ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers
Abstract
Simple Summary
Abstract
1. Introduction
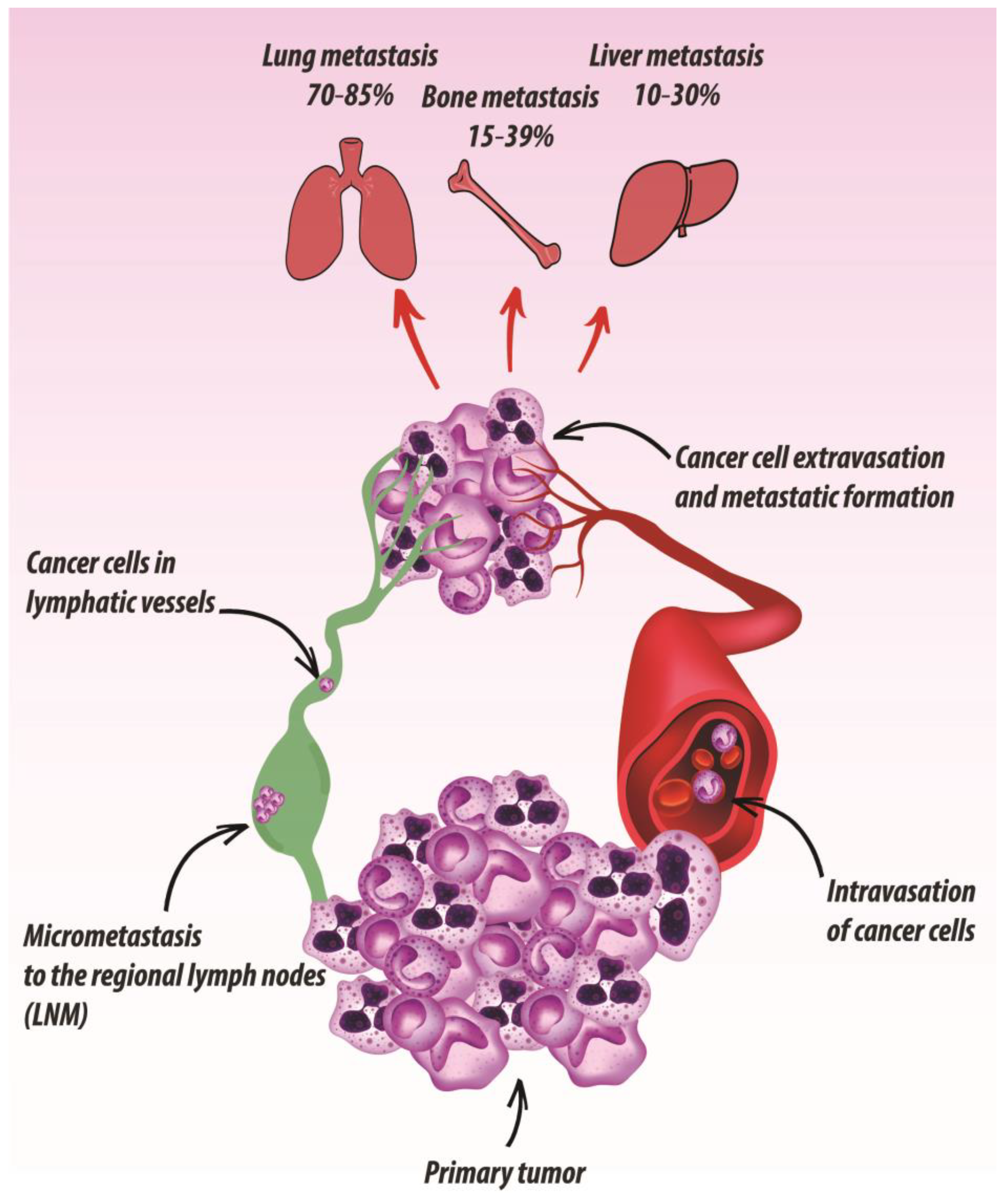
2. Metastatic Dissemination Routes of UADT SCC
2.1. Regional Metastasis
2.2. Distant Metastasis
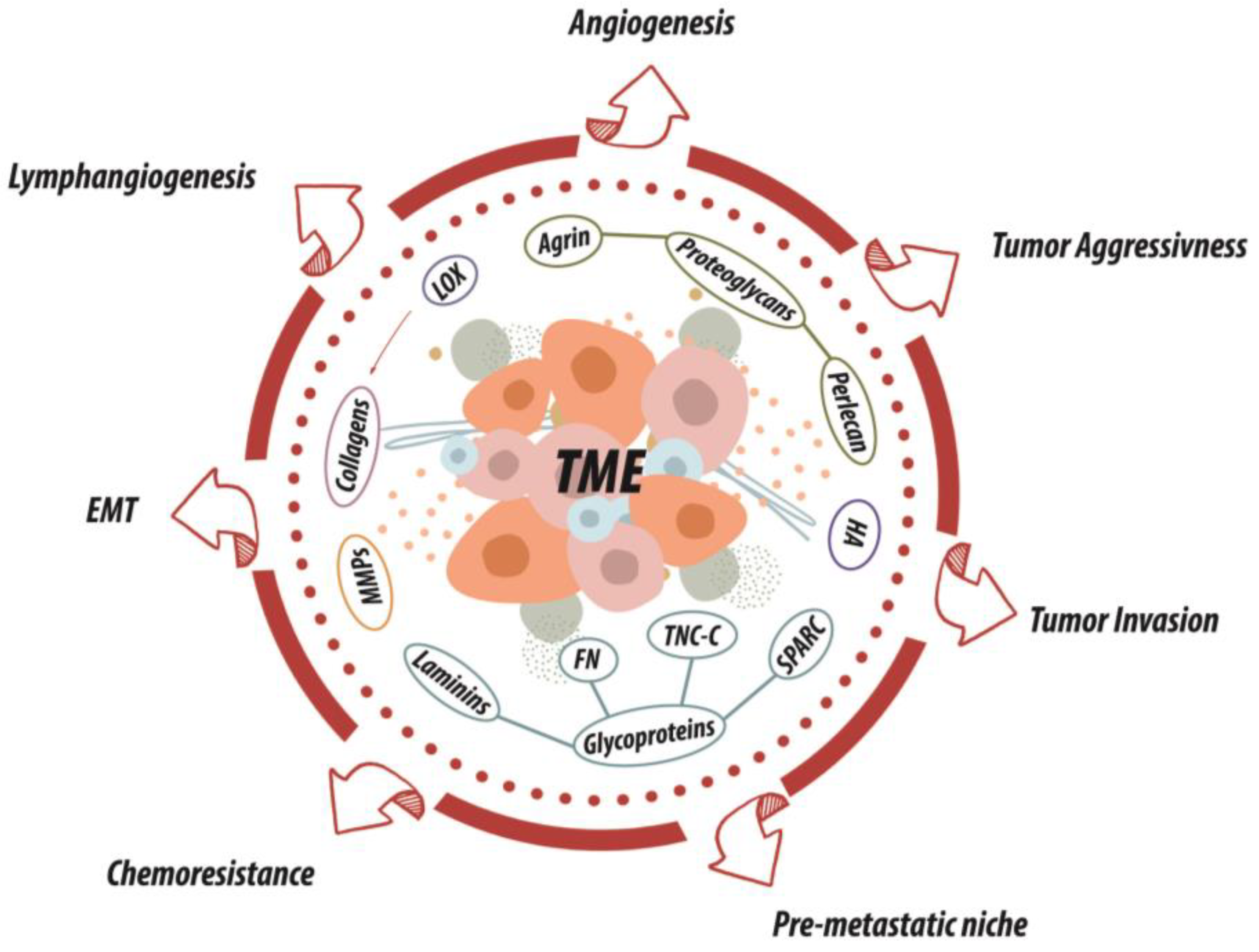
3. ECM as a Multi-Armed Warrior in SCC Dissemination
4. ECM in UADT SCC: An Intertwined Story
4.1. Collagens
4.2. Fibronectin
4.3. Laminins
4.4. Tenascin-C
4.5. SPARC
4.6. Proteoglycans
4.7. Hyaluronan
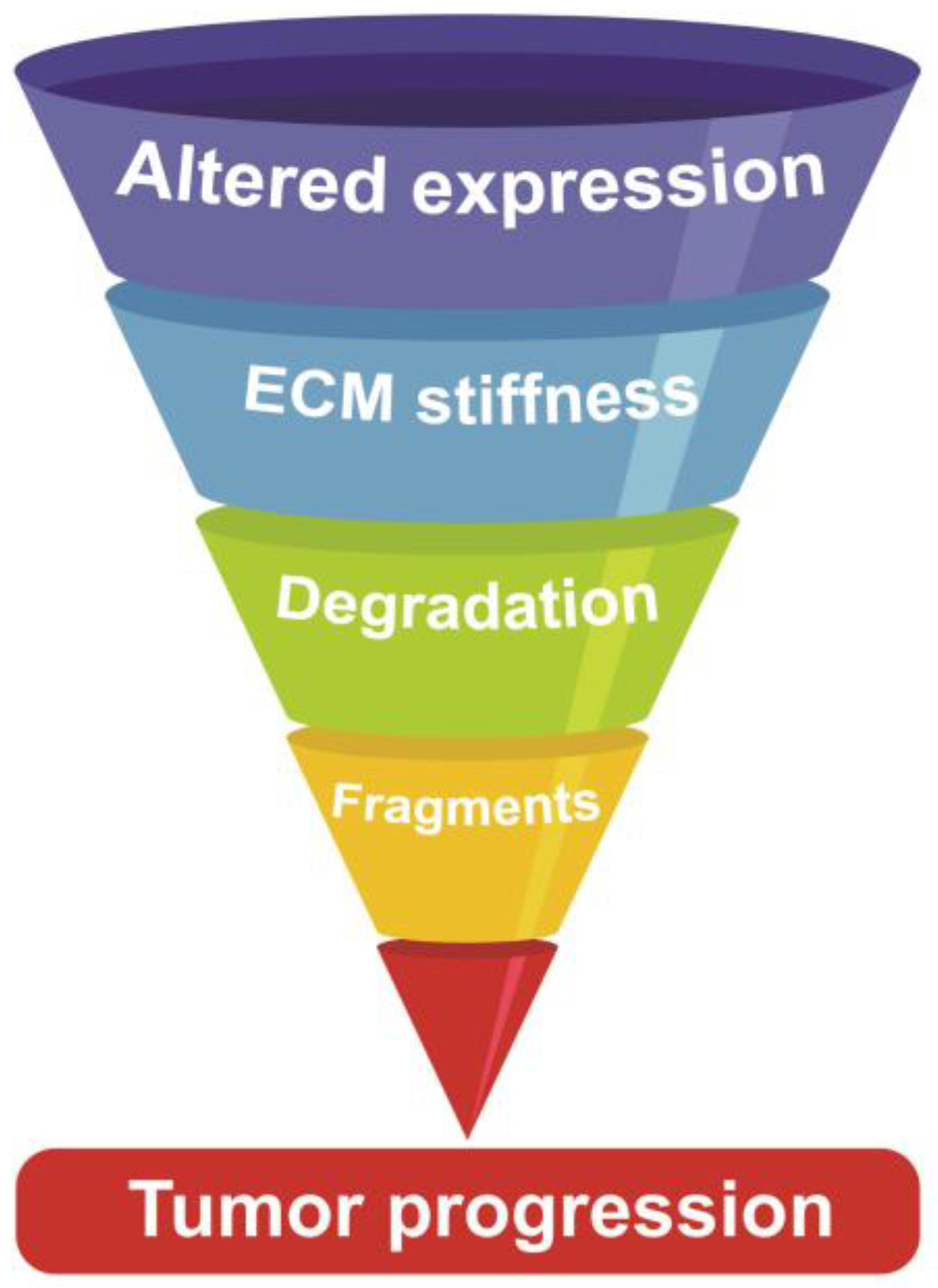
5. EMC Stiffness: The Dark Side of the Mechanical Force
6. The Turmoil of Scissor-Handed Proteases
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenig, B.M. Squamous Cell Carcinoma of the Upper Aerodigestive Tract: Dysplasia and Select Variants. Mod. Pathol. 2017, 30, S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.A.; Zugna, D.; Richiardi, L.; Merletti, F.; Marron, M.; Ahrens, W.; Pohlabeln, H.; Lagiou, P.; Trichopoulos, D.; Agudo, A.; et al. Smoking Addiction and the Risk of Upper-Aerodigestive-Tract Cancer in a Multicenter Case–Control Study. Int. J. Cancer 2013, 133, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Hsu, C.C.; Zaridze, D.; Shangina, O.; Szeszenia-Dabrowska, N.; Mates, D.; Fabiánová, E.; Rudnai, P.; Janout, V.; Holcatova, I.; et al. Dietary Risk Factors for Squamous Cell Carcinoma of the Upper Aerodigestive Tract in Central and Eastern Europe. Cancer Causes Control 2008, 19, 1161. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Alcohol and Tobacco Use, and Cancer Risk for Upper Aerodigestive Tract and Liver. Eur. J. Cancer Prev. 2008, 17, 340–344. [Google Scholar] [CrossRef]
- Bugter, O.; van de Ven, S.E.M.; Hardillo, J.A.; Bruno, M.J.; Koch, A.D.; Baatenburg de Jong, R.J. Early Detection of Esophageal Second Primary Tumors Using Lugol Chromoendoscopy in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Head Neck 2019, 41, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lu, H.-I.; Chien, C.-Y.; Lo, C.-M.; Wang, Y.-M.; Chou, S.-Y.; Li, S.-H. Efficacy of Different Chemotherapy Regimens in Patients with Locally Advanced Synchronous Esophageal and Head/Neck Squamous Cell Carcinoma Receiving Curative Concurrent Chemoradiotherapy. J. Clin. Med. 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Bognár, L.; Hegedűs, I.; Bellyei, S.; Pozsgai, É.; Zoltán, L.; Gombos, K.; Horváth, Ö.P.; Vereczkei, A.; Papp, A. Prognostic Role of HPV Infection in Esophageal Squamous Cell Carcinoma. Infect. Agent. Cancer 2018, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, F.R.; Ghosh, S.K.; Laskar, R.S.; Kannan, R.; Choudhury, B.; Bhowmik, A. Epigenetic Pathogenesis of Human Papillomavirus in Upper Aerodigestive Tract Cancers. Mol. Carcinog. 