Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Sample Size
2.2. Patient Population
2.3. Study Objectives
2.4. Radiation Treatment Preparation and Delivery
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
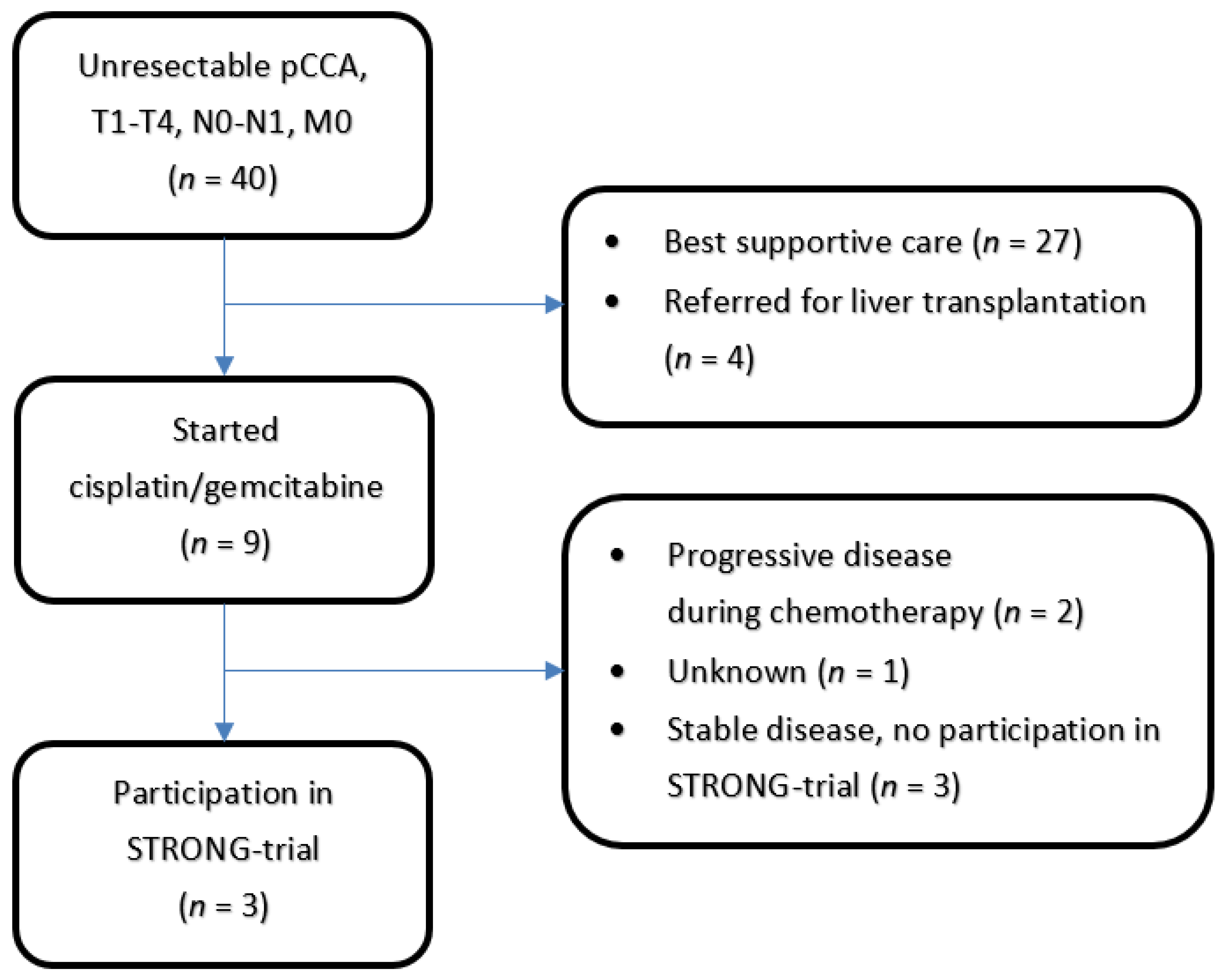
3.1. Patient Population
3.2. Radiation Treatment
3.3. Safety/Toxicity
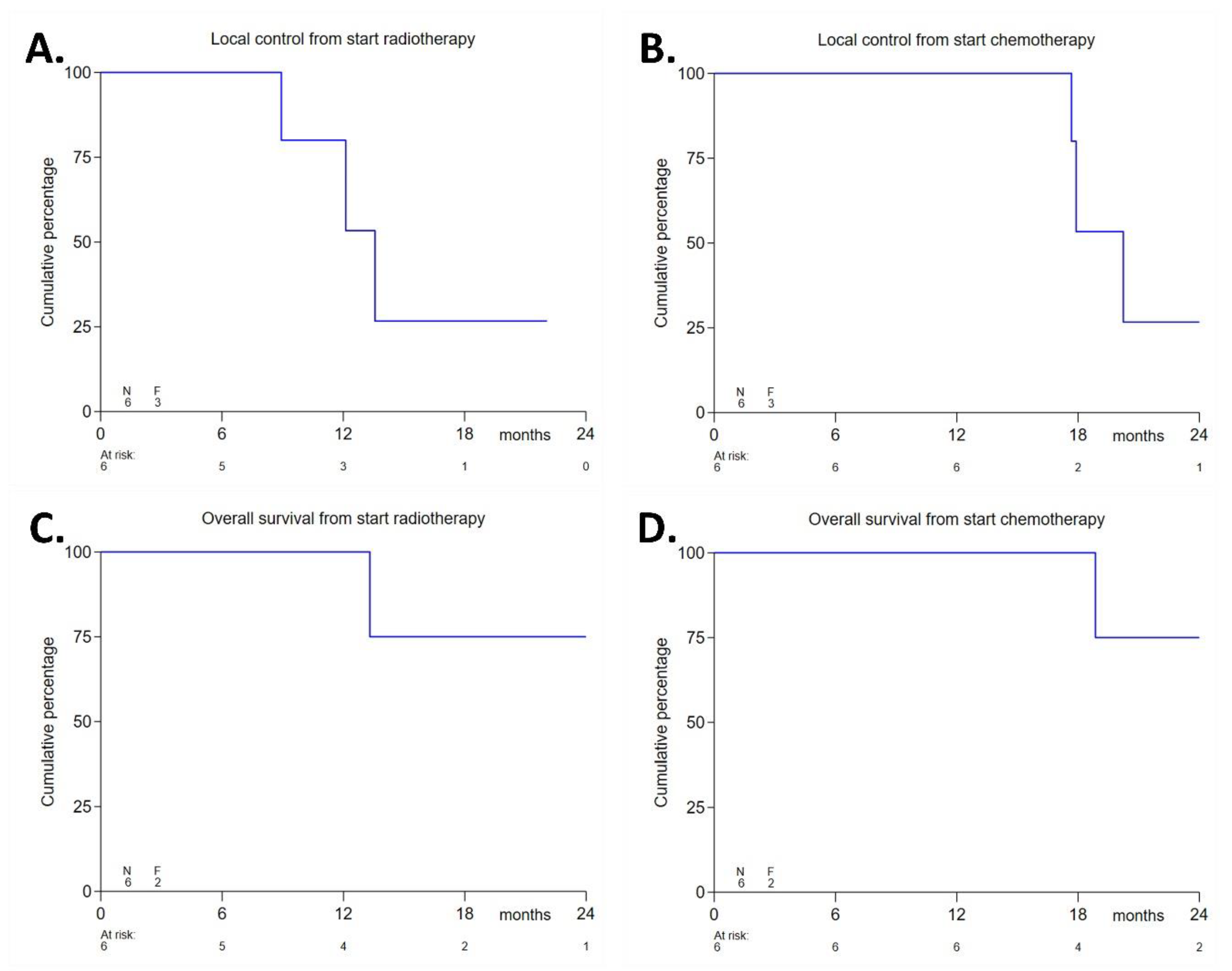
3.4. Efficacy Endpoints
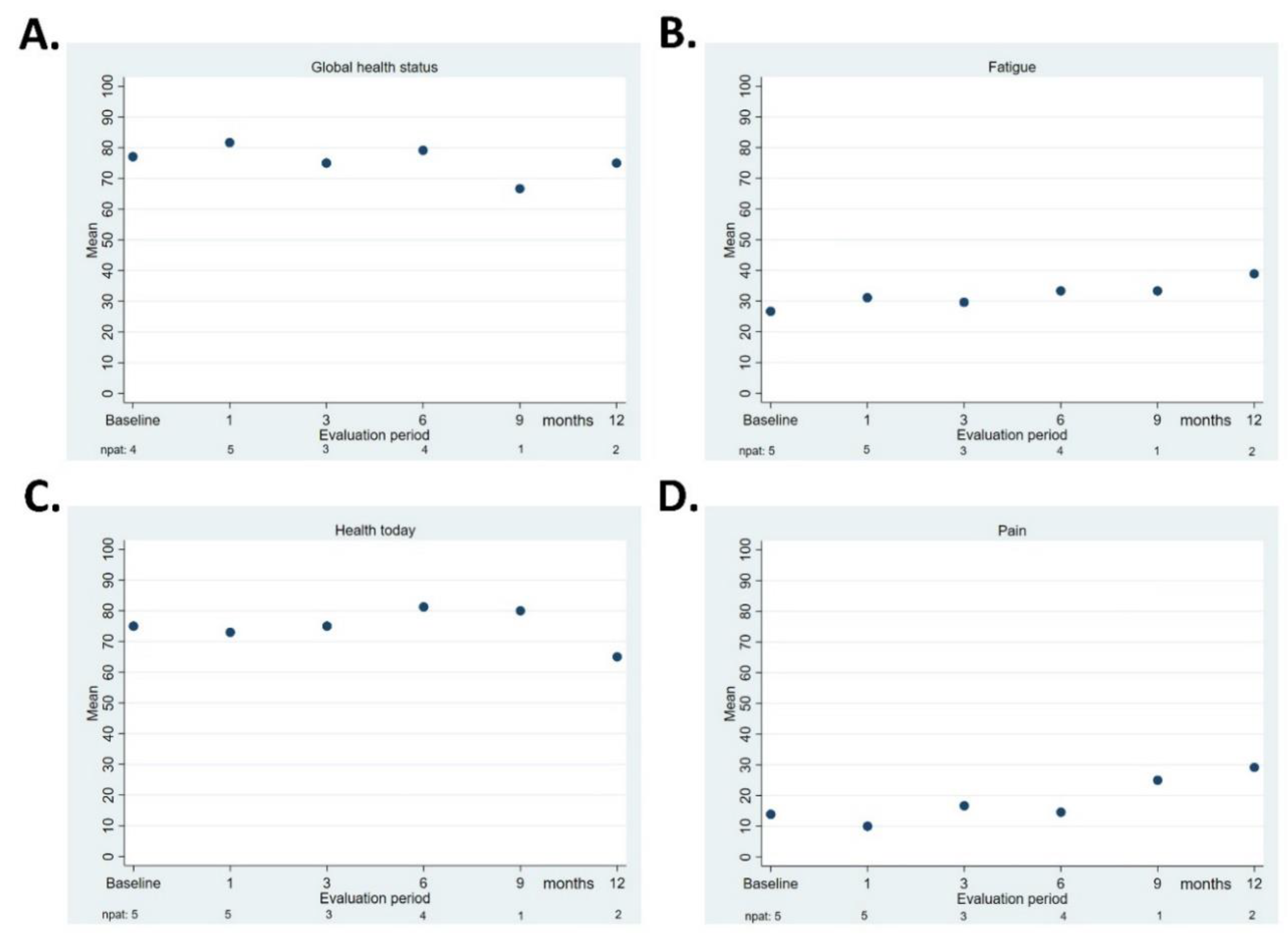
3.5. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, K.; Hawkins, M. The Role of Radiotherapy and Chemoradiation in the Management of Primary Liver Tumours. Clin. Oncol. 2014, 26, 569–580. [Google Scholar] [CrossRef]
- Weiss, M.J.; Cosgrove, D.; Herman, J.M.; Rastegar, N.; Kamel, I.; Pawlik, T.M. Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbeck’s Arch. Surg. 2014, 399, 679–692. [Google Scholar] [CrossRef]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.-N. Hilar Cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef]
- Valle, J.W.H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; Roughton, M. ABC-02 Trial Investigators, Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021. [Google Scholar] [CrossRef]
- Shin, D.W.; Kim, M.J.; Lee, J.C.; Kim, J.; Woo, S.M.; Lee, W.J.; Lee, K.H.; Hwang, J.H. Gemcitabine Plus Cisplatin Chemotherapy Prolongs the Survival in Advanced Hilar Cholangiocarcinoma: A Large Multicenter Study. Am. J. Clin. Oncol. 2020, 43, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.-J.; Yang, J.-F.; Ma, S.-R.; Wu, J.; Wang, T.-T.; Jin, H.-B.; Xia, M.-X.; Zhang, Y.-C.; Shen, H.-Z.; Ye, X.; et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: A multicenter randomized controlled trial. Gastrointest. Endosc. 2021, 94, 91–100.e2. [Google Scholar] [CrossRef] [PubMed]
