Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients, Materials and Methods
2.1. Patients
2.2. Magnetic Resonance Imaging and Processing
2.3. Surgical Procedure, Histopathology and MRI Matching
2.4. Statistical Analysis
3. Results
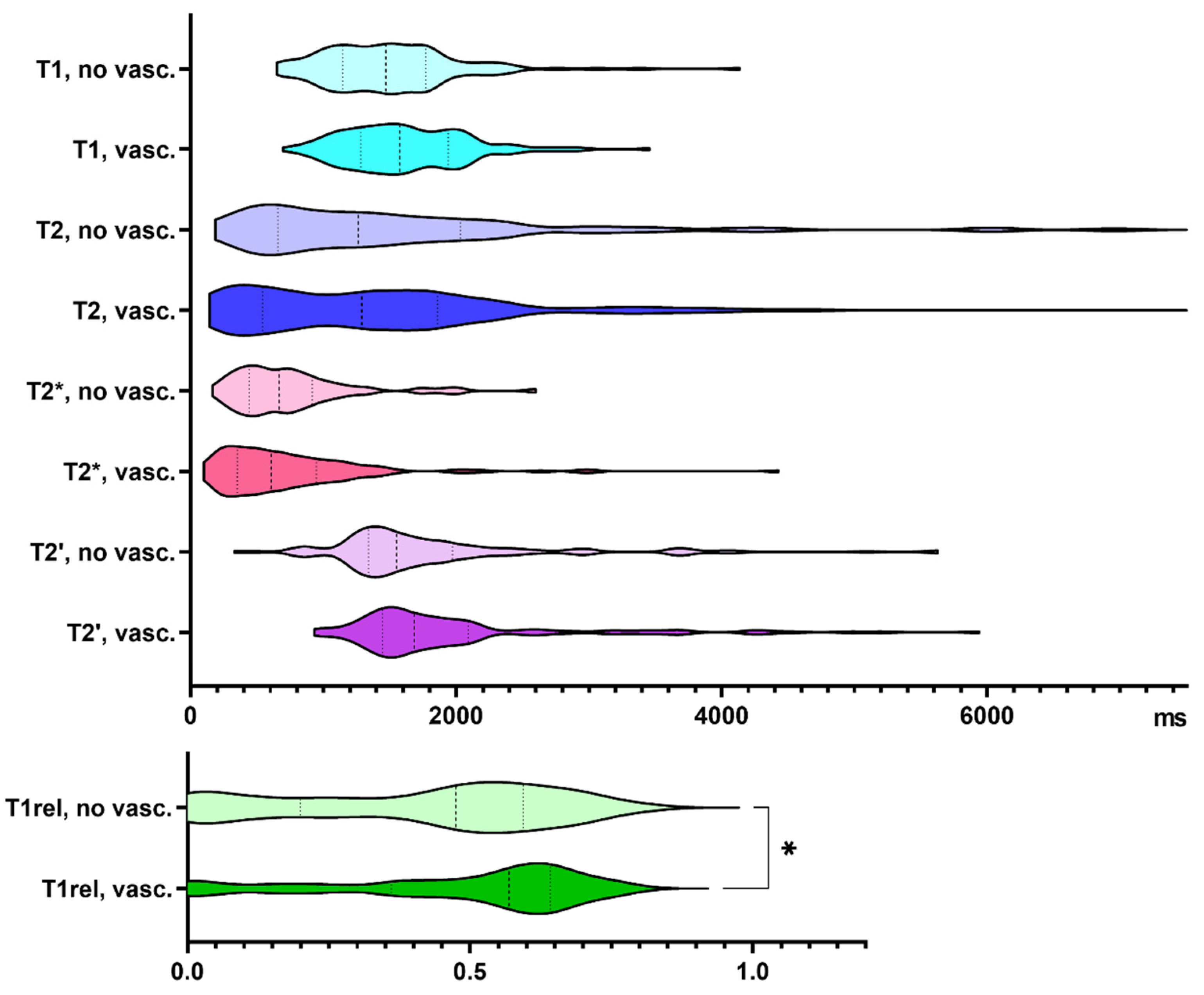
3.1. Quantitative Relaxation Times in Samples Containing Tumor Cells and Vascular Proliferates, Respectively
3.2. Association of qMRI and Histopathology
3.3. Associations between Histopathological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serai, S.D. Basics of Magnetic Resonance Imaging and Quantitative Parameters T1, T2, T2*, T1rho and Diffusion-Weighted Imaging. Pediatr. Radiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Lin, W. Quantitative Measurements of Cerebral Blood Oxygen Saturation Using Magnetic Resonance Imaging. J. Cereb. Blood Flow Metab. 2000, 20, 1225–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cercignani, M.; Dowell, N.G.; Tofts, P. (Eds.) Quantitative MRI of the Brain: Principles of Physical Measurement, 2nd ed.; Series in Medical Physics and Biomedical Engineering; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 978-1-138-03285-9. [Google Scholar]
- Hattingen, E.; Jurcoane, A.; Nelles, M.; Müller, A.; Nöth, U.; Mädler, B.; Mürtz, P.; Deichmann, R.; Schild, H.H. Quantitative MR Imaging of Brain Tissue and Brain Pathologies. Clin. Neuroradiol. 2015, 25, 219–224. [Google Scholar] [CrossRef]
- Deoni, S.C.L.; Catani, M. Visualization of the Deep Cerebellar Nuclei Using Quantitative T1 and ρ Magnetic Resonance Imaging at 3 Tesla. NeuroImage 2007, 37, 1260–1266. [Google Scholar] [CrossRef]
- Nöth, U.; Hattingen, E.; Bähr, O.; Tichy, J.; Deichmann, R. Improved Visibility of Brain Tumors in Synthetic MP-RAGE Anatomies with Pure T 1 Weighting: Improved tumor visibility in purely T 1 -weighted mp-rage. NMR Biomed. 2015, 28, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Nöth, U.; Gracien, R.; Maiworm, M.; Reif, P.S.; Hattingen, E.; Knake, S.; Wagner, M.; Deichmann, R. Detection of Cortical Malformations Using Enhanced Synthetic Contrast Images Derived from Quantitative T1 Maps. NMR Biomed. 2020, 33. [Google Scholar] [CrossRef] [Green Version]
- Maiworm, M.; Nöth, U.; Hattingen, E.; Steinmetz, H.; Knake, S.; Rosenow, F.; Deichmann, R.; Wagner, M.; Gracien, R.-M. Improved Visualization of Focal Cortical Dysplasia With Surface-Based Multiparametric Quantitative MRI. Front. Neurosci. 2020, 14, 622. [Google Scholar] [CrossRef]
- Nöth, U.; Tichy, J.; Tritt, S.; Bähr, O.; Deichmann, R.; Hattingen, E. Quantitative T1 Mapping Indicates Tumor Infiltration beyond the Enhancing Part of Glioblastomas. NMR Biomed. 2020, 33. [Google Scholar] [CrossRef]