2015, 54, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Sacks, P.-L.; Alvarado, R.; Sacks, R.; Gallagher, R.; Harvey, R. Prognostic Biomarkers of Human Papilloma Virus (HPV)-Positive Neoplasia of the Upper Aerodigestive Tract: A Systematic Review. Aust. J. Otolaryngol. 2018, 1. [Google Scholar] [CrossRef]
- Michmerhuizen, N.L.; Birkeland, A.C.; Bradford, C.R.; Brenner, J.C. Genetic Determinants in Head and Neck Squamous Cell Carcinoma and Their Influence on Global Personalized Medicine. Genes Cancer 2016, 7, 182–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raudenská, M.; Balvan, J.; Masařík, M. Cell Death in Head and Neck Cancer Pathogenesis and Treatment. Cell Death Dis. 2021, 12, 192. [Google Scholar] [CrossRef]
- Hirano, H.; Kato, K. Systemic Treatment of Advanced Esophageal Squamous Cell Carcinoma: Chemotherapy, Molecular-Targeting Therapy and Immunotherapy. Jpn. J. Clin. Oncol. 2019, 49, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Hong, P.; Xu, W.W.; He, Q.-Y.; Li, B. Advances in Targeted Therapy for Esophageal Cancer. Signal Transduct. Target. Ther. 2020, 5, 229. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current Role of Primary Surgical Treatment in Patients with Head and Neck Squamous Cell Carcinoma. Curr. Opin. Oncol. 2019, 31, 138–145. [Google Scholar] [CrossRef]
- Saeki, H.; Sohda, M.; Sakai, M.; Sano, A.; Shirabe, K. Role of Surgery in Multidisciplinary Treatment Strategies for Locally Advanced Esophageal Squamous Cell Carcinoma. Ann. Gastroenterol. Surg. 2020, 4, 490–497. [Google Scholar] [CrossRef]
- Oosting, S.F.; Haddad, R.I. Best Practice in Systemic Therapy for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primer 2020, 6, 92. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Kang, E.J.; Keam, B.; Choi, J.-H.; Kim, J.-S.; Park, K.U.; Lee, K.E.; Kwon, J.H.; Lee, K.-W.; Kim, M.K.; et al. Treatment Strategy and Outcomes in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Nationwide Retrospective Cohort Study (KCSG HN13–01). BMC Cancer 2020, 20, 813. [Google Scholar] [CrossRef]
