. Multidetector-computed tomography attenuation values between the tumor and aortic wall in response to induction therapy for esophageal cancer and its predictive value for aortic invasion. Exp. Ther. Med. 2012, 3, 243–248. [Google Scholar] [CrossRef]
- Sun, Y.; Hua, Y.; Wang, M.; Mao, D.; Jin, X.; Li, C.; Shi, K.; Xu, J. Evaluation of a High Concentrated Contrast Media Injection Protocol in Combination with Low Tube Current for Dose Reduction in Coronary Computed Tomography Angiography: A Randomized, Two-center Prospective Study. Acad. Radiol. 2017, 24, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Ishwaran, H.; Hofstetter, W.L.; Kelsen, D.P.; Apperson-Hansen, C.; Blackstone, E.H. Worldwide Esophageal Cancer Collaboration Investigators Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus 2016, 29, 897–905. [Google Scholar] [CrossRef]
- Rice, T.W.; Ishwaran, H.; Blackstone, E.H.; Hofstetter, W.L.; Kelsen, D.P.; Apperson-Hansen, C.; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus 2016, 29, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.L.; Gönen, M.; Ford, R.R. Monitoring Reader Metrics in Blinded Independent Central Review of Oncology Studies. J. Clin. Trials 2015, 2915, 4. [Google Scholar]
- Amit, O.; Mannino, F.; Stone, A.M.; Bushnell, W.; Denne, J.; Helterbrand, J.; Burger, H.U. Blinded independent central review of progression in cancer clinical trials: Results from a meta-analysis. Eur. J. Cancer 2011, 47, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Dodd, L.E.; Korn, E.L.; Freidlin, B.; Jaffe, C.C.; Rubinstein, L.V.; Dancey, J.; Mooney, M.M. Blinded independent central review of progression-free survival in phase III clinical trials: Important design element or unnecessary expense? J. Clin. Oncol. 2008, 26, 3791–3796. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Kim, J.S.; Jung, H.C.; Song, I.S. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg. Endosc. 2010, 24, 1380–1386. [Google Scholar] [CrossRef]
- Luo, L.N.; He, L.J.; Gao, X.Y.; Huang, X.X.; Shan, H.B.; Luo, G.Y.; Li, Y.; Lin, S.Y.; Wang, G.B.; Zhang, R.; et al. Endoscopic Ultrasound for Preoperative Esophageal Squamous Cell Carcinoma: A Meta-Analysis. PLoS ONE 2016, 11, e0158373. [Google Scholar] [CrossRef]
- Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, J.H.; Ajani, J.; Swisher, S.G.; Hofstetter, W.L.; Guha, S.; Bhutani, M.S. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 242–253. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Han, J.; Ye, P.; Hu, J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: A meta-analysis and systematic review. Dis. Esophagus 2015, 28, 757–771. [Google Scholar] [CrossRef]
- Misra, S.; Choi, M.; Livingstone, A.S.; Franceschi, D. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg. Endosc. 2012, 26, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, U.K.; Nagel, S.N.; Kausche, S.; Enzweiler, C. Clinical benefit of power-injectable port systems: A prospective observational study. Eur. J. Radiol. 2012, 81, 528–533. [Google Scholar] [CrossRef]