- Blystad, I.; Warntjes, J.B.M.; Smedby, Ö.; Lundberg, P.; Larsson, E.-M.; Tisell, A. Quantitative MRI Using Relaxometry in Malignant Gliomas Detects Contrast Enhancement in Peritumoral Oedema. Sci. Rep. 2020, 10, 17986. [Google Scholar] [CrossRef]
- Lescher, S.; Jurcoane, A.; Veit, A.; Bähr, O.; Deichmann, R.; Hattingen, E. Quantitative T1 and T2 Mapping in Recurrent Glioblastomas under Bevacizumab: Earlier Detection of Tumor Progression Compared to Conventional MRI. Neuroradiology 2015, 57, 11–20. [Google Scholar] [CrossRef]
- Hattingen, E.; Jurcoane, A.; Daneshvar, K.; Pilatus, U.; Mittelbronn, M.; Steinbach, J.P.; Bähr, O. Quantitative T2 Mapping of Recurrent Glioblastoma under Bevacizumab Improves Monitoring for Non-Enhancing Tumor Progression and Predicts Overall Survival. Neuro-Oncology 2013, 15, 1395–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, S.H.; Free, S.L.; Thom, M.; Martinian, L.; Symms, M.R.; Salmenpera, T.M.; McEvoy, A.W.; Harkness, W.; Duncan, J.S.; Sisodiya, S.M. Correlation of Quantitative MRI and Neuropathology in Epilepsy Surgical Resection Specimens--T2 Correlates with Neuronal Tissue in Gray Matter. NeuroImage 2007, 37, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, R.; Steins, A.; Gurney-Champion, O.J.; Bijlsma, M.F.; Tienhoven, G.; Engelbrecht, M.R.W.; Eijck, C.H.J.; Suker, M.; Wilmink, J.W.; Besselink, M.G.; et al. Pathological Validation and Prognostic Potential of Quantitative MRI in the Characterization of Pancreas Cancer: Preliminary Experience. Mol. Oncol. 2020, 14, 2176–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quick-Weller, J.; Tichy, J.; Harter, P.N.; Tritt, S.; Baumgarten, P.; Bähr, O.; Dinc, N.; Behmanesh, B.; Weise, L.; Seifert, V.; et al. “Two Is Not Enough”-Impact of the Number of Tissue Samples Obtained from Stereotactic Brain Biopsies in Suspected Glioblastoma. J. Clin. Neurosci. 2018, 47, 311–314. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Preibisch, C.; Deichmann, R. T1 Mapping Using Spoiled FLASH-EPI Hybrid Sequences and Varying Flip Angles. Magn. Reson. Med. 2009, 62, 240–246. [Google Scholar] [CrossRef]
- Howarth, C.; Hutton, C.; Deichmann, R. Improvement of the Image Quality of T1-Weighted Anatomical Brain Scans. NeuroImage 2006, 29, 930–937. [Google Scholar] [CrossRef]
