A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Systemic Treatment
2.3. Follow-Up Program and Diagnosis of mPC
2.4. Statistical Analysis
2.4.1. Developing a Prediction Model
2.4.2. Validating the Selected Prediction Model
3. Results
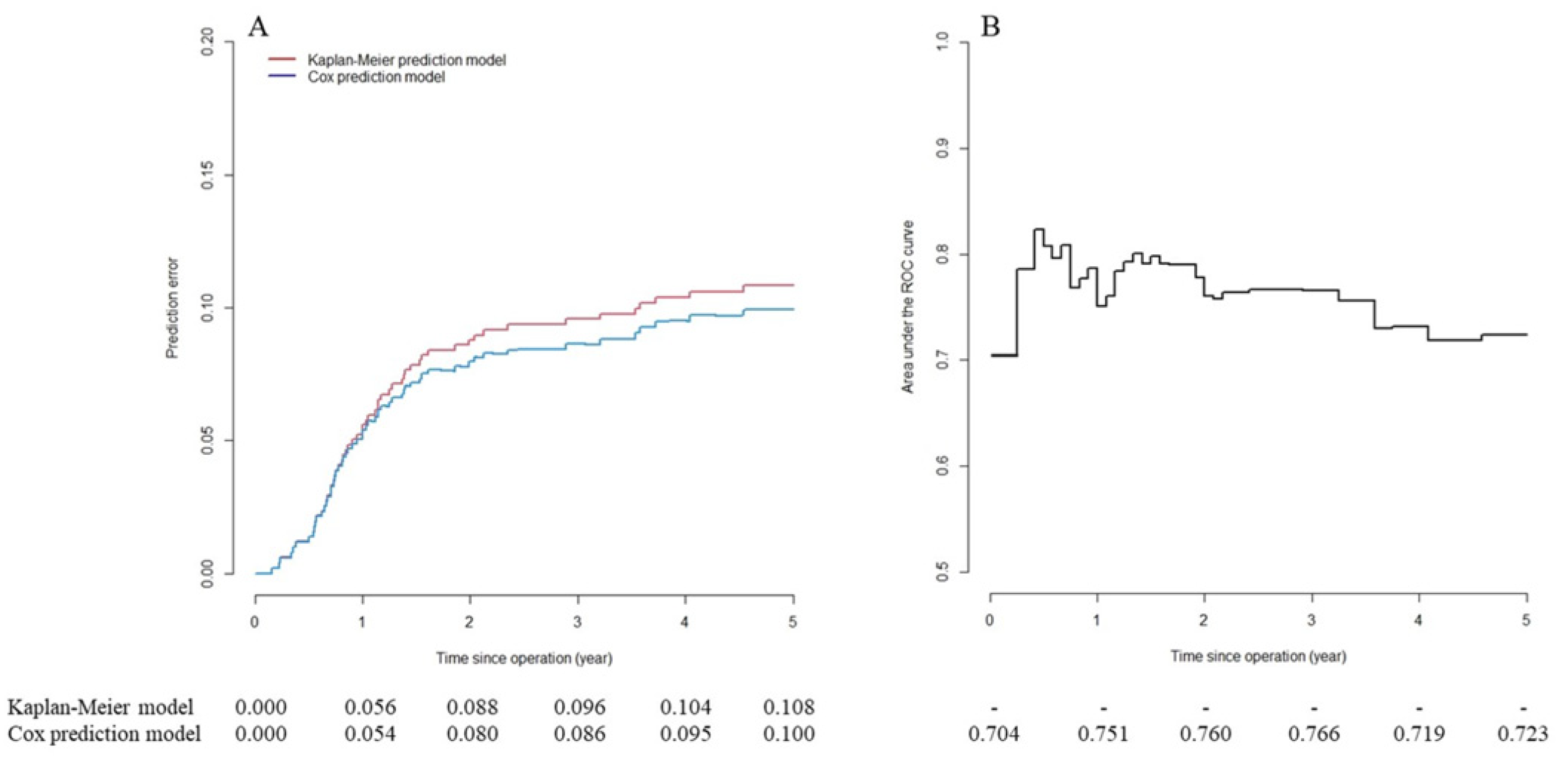
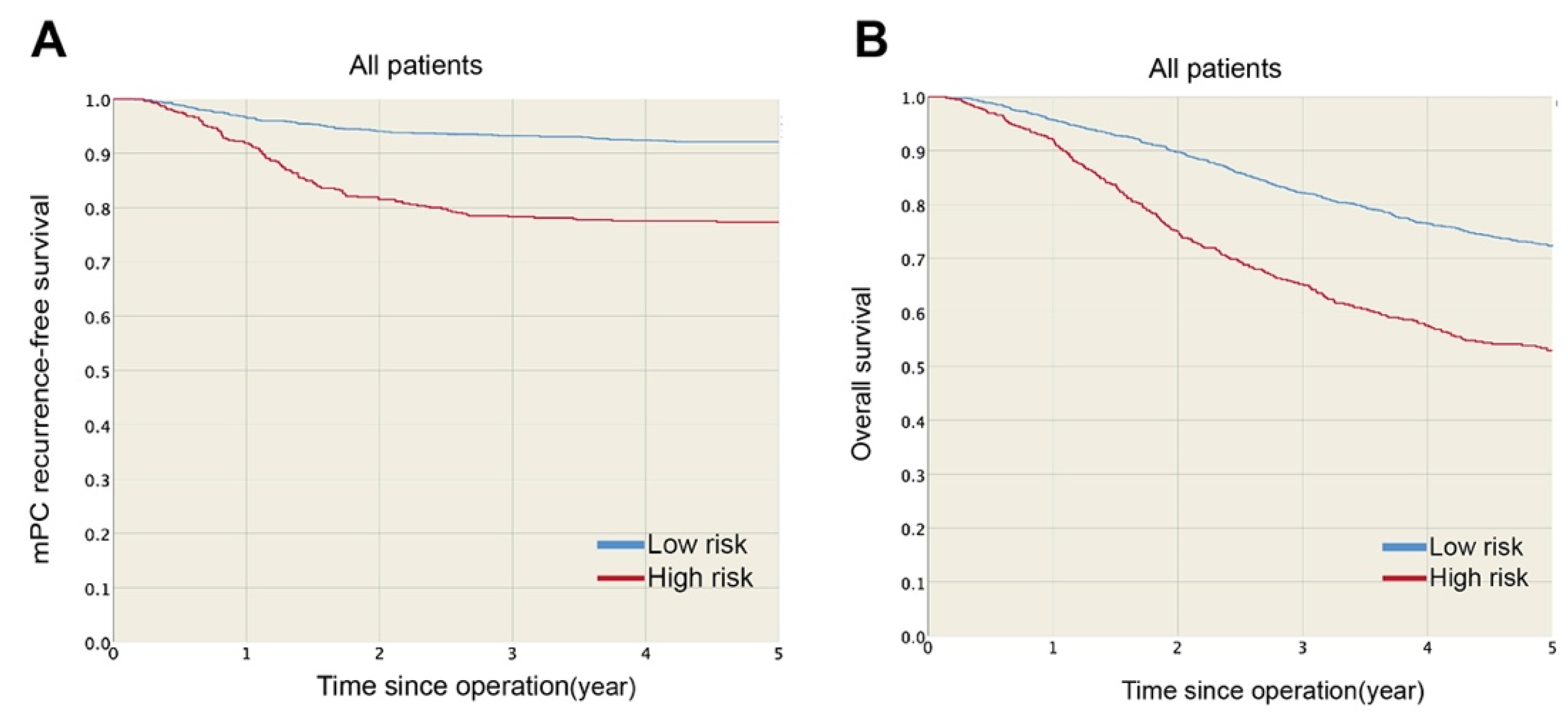
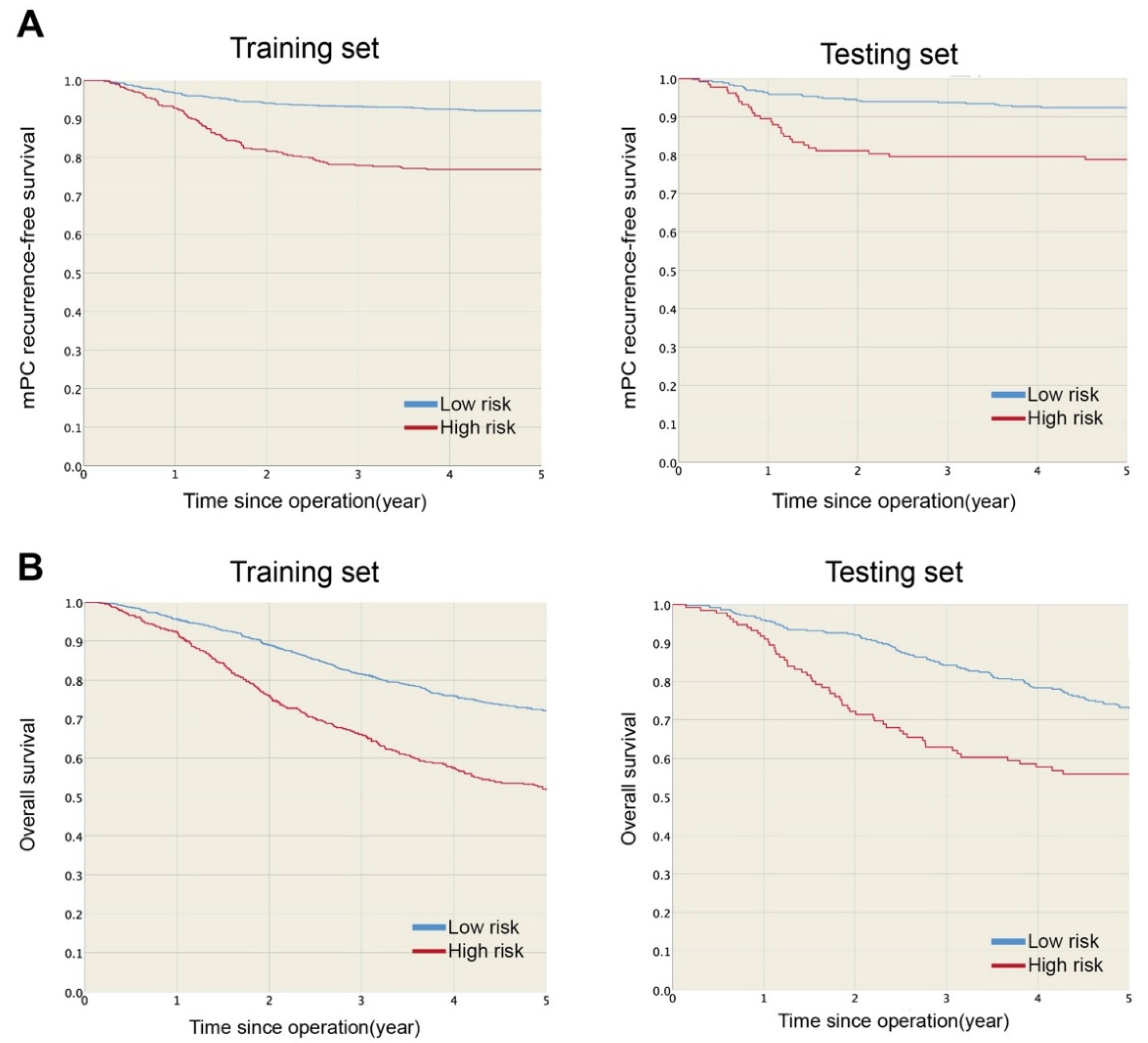
Developing and Validating a Prediction Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.; Seymour, M.T.; Saltz, L.; A Punt, C.J.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Kerscher, A.G.; Chua, T.C.; Gasser, M.; Maeder, U.; Kunzmann, V.; Isbert, C.; Germer, C.T.; Pelz, J.O.W. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer 2013, 108, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia with Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Van Ruth, S.; de Bree, E.; Van Slooten, G.W.; Van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Schellinx, M.E.; Chang, D.; Koslowe, P.; von Meyerfeldt, M. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J. Surg. 1996, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Blot, F.; El Otmany, A.; Antoun, S.; Lasser, P.; Boige, V.; Rougier, P.; Ducreux, M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001, 92, 71–76. [Google Scholar] [CrossRef]
- Jacquet, P.; Jelinek, J.S.; Steves, M.A.; Sugarbaker, P.H. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 1993, 72, 1631–1636. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Pinto, A.; Tuech, J.-J.; Kianmanesh, R.; et al. A Phase 3 Randomised Study Evaluating the Benefit of Second-Look Surgery Plus HIPEC in Patients at High Risk of Developing Colorectal Peritoneal Metastases (PROPHYLOCHIP). Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Elias, D.; Honoré, C.; Dumont, F.; Ducreux, M.; Boige, V.; Malka, D.; Burtin, P.; Dromain, C.; Goéré, D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 2011, 254, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Goéré, D.; Di Pietrantonio, D.; Boige, V.; Malka, D.; Kohneh-Shahri, N.; Dromain, C.; Ducreux, M. Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 2008, 247, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Akre, O.; Gustafsson, U.O.; Bottai, M.; Martling, A. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer. Colorectal Dis. 2016, 18, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Ravn, S.; Heide-Jorgensen, U.; Christiansen, C.F.; Verwaal, V.J.; Hagemann-Madsen, R.H.; Iversen, L.H. Overall risk and risk factors for metachronous peritoneal metastasis after colorectal cancer surgery: A nationwide cohort study. BJS Open 2020, 4, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Van Santvoort, H.C.; Braam, H.J.; Spekreijse, K.R.; Koning, N.R.; De Bruin, P.C.; Reilingh, T.S.D.V.; Boerma, D.; Smits, A.B.; Wiezer, M.J.; Van Ramshorst, B. Peritoneal carcinomatosis in t4 colorectal cancer: Occurrence and risk factors. Ann. Surg. Oncol. 2014, 21, 1686–1691. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Revised guidelines for second-look surgery in patients with colon and rectal cancer. Clin. Transl. Oncol. 2010, 12, 621–628. [Google Scholar] [CrossRef]
- Baguena, G.; Pellino, G.; Frasson, M.; Roselló, S.; Cervantes, A.; García-Granero, A.; Giner, F.; García-Granero, E. Prognostic Impact of pT Stage and Peritoneal Invasion in Locally Advanced Colon Cancer. Dis. Colon Rectum 2019, 62, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; ABremers, A.J.; ABurger, J.W.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Bastiaenen, V.P.; Klaver, C.E.L.; Kok, N.F.M.; De Wilt, J.H.W.; De Hingh, I.H.J.T.; Aalbers, A.G.J.; Boerma, D.; Bremers, A.J.A.; Burger, J.W.A.; Van Duyn, E.B.; et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer 2019, 19, 254. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Barrios, P.; Boldo-Roda, E.; Camps, B.; Carrasco-Campos, J.; Martín, V.C.; García-Fadrique, A.; Gutiérrez-Calvo, A.; Morales, R.; Ortega-Pérez, G.; et al. HIPECT4: Multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, N.B.; Navascues, J.M.E.; Echaniz, G.E.; Ansorena, Y.S.; Galán, C.P.; Martín, X.A.; Pardo, L.V. Prophylactic HIPEC in pT4 Colon Tumors: Proactive Approach or Overtreatment? Ann. Surg. Oncol. 2020, 27, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.E., 2nd; Harrell, F.E., Jr. The restricted cubic spline as baseline hazard in the proportional hazards model with step function time-dependent covariables. Stat. Med. 1995, 14, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Cai, T.; Pencina, M.J.; D’Agostino, R.B.; Wei, L.J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 2011, 30, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Schmoor, C.; Sauerbrei, W.; Schumacher, M. Assessment and comparison of prognostic classification schemes for survival data. Stat. Med. 1999, 18, 2529–2545. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Loh, W.Y. Classification and regression trees. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2011, 1, 14–23. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Cox, T.; Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Gerds, T.A.; Schumacher, M. Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom. J. 2006, 48, 1029–1040. [Google Scholar] [CrossRef]
- Nagata, H.; Ishihara, S.; Oba, K.; Tanaka, T.; Hata, K.; Kawai, K.; Nozawa, H. Development and Validation of a Prediction Model for Postoperative Peritoneal Metastasis After Curative Resection of Colon Cancer. Ann. Surg. Oncol. 2018, 25, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; van Huijgevoort, N.C.M.; de Buck van Overstraeten, A.; Wolthuis, A.M.; Tanis, P.; Van Der Bilt, J.D.W.; Sagaert, X.; D’Hoore, A. Locally Advanced Colorectal Cancer: True Peritoneal Tumor Penetration is Associated with Peritoneal Metastases. Ann. Surg. Oncol. 