- Cristina, V.; Herrera-Gómez, R.G.; Szturz, P.; Espeli, V.; Siano, M. Immunotherapies and Future Combination Strategies for Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 5399. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of Tumor Microenvironment on Pathogenesis of the Head and Neck Squamous Cell Carcinoma: A Systematic Review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-ad, V.; Pribitkin, E.; Tuluc, M. Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Tumor Microenviron. 2014, 41, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Andreuzzi, E.; Capuano, A.; Pellicani, R.; Poletto, E.; Doliana, R.; Maiero, S.; Fornasarig, M.; Magris, R.; Colombatti, A.; Cannizzaro, R.; et al. Loss of Multimerin-2 and EMILIN-2 Expression in Gastric Cancer Associate with Altered Angiogenesis. Int. J. Mol. Sci. 2018, 19, 3983. [Google Scholar] [CrossRef]
- Marastoni, S.; Andreuzzi, E.; Paulitti, A.; Colladel, R.; Pellicani, R.; Todaro, F.; Schiavinato, A.; Bonaldo, P.; Colombatti, A.; Mongiat, M. EMILIN2 Down-Modulates the Wnt Signalling Pathway and Suppresses Breast Cancer Cell Growth and Migration. J. Pathol. 2014, 232, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The Extracellular Matrix in Tumor Progression and Metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Gubbiotti, M.A. Extracellular Matrix: The Driving Force of Mammalian Diseases. Matrix Biol. J 2018, 71–72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mongiat, M.; Buraschi, S.; Andreuzzi, E.; Neill, T.; Iozzo, R.V. Extracellular Matrix: The Gatekeeper of Tumor Angiogenesis. Biochem. Soc. Trans. 2019, 47, 1543–1555. [Google Scholar] [CrossRef]
- Jin, M.-Z.; Jin, W.-L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Ye, L.-L.; Rao, J.; Fan, X.-W.; Kong, F.-F.; Hu, C.-S.; Ying, H.-M. The Prognostic Value of Tumor Depth for Cervical Lymph Node Metastasis in Hypopharyngeal and Supraglottic Carcinomas. Head Neck 2019, 41, 2116–2122. [Google Scholar] [CrossRef]
- Abdeyrim, A.; He, S.; Zhang, Y.; Mamtali, G.; Asla, A.; Yusup, M.; Liu, J. Prognostic Value of Lymph Node Ratio in Laryngeal and Hypopharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Otolaryngol-Head Neck Surg. 2020, 49, 31. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Lin, C.-S.; Yang, C.-Y.; Lin, C.-K.; Chen, Y.-W. Lymph Node Density as a Prognostic Predictor in Patients with Betel Nut-Related Oral Squamous Cell Carcinoma. Clin. Oral Investig. 2018, 22, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lu, H.-I.; Lo, C.-M.; Wang, Y.-M.; Chou, S.-Y.; Hsiao, C.-C.; Shih, L.-H.; Chen, S.-W.; Li, S.-H. Neck Lymph Node Metastasis as A Poor Prognostic Factor in Thoracic Esophageal Squamous Cell Carcinoma Patients Receiving Concurrent Chemoradiotherapy: A Propensity Score-Matched Analysis. Sci. Rep. 2018, 8, 15073. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Leeman, J.E.; Xie, P.; Li, X.; Goldman, D.A.; Zhang, Z.; Sherman, E.; McBride, S.; Riaz, N.; Lee, N.; et al. Long-Term Survival in Patients with Metastatic Head and Neck Squamous Cell Carcinoma Treated with Metastasis-Directed Therapy. Br. J. Cancer 2019, 121, 897–903. [Google Scholar] [CrossRef]