- Burbridge, B.; Plewes, C.; Stoneham, G.; Szkup, P.; Otani, R.; Babyn, P.; Bryce, R. Randomized Clinical Trial Evaluating Complications and Complication-Related Removal of Arm-Situated Power-Injectable and Non-Power-Injectable Totally Implanted Venous Access Devices among Cancer Patients. J. Vasc. Interv. Radiol. 2018, 29, 648–656.e3. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Virk, J.; Krüger, K. Power-injectable totally implantable venous access devices-analysis of success and complication rates of ultrasound-guided implantation and a patient satisfaction. VASA 2019, 48, 524–530. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Value (Mean ± SD) | ||
|---|---|---|---|
| Number of patients | 48 | ||
| Sex | Female = 2/Male = 46 | ||
| Age (years) | 57.94 ± 8.61 | ||
| Image characteristics | Value (mean ± SD) | ||
| CT (via the peripheral vein) | CT (via the central vein) | p Value | |
| Tumor measurement | |||
| Pre-Tx CT tumor volume | 46.43 ± 39.64 | 46.44 ± 39.89 | 0.999 |
| Pre-Tx wall thickness | 16.48 ± 5.79 | 16.36 ± 5.75 | 0.9185 |
| Pre Tx LN station | 3.50 ± 2.73 | 3.50 ± 2.73 | 1 |
| CT number of major structures | |||
| Pre-Tx ascending aorta | 189.71 ± 41.43 | 164.97 ± 27.51 | 0.0009 |
| Pre-Tx pulmonary artery | 173.43 ± 42.68 | 156.40 ± 25.07 | 0.0197 |
| Pre-Tx descending aorta | 186.29 ± 39.80 | 163.28 ± 26.12 | 0.0012 |
| Pre-Tx liver | 105.10 ± 17.47 | 115.90 ± 15.30 | 1 |
| Pre-Tx kidney | 173.96 ± 27.81 | 190.76 ± 30.59 | 0.0059 |
| CT measurement of tumor | |||
| Pre-Tx tumor | 77.30 ± 13.4 | 79.45 ± 13.12 | 0.4281 |
| Pre-Tx LN | 78.54 ± 20.83 | 68.65 ± 35.54 | 0.1065 |
| Pre-Tx peripheral vein regurgitation | N = 38/Y = 10 | N = 48 | 0.0008 |
| (A) Agreement of T stage (peripheral vein CT vs. EUS) | ||||||||
| Coincidence of T Stage: Peripheral Vein CT (p-CT) versus Endoscopic Sonography (EUS) | ||||||||
| EUS | T stage |  | ||||||
| p-CT | T1 | T2 | T3 | T4 | Total | |||
| T stage | T1 | 0 | 0 | 0 | 0 | 0 | ||
| T2 | 0 | 3 | 3 | 1 | 7 | |||
| T3 | 1 | 3 | 25 | 2 | 31 | |||
| T4 | 0 | 1 | 4 | 4 | 9 | |||
| Total | 1 | 7 | 32 | 7 | 47 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.3620 | 0.1236 | 0.1196-0.6403 | ||||||
| (B) Agreement of T stage (central vein CT vs. EUS) | ||||||||
| Coincidence of T Stage: Central Vein CT (c-CT) versus Endoscopic Sonography (EUS) | ||||||||
| EUS | T stage |  | ||||||
| p-CT | T1 | T2 | T3 | T4 | Total | |||
| T stage | T1 | 0 | 0 | 0 | 0 | 0 | ||
| T2 | 0 | 4 | 1 | 0 | 5 | |||
| T3 | 1 | 3 | 25 | 2 | 31 | |||
| T4 | 0 | 0 | 6 | 5 | 11 | |||
| Total | 1 | 7 | 32 | 7 | 47 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.4471 | 0.1275 | 0.1972-0.6969 | ||||||
| (A) Agreement between T stage of initial reading (peripheral vein CT) versus BICR (peripheral vein CT) | ||||||||
