Rethinking a Non-Predominant Pattern in Invasive Lung Adenocarcinoma: Prognostic Dissection Focusing on a High-Grade Pattern
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Imaging and Analysis
2.3. PET Analysis
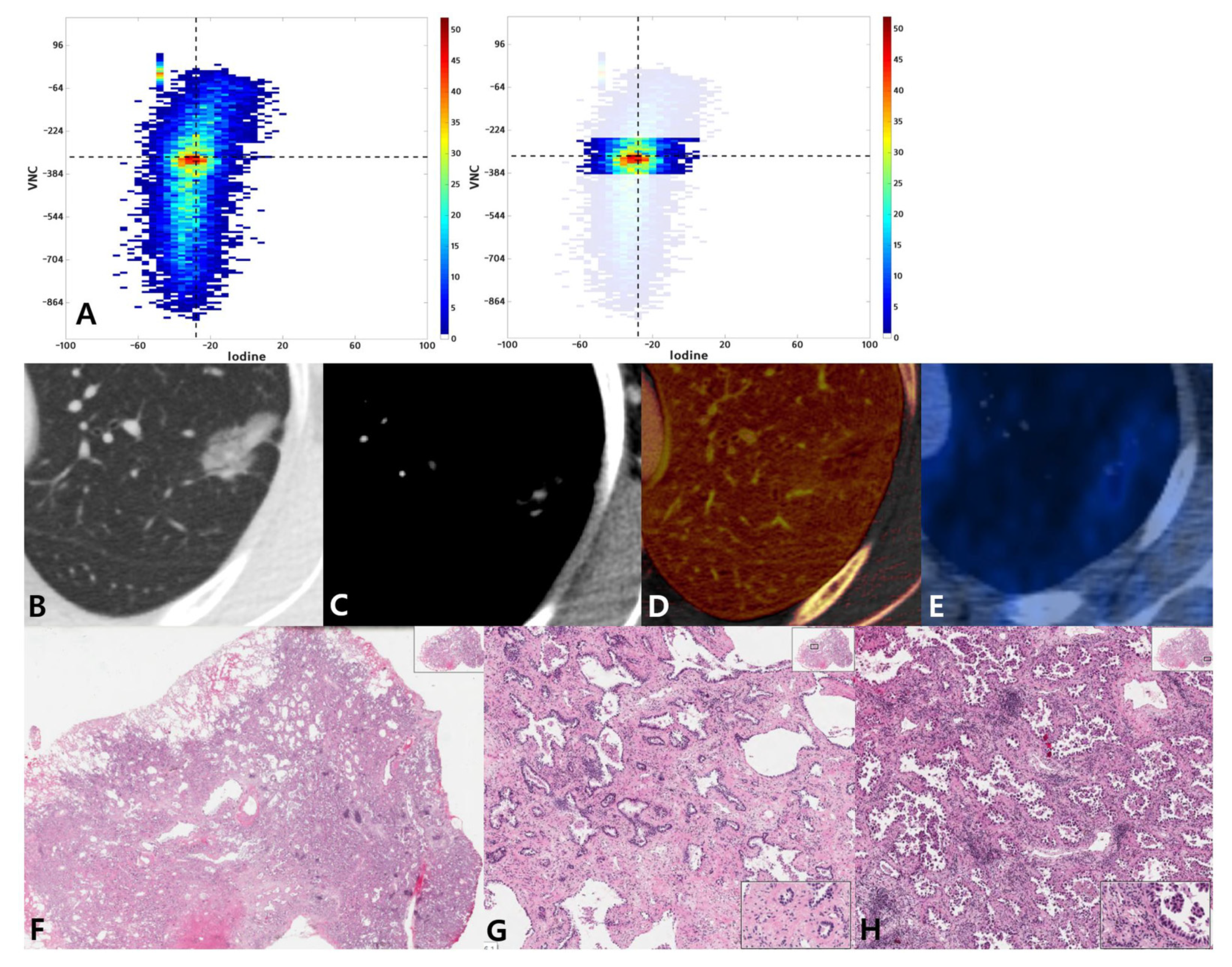
2.4. Visual Assessment of Joint Histogram Graphs
2.5. Pathologic Evaluation
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
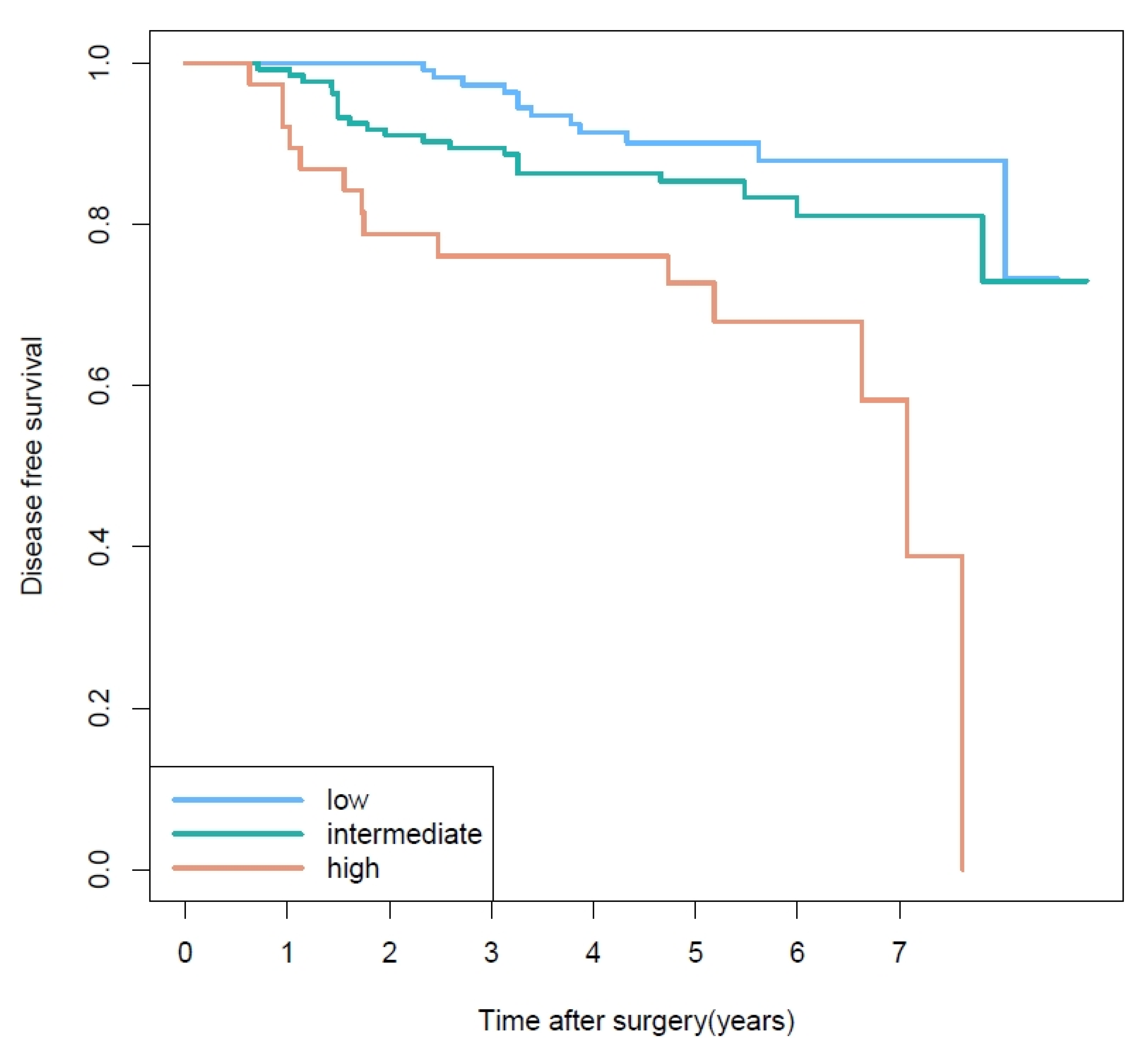
3.2. Prognostic Factors of Disease-Free Survival
3.3. Prognosis Based on Second Most Predominant Pattern
3.4. Prediction for Non-Predominant But Higher Second Most Predominant Pattern
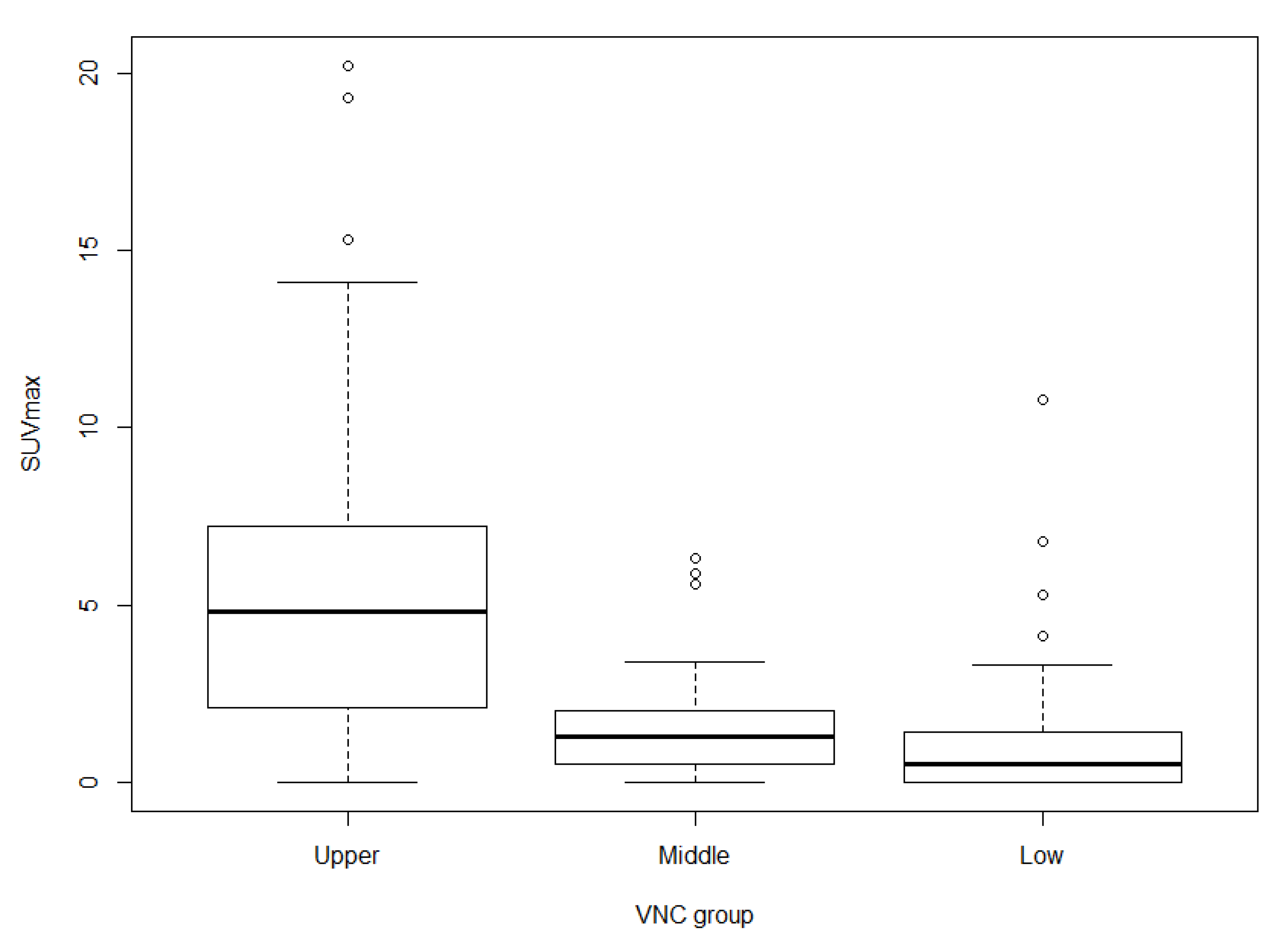
3.5. Associations among SUVmax, VNC Group, and Histologic Grade
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | adenocarcinoma |
| CI | confidence interval |
| DECT | dual-energy computed tomography |
| DFS | disease-free survival |
| GGO | ground-glass opacity |
| OR | odds ratio |
| ROC | receiver operating characteristic |
| ROI | region of interest |
| SD | standard deviation |
| VNC | virtual non-contrast |
References
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Powell, C.A.; Beer, D.; Riely, G.; Garg, K.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc. Am. Thorac. Soc. 2011, 8, 381–385. [Google Scholar] [CrossRef]
- Russell, P.A.; Wainer, Z.; Wright, G.M.; Daniels, M.; Conron, M.; Williams, R.A. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J. Thorac. Oncol. 2011, 6, 1496–1504. [Google Scholar]
- Warth, A.; Muley, T.; Meister, M.; Stenzinger, A.; Thomas, M.; Schirmacher, P.; Schnabel, P.A.; Budczies, J.; Hoffmann, H.; Weichert, W. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J. Clin. Oncol. 2012, 30, 1438–1446. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Motoi, N.; Riely, G.J.; Sima, C.S.; Gerald, W.L.; Kris, M.G.; Park, B.J.; Rusch, V.W.; Travis, W.D. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod. Pathol. 2011, 24, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Choi, E.R.; Lee, H.Y.; Jeong, J.Y.; Ahn, J.H.; Kim, S.; Bae, J.; Kim, H.K.; Choi, Y.S.; Kim, J.; et al. Pathologic heterogeneity of lung adenocarcinomas: A novel pathologic index predicts survival. Oncotarget 2016, 7, 70353–70363. [Google Scholar] [CrossRef][Green Version]
- Motoi, N.; Szoke, J.; Riely, G.J.; Seshan, V.E.; Kris, M.G.; Rusch, V.W.; Gerald, W.L.; Travis, W.D. Lung adenocarcinoma: Modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am. J. Surg. Pathol. 2008, 32, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Miyata, Y.; Yoshiya, T.; Tsutani, Y.; Mimura, T.; Murakami, S.; Ito, H.; Nakayama, H.; Okada, M. Second predominant subtype predicts outcomes of intermediate-malignant invasive lung adenocarcinomadagger. Eur. J. Cardiothorac. Surg. 2017, 51, 218–222. [Google Scholar] [PubMed]
- Takahashi, Y.; Eguchi, T.; Kameda, K.; Lu, S.; Vaghjiani, R.G.; Tan, K.S.; Travis, W.D.; Jones, D.R.; Adusumilli, P.S. Histologic subtyping in pathologic stage I-IIA lung adenocarcinoma provides risk-based stratification for surveillance. Oncotarget 2018, 9, 35742–35751. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, M.; Ohbayashi, C.; Kawaguchi, T.; Kawai, N.; Nakai, T.; Sawabata, N.; Taniguchi, S. Analysis of Histological Grade in Resected Lung-invasive Adenocarcinoma. Anticancer Res. 2019, 39, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading system for invasive pulmonary adenocarcinoma: A proposal from the IASLC pathology committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef]