- Nöth, U.; Volz, S.; Hattingen, E.; Deichmann, R. An Improved Method for Retrospective Motion Correction in Quantitative T2* Mapping. NeuroImage 2014, 92, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Volz, S.; Nöth, U.; Rotarska-Jagiela, A.; Deichmann, R. A Fast B1-Mapping Method for the Correction and Normalization of Magnetization Transfer Ratio Maps at 3 T. NeuroImage 2010, 49, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Lin, W.; Haacke, E.M. Accurate Determination of Spin-Density and T1 in the Presence of RF-Field Inhomogeneities and Flip-Angle Miscalibration. Magn. Reson. Med. 1998, 40, 592–602. [Google Scholar] [CrossRef]
- Weise, L.; Eibach, S.; Seifert, V.; Setzer, M. Intraoperative 3D Fluoroscopy in Stereotactic Surgery. Acta Neurochir. (Wien) 2012, 154, 815–821. [Google Scholar] [CrossRef]
- Weise, L.M.; Harter, P.N.; Eibach, S.; Braczynski, A.K.; Dunst, M.; Rieger, J.; Bähr, O.; Hattingen, E.; Steinbach, J.P.; Plate, K.H.; et al. Confounding Factors in Diagnostics of MGMT Promoter Methylation Status in Glioblastomas in Stereotactic Biopsies. Stereotact. Funct. Neurosurg. 2014, 92, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Braczynski, A.K.; Capper, D.; Jones, D.T.W.; Schittenhelm, J.; Stichel, D.; von Deimling, A.; Harter, P.N.; Mittelbronn, M. High Density DNA Methylation Array Is a Reliable Alternative for PCR-Based Analysis of the MGMT Promoter Methylation Status in Glioblastoma. Pathol. Res. Pract. 2020, 216, 152728. [Google Scholar] [CrossRef]
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing, 3rd ed.; Statistical Modeling and Decision Science; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-386983-8. [Google Scholar]
- Khurshed, M.; Molenaar, R.J.; Lenting, K.; Leenders, W.P.; van Noorden, C.J.F. In Silico Gene Expression Analysis Reveals Glycolysis and Acetate Anaplerosis in IDH1 Wild-Type Glioma and Lactate and Glutamate Anaplerosis in IDH1 -Mutated Glioma. Oncotarget 2017, 8, 49165–49177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Muramatsu, R.; Maedera, N.; Tsunematsu, H.; Hamaguchi, M.; Koyama, Y.; Kuroda, M.; Ono, K.; Sawada, M.; Yamashita, T. Extracellular Lactate Dehydrogenase A Release From Damaged Neurons Drives Central Nervous System Angiogenesis. EBioMedicine 2018, 27, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Proescholdt, M.A.; Merrill, M.J.; Stoerr, E.-M.; Lohmeier, A.; Pohl, F.; Brawanski, A. Function of Carbonic Anhydrase IX in Glioblastoma Multiforme. Neuro-Oncology 2012, 14, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Oros-Peusquens, A.-M.; Loução, R.; Abbas, Z.; Gras, V.; Zimmermann, M.; Shah, N.J. A Single-Scan, Rapid Whole-Brain Protocol for Quantitative Water Content Mapping With Neurobiological Implications. Front. Neurol. 2019, 10, 1333. [Google Scholar] [CrossRef]