2018, 25, 212–220. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Mucinous colorectal carcinoma. J. Surg. Oncol. 2001, 77, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Akre, O.; Gustafsson, U.O.; Bottai, M.; Martling, A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014, 16, 359–367. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. 2019, 45, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Kawai, K.; Hata, K.; Tanaka, T.; Nozawa, H.; Ishihara, S. Laparoscopic surgery for T4 colon cancer: A risk factor for peritoneal recurrences? Surgery 2020, 168, 119–124. [Google Scholar] [CrossRef]
- Macari, D.; Kawak, S.; Raofi, V.; Wasvary, H.; Jaiyesimi, I. Recurrence pattern and outcomes in T4 colon cancer: A single institution analysis. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
| Cumulative mPC Incidence (%) | Overall Survival (%) | ||||||
|---|---|---|---|---|---|---|---|
| Patients, N | One-Year | Three-Year | Five-Year | p-Value | Five-Year | p-Value | |
| All patients | 2003 | 4.7 | 10.7 | 11.8 | 67.3 | ||
| Gender, N (%) | 0.979 | 0.017 | |||||
| Female | 961 (48.0) | 4.3 | 10.7 | 11.7 | 69.7 | ||
| Male | 1042 (52.0) | 5.1 | 10.7 | 11.9 | 65.1 | ||
| Age at diagnosis, N (%) | 0.002 | <0.001 | |||||
| <50 years | 385 (19.2) | 6 | 15.1 | 16.6 | 75 | ||
| ≥50 years | 1618 (80.8) | 4.4 | 9.6 | 10.6 | 65.5 | ||
| Tumor location, N (%) | <0.001 | 0.633 | |||||
| Right colon | 941 (47.0) | 5.8 | 13.2 | 14.3 | 67.1 | ||
| Left colon | 1062 (53.0) | 3.7 | 8.5 | 9.5 | 67.5 | ||
| Preoperative CEA, N (%) | <0.001 | <0.001 | |||||
| ≤5 ng/mL | 1132 (56.5) | 3.5 | 8.1 | 9.3 | 74.2 | ||
| >5 ng/mL | 871 (43.5) | 6.2 | 14 | 15 | 58.4 | ||
| Histological grade, N (%) | <0.001 | 0.001 | |||||
| Well-to-moderately differentiated | 1782 (89.0) | 4.2 | 9.7 | 10.9 | 68.4 | ||