- Coelen, R.J.S.; Vogel, J.A.; Vroomen, L.G.P.H.; Roos, E.; Busch, O.R.C.; Van Delden, O.M.; Van Delft, F.; Heger, M.; Van Hooft, J.E.; Kazemier, G.; et al. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): A multicentre phase I/II feasibility study protocol. BMJ Open 2017, 7, e015810. [Google Scholar] [CrossRef]
- Wagner, A.; Denzer, U.W.; Neureiter, D.; Kiesslich, T.; Puespoeck, A.; Rauws, E.A.J.; Emmanuel, K.; Degenhardt, N.; Frick, U.; Beuers, U.; et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: A multicenter prospective phase II study. Hepatology 2015, 62, 1456–1465. [Google Scholar] [CrossRef]
- Martin, E.K.; Bhutiani, N.; Egger, M.E.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Kelly, L.R.; Vitale, G.C.; Martin, R.C. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB 2018, 20, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Autorino, R.; Bisiello, S.; Pappalardi, B.; Privitera, V.; Buwenge, M.; Piccolo, F.; Masciocchi, C.; Tagliaferri, L.; Macchia, G.; Curti, C.D.; et al. Intraluminal Brachytherapy in Unresectable Extrahepatic Biliary Duct Cancer: An Italian Pooled Analysis. Anticancer. Res. 2020, 40, 3417–3421. [Google Scholar] [CrossRef]
- Makita, C.; Nakamura, T.; Takada, A.; Takayama, K.; Suzuki, M.; Ishikawa, Y.; Azami, Y.; Kato, T.; Tsukiyama, I.; Kikuchi, Y.; et al. Clinical outcomes and toxicity of proton beam therapy for advanced cholangiocarcinoma. Radiat. Oncol. 2014, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadevan, A.; Dagoglu, N.; Mancias, J.; Raven, K.; Khwaja, K.; Tseng, J.F.; Ng, K.; Enzinger, P.; Miksad, R.; Bullock, A.; et al. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J. Cancer 2015, 6, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopek, N.; Holt, M.I.; Hansen, A.T.; Høyer, M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother. Oncol. 2010, 94, 47–52. [Google Scholar] [CrossRef]
- Polistina, F.A.; Guglielmi, R.; Baiocchi, C.; Francescon, P.; Scalchi, P.; Febbraro, A.; Costantin, G.; Ambrosino, G. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother. Oncol. 2011, 99, 120–123. [Google Scholar] [CrossRef]