- Jones, G.C.; Kehrer, J.D.; Kahn, J.; Koneru, B.N.; Narayan, R.; Thomas, T.O.; Camphausen, K.; Metha, M.P.; Kaushal, A. Primary Treatment Options for High-Risk/Medically Inoperable Early Stage NSCLC Patients. Clin. Lung Cancer 2015, 16, 413–430. [Google Scholar] [CrossRef]
- Sauter, A.P.; Muenzel, D.; Dangelmaier, J.; Braren, R.; Pfeiffer, F.; Rummeny, E.J.; Noel, P.B.; Fingerle, A.A. Dual-layer spectral computed tomography: Virtual non-contrast in comparison to true non-contrast images. Eur. J. Radiol. 2018, 104, 108–114. [Google Scholar] [CrossRef]
- Son, J.Y.; Lee, H.Y.; Kim, J.H.; Han, J.; Jeong, J.Y.; Lee, K.S.; Kwon, O.J.; Shim, Y.M. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: The added value of using iodine mapping. Eur. Radiol. 2016, 26, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, S.W.; Lee, K.S.; Jeong, J.Y.; Choi, J.Y.; Kwon, O.J.; Song, S.H.; Kim, E.Y.; Kim, J.; Shim, Y.M. Role of CT and PET Imaging in Predicting Tumor Recurrence and Survival in Patients with Lung Adenocarcinoma: A Comparison with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2015, 10, 1785–1794. [Google Scholar]
- Lee, H.Y.; Han, J.; Lee, K.S.; Koo, J.H.; Jeong, S.Y.; Kim, B.T.; Cho, Y.; Shim, Y.M.; Kim, J.; Kim, K.; et al. Lung adenocarcinoma as a solitary pulmonary nodule: Prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer 2009, 66, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Jeong, J.Y.; Lee, K.S.; Yi, C.A.; Kim, B.T.; Kang, H.; Kwon, O.J.; Shim, Y.M.; Han, J.H. Histopathology of lung adenocarcinoma based on new IASLC/ATS/ERS classification: Prognostic stratification with functional and metabolic imaging biomarkers. J. Magn. Reson. Imaging 2013, 38, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Nitadori, J.; Bograd, A.J.; Kadota, K.; Sima, C.S.; Rizk, N.P.; Morales, E.A.; Rusch, V.W.; Travis, W.D.; Adusumilli, P. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J. Natl. Cancer Inst. 2013, 105, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Lian, F.; Guo, L.; Wen, P.; Liu, X.Y.; Lin, D. Lepidic and micropapillary growth pattern and expression of Napsin A can stratify patients of stage I lung adenocarcinoma into different prognostic subgroup. Int. J. Clin. Exp. Pathol. 2014, 7, 1459–1468. [Google Scholar]
- Lee, K.H.; Goo, J.M.; Park, S.J.; Wi, J.Y.; Chung, D.H.; Go, H.; Park, H.S.; Park, C.M.; Lee, S.M. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J. Thorac. Oncol. 2014, 9, 74–82. [Google Scholar] [CrossRef]
- Sica, G.; Yoshizawa, A.; Sima, C.S.; Azzoli, C.G.; Downey, R.J.; Rusch, V.W.; Travis, W.D.; Moreira, A.L. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am. J. Surg. Pathol. 2010, 34, 1155–1162. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Muller-Hemelink, H.K.; Harris, C.C. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. World Health Organisation Classification of Tumours; IARC Press: Lyon, France, 2004. [Google Scholar]
- Okada, M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: Correlation with patient prognosis. Thorac. Surg. Clin. 2013, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tavora, F.; Burke, A. Histologic features associated with metastatic potential in invasive adenocarcinomas of the lung. Am. J. Surg. Pathol. 2013, 37, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Chandrakumar, D.; Gupta, S.; Yan, T.D.; Tian, D.H. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015, 89, 121–132. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, M.K.; Hamed, D.; Routledge, T.; Scarci, M. Is limited pulmonary resection equivalent to lobectomy for surgical management of stage I non-small-cell lung cancer? Interact. Cardiovasc. Thorac. Surg. 2012, 14, 816–820. [Google Scholar] [CrossRef]