- Sahai, E. Illuminating the Metastatic Process. Nat. Rev. Cancer 2007, 7, 737–749. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Fukusumi, T.; Guo, T.W.; Sakai, A.; Ando, M.; Ren, S.; Haft, S.; Liu, C.; Amornphimoltham, P.; Gutkind, J.S.; Califano, J.A. The NOTCH4-HEY1 Pathway Induces Epithelial-Mesenchymal Transition in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Forghanifard, M.M.; Azaraz, S.; Ardalan Khales, S.; Morshedi Rad, D.; Abbaszadegan, M.R. MAML1 Promotes ESCC Aggressiveness through Upregulation of EMT Marker TWIST1. Mol. Biol. Rep. 2020, 47, 2659–2668. [Google Scholar] [CrossRef]
- Natsuizaka, M.; Whelan, K.A.; Kagawa, S.; Tanaka, K.; Giroux, V.; Chandramouleeswaran, P.M.; Long, A.; Sahu, V.; Darling, D.S.; Que, J.; et al. Interplay between Notch1 and Notch3 Promotes EMT and Tumor Initiation in Squamous Cell Carcinoma. Nat. Commun. 2017, 8, 1758. [Google Scholar] [CrossRef]
- Jung, A.R.; Jung, C.-H.; Noh, J.K.; Lee, Y.C.; Eun, Y.-G. Epithelial-Mesenchymal Transition Gene Signature Is Associated with Prognosis and Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 3652. [Google Scholar] [CrossRef]
- Wu, S.-G.; Zhang, W.-W.; Sun, J.-Y.; Li, F.-Y.; Lin, Q.; He, Z.-Y. Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front. Oncol. 2018, 8, 302. [Google Scholar] [CrossRef]
- Han, P.; Cao, P.; Hu, S.; Kong, K.; Deng, Y.; Zhao, B.; Li, F. Esophageal Microenvironment: From Precursor Microenvironment to Premetastatic Niche. Cancer Manag. Res. 2020, 12, 5857–5879. [Google Scholar] [CrossRef]
- Bhat, A.A.; Yousuf, P.; Wani, N.A.; Rizwan, A.; Chauhan, S.S.; Siddiqi, M.A.; Bedognetti, D.; El-Rifai, W.; Frenneaux, M.P.; Batra, S.K.; et al. Tumor Microenvironment: An Evil Nexus Promoting Aggressive Head and Neck Squamous Cell Carcinoma and Avenue for Targeted Therapy. Signal Transduct. Target. Ther. 2021, 6, 12. [Google Scholar] [CrossRef]
- Otto, B.; Koenig, A.M.; Tolstonog, G.V.; Jeschke, A.; Klaetschke, K.; Vashist, Y.K.; Wicklein, D.; Wagener, C.; Izbicki, J.R.; Streichert, T. Molecular Changes in Pre-Metastatic Lymph Nodes of Esophageal Cancer Patients. PLoS ONE 2014, 9, e102552. [Google Scholar] [CrossRef]
- Werner, J.A.; Dünne, A.A.; Myers, J.N. Functional Anatomy of the Lymphatic Drainage System of the Upper Aerodigestive Tract and Its Role in Metastasis of Squamous Cell Carcinoma. Head Neck 2003, 25, 322–332. [Google Scholar] [CrossRef]