- Barney, B.M.; Olivier, K.R.; Miller, R.C.; Haddock, M.G. Clinical outcomes and toxicity using Stereotactic Body Radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat. Oncol. 2012, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Momm, F.; Schubert, E.; Henne, K.; Hodapp, N.; Frommhold, H.; Harder, J.; Grosu, A.-L.; Becker, G. Stereotactic fractionated radiotherapy for Klatskin tumours. Radiother. Oncol. 2010, 95, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Kim, M.-S.; Cho, C.K.; Yoo, H.J.; Jang, W.I.; Seo, Y.S.; Paik, E.K.; Kim, K.B.; Han, C.J.; Kim, S.B. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat. Oncol. J. 2014, 32, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Sandler, K.A.; Veruttipong, D.; Agopian, V.G.; Finn, R.S.; Hong, J.C.; Kaldas, F.M.; Sadeghi, S.; Busuttil, R.W.; Lee, P. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv. Radiat. Oncol. 2016, 1, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkika, E.; Hallauer, L.; Kirste, S.; Adebahr, S.; Bartl, N.; Neeff, H.P.; Fritsch, R.; Brass, V.; Nestle, U.; Grosu, A.L.; et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer 2017, 17, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koedijk, M.S.; Heijmen, B.J.M.; Koerkamp, B.G.; Eskens, F.A.L.M.; Sprengers, D.; Poley, J.-W.; Van Gent, D.C.; van der Laan, L.; Van Der Holt, B.; Willemssen, F.E.J.A.; et al. Protocol for the STRONG trial: Stereotactic body radiation therapy following chemotherapy for unresectable perihilar cholangiocarcinoma, a phase I feasibility study. BMJ Open 2018, 8, e020731. [Google Scholar] [CrossRef] [Green Version]
- Welling, T.H.; Feng, M.; Wan, S.; Hwang, S.Y.; Volk, M.L.; Lawrence, T.S.; Zalupski, M.M.; Sonnenday, C.J. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl. 2014, 20, 81–88. [Google Scholar] [CrossRef] [Green Version]