- von der Thüsen, J.H.; Tham, Y.S.; Pattenden, H.; Rice, A.; Dusmet, M.; Lim, E.; Nicholson, A.G. Prognostic significance of predominant histologic pattern and nuclear grade in resected adenocarcinoma of the lung: Potential parameters for a grading system. J. Thorac. Oncol. 2013, 8, 37–44. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef]
- Ganeshan, B.; Abaleke, S.; Young, R.C.; Chatwin, C.R.; Miles, K.A. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010, 10, 137–143. [Google Scholar] [CrossRef]
- Ikeda, K.; Awai, K.; Mori, T.; Kawanaka, K.; Yamashita, Y.; Nomori, H. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest 2007, 132, 984–990. [Google Scholar] [CrossRef]
- Yi, C.A.; Lee, K.S.; Kim, E.A.; Han, J.; Kim, H.; Kwon, O.J.; Jeong, Y.J.; Kim, S. Solitary pulmonary nodules: Dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004, 233, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Morin, R.L.; Schueler, B.A.; Brown, L.R.; Cortese, D.A.; Pairolero, P.C.; Brutinel, W.M. Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material—A preliminary report. Radiology 1992, 182, 343–347. [Google Scholar] [CrossRef]
- Yamashita, K.; Matsunobe, S.; Takahashi, R.; Tsuda, T.; Matsumoto, K.; Miki, H.; Oyanagi, H.; Konishi, J. Small peripheral lung carcinoma evaluated with incremental dynamic CT: Radiologic-pathologic correlation. Radiology 1995, 196, 401–408. [Google Scholar] [CrossRef]