| T Stage Agreement: Reading Result of Peripheral Vein CT (p-CT): Initial Reading versus BICR | ||||||||
| Initial p-CT | T stage | 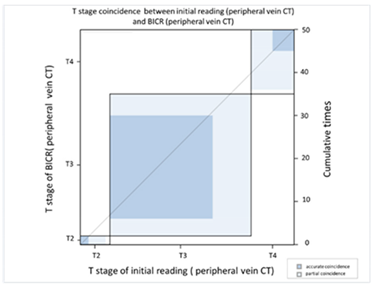 | ||||||
| BICR p-CT | T1 | T2 | T3 | T4 | Total | |||
| T stage | T1 | 0 | 0 | 0 | 0 | 0 | ||
| T2 | 0 | 2 | 4 | 1 | 7 | |||
| T3 | 0 | 0 | 24 | 9 | 33 | |||
| T4 | 0 | 0 | 5 | 5 | 10 | |||
| Total | 0 | 2 | 35 | 15 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.2382 | 0.1273 | 0.0114-0.4788 | ||||||
| (B) Agreement between T stage initial reading (central vein CT) versus BICR (central vein CT) | ||||||||
| T Stage Agreement: Reading Result of Central Vein CT (c-CT): Initial Reading versus BICR | ||||||||
| Initial p-CT | T stage | 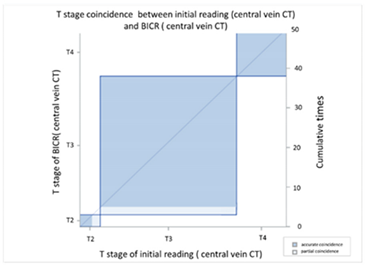 | ||||||
| BICR p-CT | T1 | T2 | T3 | T4 | Total | |||
| T stage | T1 | 0 | 0 | 0 | 0 | 0 | ||
| T2 | 0 | 3 | 2 | 0 | 5 | |||
| T3 | 0 | 0 | 33 | 0 | 33 | |||
| T4 | 0 | 0 | 0 | 12 | 12 | |||
| Total | 0 | 3 | 35 | 12 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.9157 | 0.0578 | 0.8025-1.0000 | ||||||
| (C) Agreement between T stage initial reading (peripheral vein CT) versus BICR (central vein CT) | ||||||||
| T Stage Agreement: Initial Reading of Peripheral Vein CT (p-CT) versus BICR of Central Vein CT (c-CT) | ||||||||
| Initial p-CT | T stage | 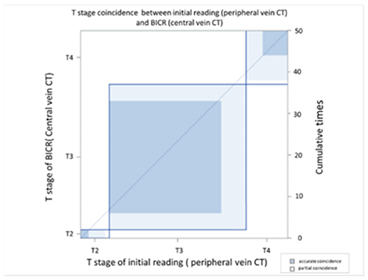 | ||||||
| BICR p-CT | T1 | T2 | T3 | T4 | Total | |||
| T stage | T1 | 0 | 0 | 0 | 0 | 0 | ||
| T2 | 0 | 2 | 4 | 1 | 7 | |||
| T3 | 0 | 0 | 27 | 6 | 33 | |||
| T4 | 0 | 0 | 4 | 6 | 10 | |||
| Total | 0 | 2 | 35 | 13 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.3755 | 0.1257 | 0.1293-0.6218 | ||||||
| (A) Agreement of N stage (peripheral vein CT vs. PET) | ||||||||
| N Stage Agreement: Initial Reading of Peripheral Vein CT (p-CT) versus Positron Emission Tomography (PET) | ||||||||
| p-CT | N stage | 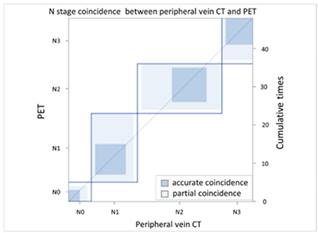 | ||||||
| PET | N0 | N1 | N2 | N3 | Total | |||
| N stage | N0 | 3 | 2 | 1 | 0 | 6 | ||
| N1 | 1 | 8 | 2 | 1 | 12 | |||
| N2 | 1 | 8 | 9 | 4 | 22 | |||
| N3 | 0 | 0 | 1 | 7 | 8 | |||
| Total | 5 | 18 | 13 | 12 | 48 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.3986 | 0.0979 | 0.2068-0.5904 | ||||||
| (B) Agreement of N stage (central vein CT vs. PET) | ||||||||
| N Stage Agreement: Initial Reading of Peripheral Vein CT (p-CT) versus Positron Emission Tomography (PET) | ||||||||
| p-CT | N stage |  | ||||||
| PET | N0 | N1 | N2 | N3 | Total | |||
| N stage | N0 | 4 | 2 | 0 | 0 | 6 | ||
| N1 | 1 | 7 | 2 | 1 | 11 | |||
| N2 | 0 | 8 | 9 | 3 | 20 | |||
| N3 | 0 | 1 | 2 | 8 | 11 | |||
| Total | 5 | 18 | 13 | 12 | 48 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.4299 | 0.0981 | 0.2367-0.6223 | ||||||
| (A) Agreement between N stage of the initial reading (peripheral vein CT) versus BICR (peripheral vein CT) | ||||||||
| N Stage Agreement of Peripheral Vein CT (p-CT): Initial Reading versus BICR | ||||||||