- US National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03; Cancer Therapy Evaluation Program; National Cancer Institute: Bethesda, MD, USA, 2010.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schefter, T.E.; Kavanagh, B.D.; Timmerman, R.D.; Cardenes, H.R.; Baron, A.; Gaspar, L.E. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int. J. Radiat. Oncol. 2005, 62, 1371–1378. [Google Scholar] [CrossRef]
- Dawson, L.A.; Eccles, C.; Craig, T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006, 45, 856–864. [Google Scholar] [CrossRef]
- Son, S.H.; Choi, B.O.; Ryu, M.R.; Kang, Y.N.; Jang, J.S.; Bae, S.H.; Yoon, S.K.; Choi, I.B.; Kang, K.M.; Jang, H.S. Stereotactic Body Radiotherapy for Patients With Unresectable Primary Hepatocellular Carcinoma: Dose-Volumetric Parameters Predicting the Hepatic Complication. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1073–1080. [Google Scholar] [CrossRef]
- Kavanagh, B.D.; Pan, C.C.; Dawson, L.; Das, S.K.; Li, X.A.; Haken, R.T.; Miften, M. Radiation Dose–Volume Effects in the Stomach and Small Bowel. Int. J. Radiat. Oncol. 2010, 76, S101–S107. [Google Scholar] [CrossRef]
- Kong, F.-M.; Ritter, T.; Quint, D.J.; Senan, S.; Gaspar, L.E.; Komaki, R.U.; Hurkmans, C.W.; Timmerman, R.; Bezjak, A.; Bradley, J.D.; et al. Consideration of Dose Limits for Organs at Risk of Thoracic Radiotherapy: Atlas for Lung, Proximal Bronchial Tree, Esophagus, Spinal Cord, Ribs, and Brachial Plexus. Int. J. Radiat. Oncol. 2011, 81, 1442–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vugt, J.L.; Gaspersz, M.P.; Coelen, R.; Vugts, J.; Labeur, T.; de Jonge, J.; Polak, W.G.; Busch, O.R.; Besselink, M.G.; Ijzermans, J.N.; et al. The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB 2018, 20, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Organs at Risk | Hard Constraints |
|---|---|
| Healthy liver | ≥700 mL liver-GTV, |
| dose <25.5 Gy [28] | |
| If cirrhosis is present: NTCP | |