- Nakayama, H.; Okumura, S.; Daisaki, H.; Kato, Y.; Uehara, H.; Adachi, S.; Yoshimura, M.; Okada, M. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: A multicenter study. Cancer 2010, 116, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Abiko, M.; Sato, T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J. Thorac. Oncol. 2011, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Goodgame, B.; Pillot, G.A.; Yang, Z.; Shriki, J.; Meyers, B.F.; Zoole, J.; Gao, F.; Dehdashti, F.; Patterson, A.; Siegel, B.A.; et al. Prognostic value of preoperative positron emission tomography in resected stage I non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 130–134. [Google Scholar] [CrossRef]
- Nair, V.S.; Barnett, P.G.; Ananth, L.; Gould, M.K. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non-small cell lung cancer. Chest 2010, 137, 1150–1156. [Google Scholar] [CrossRef]
- Kadota, K.; Colovos, C.; Suzuki, K.; Rizk, N.P.; Dunphy, M.P.; Zabor, E.C.; Sima, C.S.; Yoshizawa, A.; Travis, W.D.; Rusch, V.W.; et al. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann. Surg. Oncol. 2012, 19, 3598–3605. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.H.; Yen, T.C.; Chang, J.T.; Chan, S.C.; Ko, S.F.; Wang, H.M.; Lee, L.; Kang, C.; Wong, A.M.; Liao, C. Prospective study of [18F] fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J. Clin. Oncol. 2006, 24, 4371–4376. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Jeong, H.S.; Park, S.G.; Jang, J.Y.; Son, Y.I.; Choi, J.Y.; Hyun, S.H.; Park, K.; Ahn, M.; Ahn, Y.C.; et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin. Cancer Res. 2009, 15, 5861–5868. [Google Scholar] [CrossRef]