- Spoerl, S.; Gerken, M.; Mamilos, A.; Fischer, R.; Wolf, S.; Nieberle, F.; Klingelhöffer, C.; Meier, J.K.; Spoerl, S.; Ettl, T.; et al. Lymph Node Ratio as a Predictor for Outcome in Oral Squamous Cell Carcinoma: A Multicenter Population-Based Cohort Study. Clin. Oral Investig. 2021, 25, 1705–1713. [Google Scholar] [CrossRef]
- Pisani, P.; Airoldi, M.; Allais, A.; Aluffi Valletti, P.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic Disease in Head & Neck Oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar] [CrossRef]
- Hasmat, S.; Mooney, C.; Gao, K.; Palme, C.E.; Ebrahimi, A.; Ch’ng, S.; Gupta, R.; Low, T.-H.; Clark, J. Regional Metastasis in Head and Neck Cutaneous Squamous Cell Carcinoma: An Update on the Significance of Extra-Nodal Extension and Soft Tissue Metastasis. Ann. Surg. Oncol. 2020, 27, 2840–2845. [Google Scholar] [CrossRef]
- Roy, R.; Kandimalla, R.; Sonohara, F.; Koike, M.; Kodera, Y.; Takahashi, N.; Yamada, Y.; Goel, A. A Comprehensive Methylation Signature Identifies Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma. Int. J. Cancer 2019, 144, 1160–1169. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Distribution of Lymph Node Metastases in Esophageal Carcinoma Patients Undergoing Upfront Surgery: A Systematic Review. Cancers 2020, 12, 1592. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; Künzli, H.T.; van Rijswijk, A.-S.; Meijer, S.L.; Mijnals, R.C.D.; Weusten, B.L.A.M.; Geijsen, E.D.; van Laarhoven, H.W.M.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Distribution of Lymph Node Metastases in Esophageal Adenocarcinoma after Neoadjuvant Chemoradiation Therapy: A Prospective Study. Surg. Endosc. 2020, 34, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Castoro, C.; Scarpa, M.; Cagol, M.; Ruol, A.; Cavallin, F.; Alfieri, R.; Zanchettin, G.; Rugge, M.; Ancona, E. Nodal Metastasis From Locally Advanced Esophageal Cancer: How Neoadjuvant Therapy Modifies Their Frequency and Distribution. Ann. Surg. Oncol. 2011, 18, 3743–3754. [Google Scholar] [CrossRef]
- Liu, J.C.; Bhayani, M.; Kuchta, K.; Galloway, T.; Fundakowski, C. Patterns of Distant Metastasis in Head and Neck Cancer at Presentation: Implications for Initial Evaluation. Oral Oncol. 2019, 88, 131–136. [Google Scholar] [CrossRef]