| liver-GTV ≤ 5% [29] and >800 mL | |
| liver-GTV, dose <31.5 Gy [30] | |
| Stomach Duodenum Small and large bowel (combined in one structure when needed) | Max point dose <57 Gy [31] Volume receiving ≥41 Gy should be ≤5 cc |
| Esophagus | Max point dose ≤50.25 Gy [32] |
| Spinal cord | Max point dose ≤33.8 Gy [28] |
| Kidney | 2/3 right kidney <25.5 Gy [31] |
| Organs at Risk | Objectives |
|---|---|
| Central biliary tract | Less than 0.5 cc ≥ 70 Gy |
| (NRG-GI001—http://www.cancer.gov/clinicaltrials) (accessed on 1 July 2020) | |
| Heart | Max dose < 57 Gy |
| (RTOG 1112—http://www.cancer.gov/clinicaltrials) (accessed on 1 July 2020) | |
| Gallbladder | Max dose < 86.7 Gy |
| (RTOG 1112—http://www.cancer.gov/clinicaltrials) * (accessed on 1 July 2020) | |
| Skin (external contour) | Less than 0.5 cc ≥ 50.25 Gy |
| (RTOG 1112—http://www.cancer.gov/clinicaltrials) (accessed on 1 July 2020) |
| Age in Years | |
|---|---|
| Median | 65 |
| Range | 37–73 |
| ECOG Performance Status | |
| 1 | 6 (100%) |
| Sex | |
| Male | 3 (50%) |
| Female | 3 (50%) |
| Previous Chemotherapy for Cholangiocarcinoma? | |
| Gemcitabine + Cisplatinum | 6 (100%) |
| Number of Cycles Administered | |
| 6 | 2 (33%) |
| 8 | 4 (67%) |
| Response to Previous Chemotherapy (RECIST v1.1) | |
| Partial response | 1 (17%) |
| Stable disease | 5 (83%) |
| Stent in Situ at Start of Radiotherapy | |
| Metal | 3 (50%) |
| Plastic | 2 (33%) |
| No | 1 (17%) |
| cTNM-Classification before and after Chemotherapy | |
| T4 | 6 (100%) |
| cTNM-Classification before and after Chemotherapy | |
| N0 | 3 (50%) |
| N1 | 3 (50%) |
| Bismuth–Corlette Classification | |
| II | 2 (33%) |
| IIIa | 1 (17%) |
| IIIb | 1 (17%) |
| IV | 2 (33%) |
| Tumor Size in Millimeters | |
| Median | 26 |
| Range | 21–36 |
| Pathologic Diagnosis of Tumor | |
| Adenocarcinoma | 5 (83%) |
| Unknown | 1 (17%) |
| Fatigue | |
|---|---|