- Luo, J.; Huang, Q.; Wang, R.; Han, B.; Zhang, J.; Zhao, H.; Fang, W.; Luo, Q.; Yang, J.; Yang, Y.; et al. Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J. Cancer Res. Clin. Oncol. 2016, 142, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.S.; Marguet, S.; Le Teuff, G.; Lantuejoul, S.; Shepherd, F.A.; Seymour, L.; Kratzke, R.; Graziano, S.L.; Popper, H.H.; Rosell, R.; et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J. Clin. Oncol. 2015, 33, 3439–3446. [Google Scholar] [CrossRef]
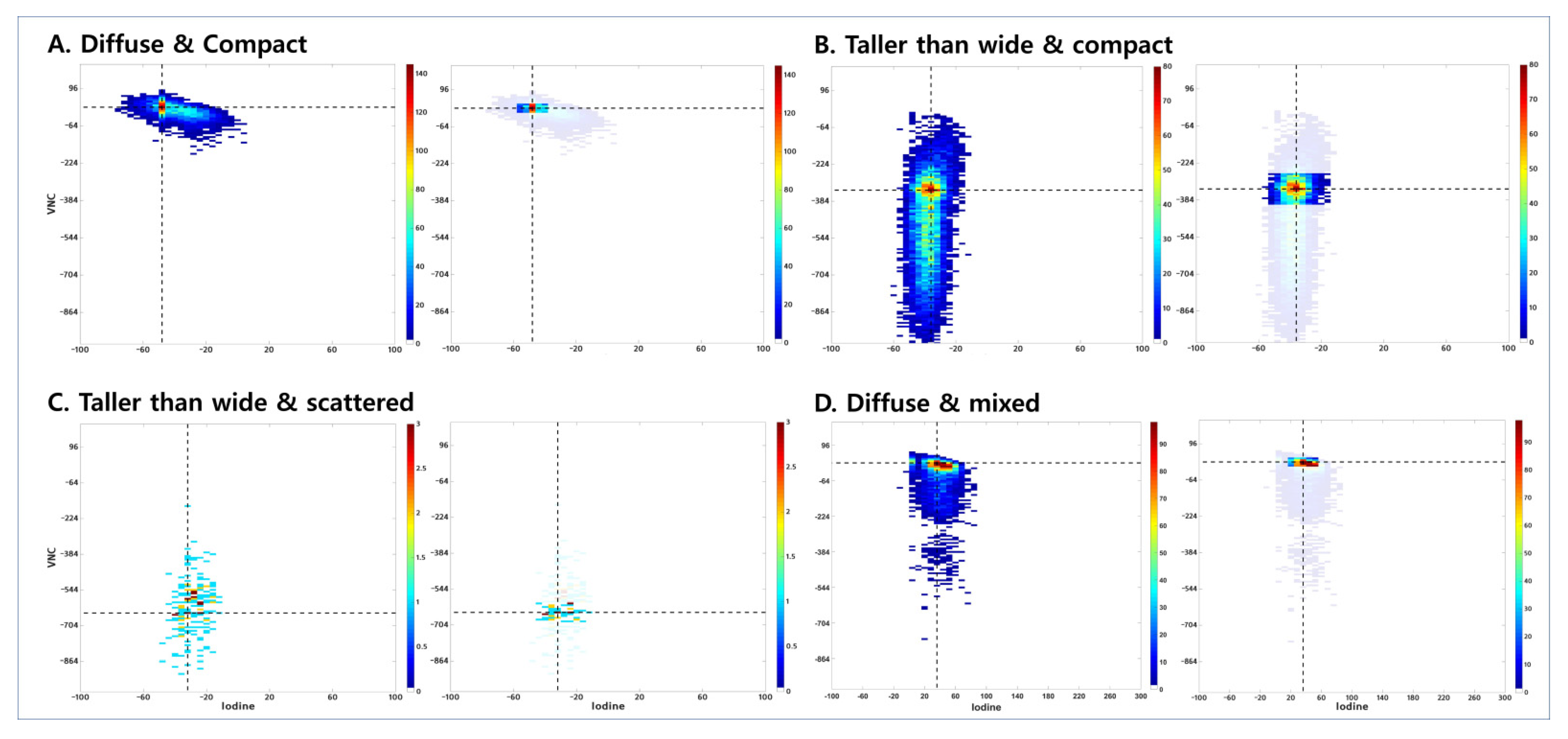
| Most Predominant Pattern | No. of Lesions with Second Most Predominant Pattern | Total No. (%) | ||||
|---|---|---|---|---|---|---|
| Lepidic | Acinar | Papillary | MP | Solid | ||
| Lepidic | 3 † | 45 | 0 | 0 | 0 | 48 (16.6) |
| Acinar | 90 | 44 † | 31 | 6 | 12 | 183 (63.1) |
| Papillary | 2 | 15 | 6 † | 4 | 4 | 31 (10.7) |
| MP | 1 | 4 | 3 | 0 † | 0 | 8 (2.7) |
| Solid | 1 | 11 | 0 | 2 | 6 † | 20 (6.9) |
| Total No. (%) | 97 (33.5) | 119 (41.0) | 40 (13.8) | 12 (4.1) | 22 (7.6) | 290 (100) |
| Imaging Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Low grade as the most-predominant and intermediate grade as the second most predominant pattern | ||||||
| Shape | 2.28 | 1.05–4.97 | 0.038 | 1.58 | 0.65–3.85 | 0.316 |
| Distribution (scattered) | 1.97 | 0.87–4.44 | 0.103 | NA | ||
| Distribution (mixed) | 0.53 | 0.23–1.24 | 0.146 | NA | ||
| Distribution (compact) | Ref | NA | ||||
| Area 30 | 1.70 | 0.75–3.83 | 0.205 | NA | ||
| VNC group (middle) | 2.17 | 0.76–6.21 | 0.148 | NA | ||
| VNC group (low) | 10.29 | 3.99–26.49 | <0.0001 | 6.15 | 1.72–21.95 | 0.005 |
| VNC group (upper) | Ref | NA | ||||
| Mean iodine | 0.97 | 0.94–0.99 | 0.007 | 0.98 | 0.95–1.02 | 0.318 |
| SUVmax | 0.77 | 0.64–0.94 | 0.008 | 0.93 | 0.77–1.12 | 0.421 |
| Intermediate grade as the most-predominant and high grade as the second most predominant pattern | ||||||
| Shape | 0.38 | 0.18–0.79 | 0.010 | 0.63 | 0.26–1.50 | 0.298 |