| p-CT | N stage | 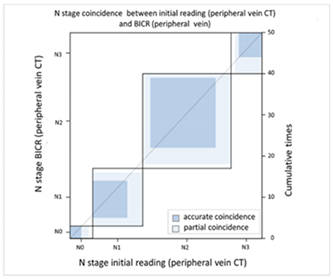 | ||||||
| PET | N0 | N1 | N2 | N3 | Total | |||
| N stage | N0 | 3 | 2 | 1 | 0 | 6 | ||
| N1 | 0 | 9 | 4 | 0 | 13 | |||
| N2 | 0 | 2 | 17 | 4 | 23 | |||
| N3 | 0 | 1 | 1 | 6 | 8 | |||
| Total | 3 | 14 | 23 | 10 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.5565 | 0.0961 | 0.3681-0.7499 | ||||||
| (B) Agreement between N stage of the initial reading (central vein CT) versus BICR (central vein CT) | ||||||||
| N Stage Agreement of Central Vein CT (c-CT): Initial Reading versus BICR | ||||||||
| p-CT | N stage | 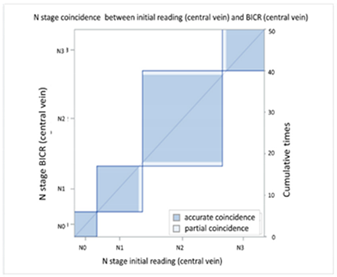 | ||||||
| PET | N0 | N1 | N2 | N3 | Total | |||
| N stage | N0 | 6 | 0 | 0 | 0 | 6 | ||
| N1 | 0 | 11 | 1 | 0 | 12 | |||
| N2 | 0 | 0 | 21 | 0 | 21 | |||
| N3 | 0 | 0 | 1 | 10 | 11 | |||
| Total | 6 | 11 | 23 | 10 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.9425 | 0.04 | 0.8641-1.0000 | ||||||
| (C) Agreement between N stage of the initial reading (peripheral vein CT) versus BICR (central vein CT) | ||||||||
| N Stage Agreement of Central Vein CT (c-CT): Initial Reading versus BICR | ||||||||
| p-CT | N stage | 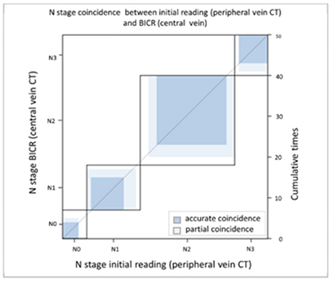 | ||||||
| PET | N0 | N1 | N2 | N3 | Total | |||
| N stage | N0 | 4 | 0 | 2 | 0 | 6 | ||
| N1 | 1 | 8 | 3 | 1 | 13 | |||
| N2 | 2 | 2 | 17 | 2 | 23 | |||
| N3 | 0 | 1 | 0 | 7 | 8 | |||
| Total | 7 | 11 | 22 | 10 | 50 | |||
| Kappa coefficient | Adjust standard error | 95% confidential interval | ||||||
| 0.5951 | 0.092 | 0.4149–0.7754 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-B.; Chen, C.-C.; Chen, H.-W.; Wu, C.-F.; Fu, J.-Y.; Hsieh, M.-J.; Peng, Y.-T.; Lu, S.-Y.; Wu, C.-Y. Difference in Computed Tomography Image Quality between Central Vein and Peripheral Vein Enhancement in Treatment Naive Esophageal Cancer Patients. Cancers 2021, 13, 4172. https://doi.org/10.3390/cancers13164172
Chang C-B, Chen C-C, Chen H-W, Wu C-F, Fu J-Y, Hsieh M-J, Peng Y-T, Lu S-Y, Wu C-Y. Difference in Computed Tomography Image Quality between Central Vein and Peripheral Vein Enhancement in Treatment Naive Esophageal Cancer Patients. Cancers. 2021; 13(16):4172. https://doi.org/10.3390/cancers13164172
Chicago/Turabian StyleChang, Chun-Bi, Chien-Cheng Chen, Huan-Wu Chen, Ching-Feng Wu, Jui-Ying Fu, Ming-Ju Hsieh, Yang-Teng Peng, Ssu-Ying Lu, and Ching-Yang Wu. 2021. "Difference in Computed Tomography Image Quality between Central Vein and Peripheral Vein Enhancement in Treatment Naive Esophageal Cancer Patients" Cancers 13, no. 16: 4172. https://doi.org/10.3390/cancers13164172
APA StyleChang, C.-B., Chen, C.-C., Chen, H.-W., Wu, C.-F., Fu, J.-Y., Hsieh, M.-J., Peng, Y.-T., Lu, S.-Y., & Wu, C.-Y. (2021). Difference in Computed Tomography Image Quality between Central Vein and Peripheral Vein Enhancement in Treatment Naive Esophageal Cancer Patients. Cancers, 13(16), 4172. https://doi.org/10.3390/cancers13164172