| 0 | 1 (17%) |
| 1 | 5 (83%) |
| Pain | |
| 0 | 5 (83%) |
| 1 | 1 (17%) |
| Infection | |
| 0 | 5 (83%) |
| 2 | 1 (17%) |
| ALT Elevation | |
| 0 | 4 (67%) |
| 1 | 2 (33%) |
| AST Elevation | |
| 0 | 3 (50%) |
| 1 | 3 (50%) |
| Gamma-GT Elevation | |
| 0 | 2 (33%) |
| 1 | 1 (17%) |
| 3 | 3 (50%) |
| Bilirubin Elevation | |
| 0 | 6 (100%) |
| Time from end of chemotherapy to start of radiotherapy in days | |
|---|---|
| Median | 67 |
| Range | 42–78 |
| Overall radiotherapy treatment time in days | |
| Median | 21.5 |
| Range | 20–22 |
| GTV Coverage with 60Gy (%) | |
| Median | 91.7 |
| Range | 86.8–98.6 |
| GTV Coverage with 45Gy (%) | |
| Median | 99.4 |
| Range | 99.3–100 |
| GTV D2 (Gy) | |
| Median | 73.1 |
| Range | 70.5–73.4 |
| GTV D50 (Gy) | |
| Median | 67.1 |
| Range | 65.3–69.4 |
| PTV Coverage with 60Gy (%) | |
| Median | 82.4 |
| Range | 75.7–89.6 |
| PTV Coverage with 45Gy (%) | |
| Median | 95.1 |
| Range | 89.1–97.6 |
| Hepatobiliary Disorders | |
|---|---|
| 0 | 1 (17%) |
| 3 | 5 (83%) |
| Fatigue | |
| 0 | 2 (33%) |
| 1 | 4 (67%) |
| Nausea | |
| 0 | 3 (50%) |
| 1 | 3 (50%) |
| Vomiting | |
| 0 | 5 (83%) |
| 1 | 1 (17%) |
| Diarrhea | |
| 0 | 5 (83%) |
| 1 | 1 (17%) |
| Pain | |
| 0 | 1 (17%) |
| 1 | 4 (67%) |
| 2 | 1 (17%) |
| ALT elevation | |
| 0 | 1 (17%) |
| 1 | 2 (33%) |
| 2 | 1 (17%) |
| 3 | 2 (33%) |
| AST elevation | |
| 1 | 4 (67%) |
| 2 | 2 (33%) |
| Gamma-GT elevation | |
| 1 | 1 (17%) |
| 2 | 1 (17%) |
| 3 | 2 (33%) |
| 4 | 2 (33%) |
| Bilirubin elevation | |
| 0 | 4 (67%) |
| 1 | 1 (17%) |
| 3 | 1 (17%) |
| PID | TNM- Staging | Bismuth–Corlette Staging | PTV Coverage 60Gy (%) | Type of Stent (Time Between Last Placement and Start of RT in Months) | Number of Cholangitis Episodes during FU (Time from Start of RT in Months) | Local Recurrence during FU (in Months) | Distant Metastasis during FU (in Months) | Death during FU (in Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | cT4N0M0 | II | 80.1 | Metal (4.1 m) | 2 (10.8 m, 12.1 m) | X (13.6 m) | X (20.8 m) | X (24.9 m) |