| Distribution (scattered) | 0.60 | 0.13–2.81 | 0.521 | NA | ||
| Distribution (mixed) | 2.98 | 1.36–6.52 | 0.006 | 2.01 | 0.82–4.97 | 0.128 |
| Distribution (compact) | Ref | NA | ||||
| Area 30 | 0.48 | 0.23–1.03 | 0.060 | NA | ||
| VNC group (middle) | 0.25 | 0.09–0.63 | 0.004 | 0.47 | 0.16–1.38 | 0.170 |
| VNC group (low) | 0.05 | 0.01–0.38 | 0.004 | 0.10 | 0.01–0.83 | 0.033 |
| VNC group (upper) | Ref | NA | ||||
| Mean iodine | 1.02 | 0.99–1.04 | 0.221 | NA | ||
| SUVmax | 1.22 | 1.12–1.34 | <0.0001 | 1.14 | 1.02–1.27 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kim, J.; Park, H.; Kim, H.K.; Kim, J.; Jeong, J.Y.; Ahn, J.H.; Lee, H.Y. Rethinking a Non-Predominant Pattern in Invasive Lung Adenocarcinoma: Prognostic Dissection Focusing on a High-Grade Pattern. Cancers 2021, 13, 2785. https://doi.org/10.3390/cancers13112785
Choi Y, Kim J, Park H, Kim HK, Kim J, Jeong JY, Ahn JH, Lee HY. Rethinking a Non-Predominant Pattern in Invasive Lung Adenocarcinoma: Prognostic Dissection Focusing on a High-Grade Pattern. Cancers. 2021; 13(11):2785. https://doi.org/10.3390/cancers13112785
Chicago/Turabian StyleChoi, Yeonu, Jonghoon Kim, Hyunjin Park, Hong Kwan Kim, Jhingook Kim, Ji Yun Jeong, Joong Hyun Ahn, and Ho Yun Lee. 2021. "Rethinking a Non-Predominant Pattern in Invasive Lung Adenocarcinoma: Prognostic Dissection Focusing on a High-Grade Pattern" Cancers 13, no. 11: 2785. https://doi.org/10.3390/cancers13112785
APA StyleChoi, Y., Kim, J., Park, H., Kim, H. K., Kim, J., Jeong, J. Y., Ahn, J. H., & Lee, H. Y. (2021). Rethinking a Non-Predominant Pattern in Invasive Lung Adenocarcinoma: Prognostic Dissection Focusing on a High-Grade Pattern. Cancers, 13(11), 2785. https://doi.org/10.3390/cancers13112785