- Van der Kamp, M.F.; Muntinghe, F.O.W.; Iepsma, R.S.; Plaat, B.E.C.; van der Laan, B.F.A.M.; Algassab, A.; Steenbakkers, R.J.H.M.; Witjes, M.J.H.; van Dijk, B.A.C.; de Bock, G.H.; et al. Predictors for Distant Metastasis in Head and Neck Cancer, with Emphasis on Age. Eur. Arch. Otorhinolaryngol. 2021, 278, 181–190. [Google Scholar] [CrossRef]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Wu, S.-G.; Zhang, W.-W.; He, Z.-Y.; Sun, J.-Y.; Chen, Y.-X.; Guo, L. Sites of Metastasis and Overall Survival in Esophageal Cancer: A Population-Based Study. Cancer Manag. Res. 2017, 9, 781–788. [Google Scholar] [CrossRef]
- Imura, Y.; Yamamoto, S.; Wakamatsu, T.; Tanaka, T.; Tamiya, H.; Sugimura, K.; Miyata, H.; Ishihara, R.; Yano, M.; Naka, N. Clinical Features and Prognostic Factors in Patients with Esophageal Cancer with Bone Metastasis. Oncol. Lett. 2020, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chu, X.; Ren, Z.; Wang, B. Relationship between T Stage and Survival in Distantly Metastatic Esophageal Cancer: A STROBE-Compliant Study. Medicine 2020, 99, e20064. [Google Scholar] [CrossRef]
- Cheng, Y.-F.; Chen, H.-S.; Wu, S.-C.; Chen, H.-C.; Hung, W.-H.; Lin, C.-H.; Wang, B.-Y. Esophageal Squamous Cell Carcinoma and Prognosis in Taiwan. Cancer Med. 2018, 7, 4193–4201. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, S.; Li, H.; Hassan, M.O.O.; Lu, T.; Zhao, J.; Zhang, L. Lung Metastases in Newly Diagnosed Esophageal Cancer: A Population-Based Study. Front. Oncol. 2021, 11, 603953. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Zhu, H.; Ren, W.; Chen, Y.; Liu, Q.; Deng, J.; Ye, J.; Fan, J.; Zhao, K. Patterns of Distant Organ Metastases in Esophageal Cancer: A Population-Based Study. J. Thorac. Dis. 2017, 9, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, O.; Ghibour, A.; Alsaid, B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol. Res. Pract. 2017, 2017, 1657310. [Google Scholar] [CrossRef]
- Takes, R.P.; Rinaldo, A.; Silver, C.E.; Haigentz, M.J.; Woolgar, J.A.; Triantafyllou, A.; Mondin, V.; Paccagnella, D.; de Bree, R.; Shaha, A.R.; et al. Distant Metastases from Head and Neck Squamous Cell Carcinoma. Part I. Basic Aspects. Oral Oncol. 2012, 48, 775–779. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Capuano, A.; Pivetta, E.; Sartori, G.; Bosisio, G.; Favero, A.; Cover, E.; Andreuzzi, E.; Colombatti, A.; Cannizzaro, R.; Scanziani, E.; et al. Abrogation of EMILIN1-β1 Integrin Interaction Promotes Experimental Colitis and Colon Carcinogenesis. Matrix Biol 2019, 83, 97–115. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Andreuzzi, E.; Fejza, A.; Capuano, A.; Poletto, E.; Pivetta, E.; Doliana, R.; Pellicani, R.; Favero, A.; Maiero, S.; Fornasarig, M.; et al. Deregulated Expression of Elastin Microfibril Interfacer 2 (EMILIN2) in Gastric Cancer Affects Tumor Growth and Angiogenesis. Matrix Biol. Plus 2020, 6–7, 100029. [Google Scholar] [CrossRef]