- Whittall, K.P.; MacKay, A.L.; Graeb, D.A.; Nugent, R.A.; Li, D.K.; Paty, D.W. In Vivo Measurement of T2 Distributions and Water Contents in Normal Human Brain. Magn. Reson. Med. 1997, 37, 34–43. [Google Scholar] [CrossRef]
- Ghassaban, K.; Liu, S.; Jiang, C.; Haacke, E.M. Quantifying Iron Content in Magnetic Resonance Imaging. NeuroImage 2019, 187, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zormpas-Petridis, K.; Poon, E.; Clarke, M.; Jerome, N.P.; Boult, J.K.R.; Blackledge, M.D.; Carceller, F.; Koers, A.; Barone, G.; Pearson, A.D.J.; et al. Noninvasive MRI Native T 1 Mapping Detects Response to MYCN -Targeted Therapies in the Th- MYCN Model of Neuroblastoma. Cancer Res. 2020, 80, 3424–3435. [Google Scholar] [CrossRef]
- Reuter, G.; Lommers, E.; Balteau, E.; Simon, J.; Phillips, C.; Scholtes, F.; Martin, D.; Lombard, A.; Maquet, P. Multiparameter Quantitative Histological MRI Values in High-Grade Gliomas: A Potential Biomarker of Tumor Progression. Neuro-Oncol. Pract. 2020, 7, 646–655. [Google Scholar] [CrossRef]
- Le Bas, J.F.; Leviel, J.L.; Decorps, M.; Benabid, A.L. NMR Relaxation Times from Serial Stereotactic Biopsies in Human Brain Tumors. J. Comput. Assist. Tomogr. 1984, 8, 1048–1057. [Google Scholar] [CrossRef]
- Englund, E.; Brun, A.; Larsson, E.M.; Györffy-Wagner, Z.; Persson, B. Tumours of the Central Nervous System. Proton Magnetic Resonance Relaxation Times T1 and T2 and Histopathologic Correlates. Acta Radiol. Diagn. (Stockh.) 1986, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Chatel, M.; Darcel, F.; de Certaines, J.; Benoist, L.; Bernard, A.M. T1 and T2 Proton Nuclear Magnetic Resonance (N.M.R.) Relaxation Times in Vitro and Human Intracranial Tumours. Results from 98 Patients. J. Neurooncol. 1986, 3, 315–321. [Google Scholar] [CrossRef]
- Calamante, F.; Lythgoe, M.F.; Pell, G.S.; Thomas, D.L.; King, M.D.; Busza, A.L.; Sotak, C.H.; Williams, S.R.; Ordidge, R.J.; Gadian, D.G. Early Changes in Water Diffusion, Perfusion, T1, and T2 during Focal Cerebral Ischemia in the Rat Studied at 8.5 T. Magn. Reson. Med. 1999, 41, 479–485. [Google Scholar] [CrossRef]
- Price, J.M.; Robinson, S.P.; Koh, D.M. Imaging Hypoxia in Tumours with Advanced MRI. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 257–270. [Google Scholar]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in Brain Tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef]
- Barajas, R.F.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional Variation in Histopathologic Features of Tumor Specimens from Treatment-Naive Glioblastoma Correlates with Anatomic and Physiologic MR Imaging. Neuro-Oncology 2012, 14, 942–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [Green Version]