| Poorly differentiated | 221 (11.0) | 9 | 18.6 | 19 | 58.7 | ||
| Histological type, N (%) | <0.001 | 0.88 | |||||
| Adenocarcinoma | 1755 (87.6) | 4.3 | 9.2 | 10.3 | 67.8 | ||
| Mucinous adenocarcinoma | 248 (12.4) | 7.3 | 21.4 | 22.6 | 63.8 | ||
| pT stage, N (%) | <0.001 | 0.001 | |||||
| 4a | 1662 (83.0) | 4.3 | 9.1 | 10.2 | 70.4 | ||
| 4b | 341 (17.0) | 6.7 | 18.2 | 19.4 | 63.6 | ||
| pN stage, N (%) | <0.001 | <0.001 | |||||
| N0 | 958 (47.8) | 3.3 | 7.2 | 8.4 | 76.7 | ||
| N1 | 640 (32.0) | 4.8 | 10.9 | 11.7 | 63.5 | ||
| N2 | 405 (20.2) | 7.7 | 18.5 | 20 | 50.3 | ||
| Adjuvant chemotherapy, N (%) | 0.28 | <0.001 | |||||
| None | 1054 (52.6) | 5.3 | 11.6 | 12.8 | 61.5 | ||
| 5-FU-based only | 841 (42.0) | 3.9 | 9.6 | 10.7 | 73.2 | ||
| Oxaliplatin-based | 108 (5.4) | 4.6 | 10.2 | 10.2 | 88 | ||
| Examined lymph node, N (%) | 0.022 | <0.001 | |||||
| <12 | 294 (14.7) | 3.4 | 7.1 | 8.2 | 61.9 | ||
| ≥12 | 1709 (85.3) | 4.9 | 11.3 | 12.4 | 68.3 | ||
| Training Set | Testing Set | p-Value | |
|---|---|---|---|
| N = 1503 | N = 500 | ||
| Gender, N (%) | 0.054 | ||
| Female | 702 (46.71) | 259 (51.80) | |
| Male | 801 (53.29) | 241 (48.20) | |
| Age at diagnosis, N (%) | 0.833 | ||
| <50 years | 291 (19.36) | 94 (18.80) | |
| ≥50 years | 1212 (80.64) | 406 (81.20) | |
| Tumor location, N (%) | 1 | ||
| Right colon | 706 (46.97) | 235 (47.00) | |
| Left colon | 797 (53.03) | 265 (53.00) | |
| Pre-operation CEA, N (%) | 0.209 | ||
| ≤5 ng/mL | 862 (57.35) | 270 (54.00) | |
| >5 ng/mL | 641 (42.65) | 230 (46.00) | |
| Histological grade, N (%) | 0.272 | ||
| Well-to-moderately differentiated | 1330 (88.49) | 452 (90.40) | |
| Poorly differentiated | 173 (11.51) | 48 (9.60) | |
| Histological type, N (%) | 0.825 | ||
| Adenocarcinoma | 1315 (87.49) | 440 (88.00) | |
| Mucinous adenocarcinoma | 188 (12.51) | 60 (12.00) | |
| pT stage, N (%) | 0.44 | ||
| 4a | 1241 (82.57) | 421 (84.20) | |