- Ziober, A.F.; Falls, E.M.; Ziober, B.L. The Extracellular Matrix in Oral Squamous Cell Carcinoma: Friend or Foe? Head Neck 2006, 28, 740–749. [Google Scholar] [CrossRef]
- Tanis, T.; Cincin, Z.B.; Gokcen-Rohlig, B.; Bireller, E.S.; Ulusan, M.; Tanyel, C.R.; Cakmakoglu, B. The Role of Components of the Extracellular Matrix and Inflammation on Oral Squamous Cell Carcinoma Metastasis. Arch. Oral Biol. 2014, 59, 1155–1163. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Hamidi, H.; Pietilä, M.; Ivaska, J. The Complexity of Integrins in Cancer and New Scopes for Therapeutic Targeting. Br. J. Cancer 2016, 115, 1017–1023. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Janes, S.M.; Watt, F.M. New Roles for Integrins in Squamous-Cell Carcinoma. Nat. Rev. Cancer 2006, 6, 175–183. [Google Scholar] [CrossRef]
- Fan, Q.-C.; Tian, H.; Wang, Y.; Liu, X.-B. Integrin-A5 Promoted the Progression of Oral Squamous Cell Carcinoma and Modulated PI3K/AKT Signaling Pathway. Arch. Oral Biol. 2019, 101, 85–91. [Google Scholar] [CrossRef]
- Vay, C.; Hosch, S.B.; Stoecklein, N.H.; Klein, C.A.; Vallböhmer, D.; Link, B.-C.; Yekebas, E.F.; Izbicki, J.R.; Knoefel, W.T.; Scheunemann, P. Integrin Expression in Esophageal Squamous Cell Carcinoma: Loss of the Physiological Integrin Expression Pattern Correlates with Disease Progression. PLoS ONE 2014, 9, e109026. [Google Scholar] [CrossRef]
- Xie, J.-J.; Guo, J.-C.; Wu, Z.-Y.; Xu, X.-E.; Wu, J.-Y.; Chen, B.; Ran, L.-Q.; Liao, L.-D.; Li, E.-M.; Xu, L.-Y. Integrin A5 Promotes Tumor Progression and Is an Independent Unfavorable Prognostic Factor in Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2016, 48, 69–75. [Google Scholar] [CrossRef]
- Fennewald, S.M.; Kantara, C.; Sastry, S.K.; Resto, V.A. Laminin Interactions with Head and Neck Cancer Cells under Low Fluid Shear Conditions Lead to Integrin Activation and Binding. J. Biol. Chem. 2012, 287, 21058–21066. [Google Scholar] [CrossRef]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and Cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Gallo, O.; Massi, D.; Baroni, G.; Santucci, M. Tumor Lymphangiogenesis in Head and Neck Squamous Cell Carcinoma: A Morphometric Study with Clinical Correlations. Cancer 2004, 101, 973–978. [Google Scholar] [CrossRef]
- Kumagai, Y.; Tachikawa, T.; Higashi, M.; Sobajima, J.; Takahashi, A.; Amano, K.; Fukuchi, M.; Ishibashi, K.; Mochiki, E.; Yakabi, K.; et al. Vascular Endothelial Growth Factors C and D and Lymphangiogenesis at the Early Stage of Esophageal Squamous Cell Carcinoma Progression. Dis. Esophagus 2018, 31. [Google Scholar] [CrossRef]