- Seano, G.; Jain, R.K. Vessel Co-Option in Glioblastoma: Emerging Insights and Opportunities. Angiogenesis 2020, 23, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of Astrocyte–Vascular Coupling and the Blood–Brain Barrier by Invading Glioma Cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravoori, M.K.; Nishimura, M.; Singh, S.P.; Lu, C.; Han, L.; Hobbs, B.P.; Pradeep, S.; Choi, H.J.; Bankson, J.A.; Sood, A.K.; et al. Tumor T1 Relaxation Time for Assessing Response to Bevacizumab Anti-Angiogenic Therapy in a Mouse Ovarian Cancer Model. PLoS ONE 2015, 10, e0131095. [Google Scholar] [CrossRef]
- Neema, M.; Goldberg-Zimring, D.; Guss, Z.D.; Healy, B.C.; Guttmann, C.R.G.; Houtchens, M.K.; Weiner, H.L.; Horsfield, M.A.; Hackney, D.B.; Alsop, D.C.; et al. 3 T MRI Relaxometry Detects T2 Prolongation in the Cerebral Normal-Appearing White Matter in Multiple Sclerosis. NeuroImage 2009, 46, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharatishvili, I.; Sierra, A.; Immonen, R.J.; Gröhn, O.H.J.; Pitkänen, A. Quantitative T2 Mapping as a Potential Marker for the Initial Assessment of the Severity of Damage after Traumatic Brain Injury in Rat. Exp. Neurol. 2009, 217, 154–164. [Google Scholar] [CrossRef]
- Jackson, G.D.; Connelly, A.; Duncan, J.S.; Grunewald, R.A.; Gadian, D.G. Detection of Hippocampal Pathology in Intractable Partial Epilepsy: Increased Sensitivity with Quantitative Magnetic Resonance T2 Relaxometry. Neurology 1993, 43, 1793. [Google Scholar] [CrossRef]


| Sex [n] | Female | 9 |
| Male | 16 | |
| Age [years] | Median | 65 |
| Range | 27–88 | |
| Diagnosis [n] | IDH wild-type glioblastoma | 23 |
| IDH wild-type astrocytoma, WHO grade III | 1 | |
| IDH wild-type astrocytoma, WHO grade II | 1 | |
| MGMT Promoter [n] | Unmethylated | 10 |
| Methylated | 9 | |
| MGMT status not available/inconclusive | 6 | |
| Samples Per Patient [n] | Median | 13 |
| Range | 4–23 | |
| Survival [Days] | Median | 295 |
| Range | 3–930 |
| Relaxation Time | Sequence | Field of View | Matrix | Repetition Time TR [ms] | Echo Time(s) TE [ms] | Flip Angle [°] | Voxel Size [mm3] | Bandwidth Hz/Pixel | Acquisition Time [min] |
|---|---|---|---|---|---|---|---|---|---|
| T1 pre GBCA | 3D FLASH-EPI | 256 × 224 × 160 mm3 | 256 × 224 × 160 | 16.4 | 6.7 | 4/24 | 1 × 1 × 1 | 222 | 9:48 |
| T2 | 2D Turbo Spin Echo | 240 × 180 mm2 | 192 × 144 | 4670 | 16, 64, 96, 128, 176 | 90/180 | 1.25 × 1.25 × 2 | 100 | 5 × 1:12 |
| T2* | 2D Multi-Echo Gradient Echo | 240 × 180 mm2 | 192 × 144 | 1500 | 10, 16, 22, 28, 34, 40, 46, 52 | 30 | 1.25 × 1.25 × 2 | 299 | 6:36 |