| 4b | 262 (17.43) | 79 (15.80) | |
| pN stage, N (%) | 0.339 | ||
| N0 | 729 (48.50) | 229 (45.80) | |
| N1 | 467 (31.07) | 173 (34.60) | |
| N2 | 307 (20.43) | 98 (19.60) |
| Hazard Ratio (95% CI) | Coefficient | Points * | ||
|---|---|---|---|---|
| Tumor location | Left colon | 1 | - | 0 |
| Right colon | 1.41 (1.05–1.89) | 0.34 | 2 | |
| Histologic type | Adenocarcinoma | 1 | - | 0 |
| Mucinous adenocarcinoma | 1.93 (1.36–2.75) | 0.66 | 3 | |
| Preoperative CEA | ≤5 ng/mL | 1 | - | 0 |
| >5 ng/mL | 1.46 (1.09–1.96) | 0.38 | 2 | |
| pT stage | 4a | 1 | - | 0 |
| 4b | 2.04 (1.47–2.83) | 0.71 | 3 | |
| pN stage | N0 | 1 | - | 0 |
| N1 | 1.49 (1.04–2.12) | 0.4 | 2 | |
| N2 | 2.79 (1.96–3.97) | 1.03 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-Y.; You, J.-F.; Hsu, Y.-J.; Jhuang, J.-R.; Chern, Y.-J.; Hung, H.-Y.; Yeh, C.-Y.; Hsieh, P.-S.; Chiang, S.-F.; Lai, C.-C.; et al. A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection. Cancers 2021, 13, 2808. https://doi.org/10.3390/cancers13112808
Tsai T-Y, You J-F, Hsu Y-J, Jhuang J-R, Chern Y-J, Hung H-Y, Yeh C-Y, Hsieh P-S, Chiang S-F, Lai C-C, et al. A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection. Cancers. 2021; 13(11):2808. https://doi.org/10.3390/cancers13112808
Chicago/Turabian StyleTsai, Tzong-Yun, Jeng-Fu You, Yu-Jen Hsu, Jing-Rong Jhuang, Yih-Jong Chern, Hsin-Yuan Hung, Chien-Yuh Yeh, Pao-Shiu Hsieh, Sum-Fu Chiang, Cheng-Chou Lai, and et al. 2021. "A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection" Cancers 13, no. 11: 2808. https://doi.org/10.3390/cancers13112808
APA StyleTsai, T.-Y., You, J.-F., Hsu, Y.-J., Jhuang, J.-R., Chern, Y.-J., Hung, H.-Y., Yeh, C.-Y., Hsieh, P.-S., Chiang, S.-F., Lai, C.-C., Chiang, J.-M., Tang, R., & Tsai, W.-S. (2021). A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection. Cancers, 13(11), 2808. https://doi.org/10.3390/cancers13112808