| 2 | cT4N0M0 | IIIB | 81.0 | Metal * (1.5 m) | 4 (1.1 m, 12.4 m, 15.2 m, 17.3 m) | - | - | - |
| 3 | cT4N1M0 | IV | 75.7 | Plastic (7.5 m) | 2 (1.3 m, 9.7 m) | X (12.1 m) | X (3.8 m) | X (13.3 m) |
| 4 | cT4N1M0 | IIIA | 87.5 | Plastic (7.5 m) | 1 (4.3 m) | X (8.9 m) | X (12.3 m) | - |
| 5 | cT4N0M0 | IV | 89.6 | - | - | - | - | - |
| 6 | cT4N1M0 | II | 83.7 | Metal (11.4 m) | 1 (1.9 m) | - | X (1.7 m) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baak, R.; Willemssen, F.E.J.A.; van Norden, Y.; Eskens, F.A.L.M.; Milder, M.T.W.; Heijmen, B.J.M.; Koerkamp, B.G.; Sprengers, D.; van Driel, L.M.J.W.; Klümpen, H.-J.; et al. Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study. Cancers 2021, 13, 3991. https://doi.org/10.3390/cancers13163991
Baak R, Willemssen FEJA, van Norden Y, Eskens FALM, Milder MTW, Heijmen BJM, Koerkamp BG, Sprengers D, van Driel LMJW, Klümpen H-J, et al. Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study. Cancers. 2021; 13(16):3991. https://doi.org/10.3390/cancers13163991
Chicago/Turabian StyleBaak, Rogier, François E. J. A. Willemssen, Yvette van Norden, Ferry A. L. M. Eskens, Maaike T. W. Milder, Ben J. M. Heijmen, Bas Groot Koerkamp, Dave Sprengers, Lydi M. J. W. van Driel, Heinz-Josef Klümpen, and et al. 2021. "Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study" Cancers 13, no. 16: 3991. https://doi.org/10.3390/cancers13163991
APA StyleBaak, R., Willemssen, F. E. J. A., van Norden, Y., Eskens, F. A. L. M., Milder, M. T. W., Heijmen, B. J. M., Koerkamp, B. G., Sprengers, D., van Driel, L. M. J. W., Klümpen, H.-J., den Toom, W., Koedijk, M. S., IJzermans, J. N. M., & Méndez Romero, A. (2021). Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study. Cancers, 13(16), 3991. https://doi.org/10.3390/cancers13163991