| T1 post GBCA | 3D FLASH-EPI | 256 × 224 × 160 mm3 | 256 × 224 × 160 | 16.4 | 6.7 | 4/24 | 1 × 1 × 1 | 222 | 9:48 |
| Cell Density [Cells/mm2] | Vessel Density [Vessels/mm2] | Necrosis [%] | CAIX [%] | LDHA [%] | Ki67 [%] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rS | p | rS | p | rS | p | rS | p | rS | p | rS | p | |
| T1 | −0.010 | 0.853 | −0.248 | <0.001 | 0.065 | 0.245 | 0.071 | 0.263 | 0.249 | <0.001 | 0.001 | 0.988 |
| T1rel | 0.116 | 0.050 | −0.050 | 0.448 | −0.195 | 0.001 | −0.191 | 0.004 | 0.072 | 0.276 | 0.140 | 0.038 |
| T2 | 0.017 | 0.762 | −0.200 | 0.002 | −0.093 | 0.107 | −0.029 | 0.661 | 0.151 | 0.019 | 0.070 | 0.297 |
| T2* | 0.014 | 0.803 | −0.235 | <0.001 | −0.093 | 0.110 | −0.017 | 0.797 | 0.212 | 0.001 | 0.014 | 0.833 |
| T2′ | 0.009 | 0.870 | −0.264 | <0.001 | 0.002 | 0.968 | −0.125 | 0.057 | 0.066 | 0.310 | 0.078 | 0.243 |
| Cell Density [Cells/mm2] | Vessel Density [Vessels/mm2] | Necrosis [%] | CAIX [%] | LDHA [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rS | p | rS | p | rS | p | rS | p | rS | p | |
| vessel density [vessels/mm2] | 0.272 | <0.001 | ||||||||
| necrosis [%] | −0.314 | <0.001 | −0.161 | 0.012 | ||||||
| CAIX [%] | 0.053 | 0.414 | −0.082 | 0.200 | 0.536 | <0.001 | ||||
| LDHA [%] | 0.204 | 0.001 | −0.072 | 0.264 | 0.443 | <0.001 | 0.583 | <0.001 | ||
| Ki67 [%] | 0.340 | <0.001 | 0.142 | 0.030 | −0.145 | 0.029 | −0.081 | 0.221 | 0.042 | 0.525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, G.D.; Tichy, J.; Harter, P.N.; Nöth, U.; Weise, L.; Quick-Weller, J.; Deichmann, R.; Steinbach, J.P.; Bähr, O.; Hattingen, E. Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma. Cancers 2021, 13, 4060. https://doi.org/10.3390/cancers13164060
Maurer GD, Tichy J, Harter PN, Nöth U, Weise L, Quick-Weller J, Deichmann R, Steinbach JP, Bähr O, Hattingen E. Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma. Cancers. 2021; 13(16):4060. https://doi.org/10.3390/cancers13164060
Chicago/Turabian StyleMaurer, Gabriele D., Julia Tichy, Patrick N. Harter, Ulrike Nöth, Lutz Weise, Johanna Quick-Weller, Ralf Deichmann, Joachim P. Steinbach, Oliver Bähr, and Elke Hattingen. 2021. "Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma" Cancers 13, no. 16: 4060. https://doi.org/10.3390/cancers13164060
APA StyleMaurer, G. D., Tichy, J., Harter, P. N., Nöth, U., Weise, L., Quick-Weller, J., Deichmann, R., Steinbach, J. P., Bähr, O., & Hattingen, E. (2021). Matching Quantitative MRI Parameters with Histological Features of Treatment-Naïve IDH Wild-Type Glioma. Cancers, 13(16), 4060. https://doi.org/10.3390/cancers13164060