- Künnapuu, J.; Bokharaie, H.; Jeltsch, M. Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs. Biology 2021, 10, 167. [Google Scholar] [CrossRef]
- Karatzanis, A.D.; Koudounarakis, E.; Papadakis, I.; Velegrakis, G. Molecular Pathways of Lymphangiogenesis and Lymph Node Metastasis in Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2012, 269, 731–737. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Ladeira, K.; Macedo, F.; Longatto-Filho, A.; Martins, S.F. Angiogenic Factors: Role in Esophageal Cancer, a Brief Review. Esophagus 2018, 15, 53–58. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C.; et al. The Role of Tumor Microenvironment in Chemoresistance: 3D Extracellular Matrices as Accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Tumour Vessel Normalization Takes Centre Stage. Nat. Rev. Drug Discov. 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Pellicani, R.; Poletto, E.; Andreuzzi, E.; Paulitti, A.; Doliana, R.; Bizzotto, D.; Braghetta, P.; Colladel, R.; Tarticchio, G.; Sabatelli, P.; et al. Multimerin-2 Maintains Vascular Stability and Permeability. Matrix Biol. 2020, 87, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Paulitti, A.; Andreuzzi, E.; Bizzotto, D.; Pellicani, R.; Tarticchio, G.; Marastoni, S.; Pastrello, C.; Jurisica, I.; Ligresti, G.; Bucciotti, F.; et al. The Ablation of the Matricellular Protein EMILIN2 Causes Defective Vascularization Due to Impaired EGFR-Dependent IL-8 Production Affecting Tumor Growth. Oncogene 2018, 3399–3414. [Google Scholar] [CrossRef]
- Hou, S.; Jin, W.; Xiao, W.; Deng, B.; Wu, D.; Zhi, J.; Wu, K.; Cao, X.; Chen, S.; Ding, Y.; et al. Integrin A5 Promotes Migration and Cisplatin Resistance in Esophageal Squamous Cell Carcinoma Cells. Am. J. Cancer Res. 2019, 9, 2774–2788. [Google Scholar] [PubMed]
- Zou, B.; Wang, D.; Xu, K.; Yuan, D.-Y.; Meng, Z.; Zhang, B. Integrin α-5 as a Potential Biomarker of Head and Neck Squamous Cell Carcinoma. Oncol. Lett. 2019, 18, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chaudhuri, O. Beyond Proteases: Basement Membrane Mechanics and Cancer Invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef] [PubMed]
- Engbring, J.A.; Kleinman, H.K. The Basement Membrane Matrix in Malignancy. J. Pathol. 2003, 200, 465–470. [Google Scholar] [CrossRef]
- Yamauchi, M.; Gibbons, D.L.; Zong, C.; Fradette, J.J.; Bota-Rabassedas, N.; Kurie, J.M. Fibroblast Heterogeneity and Its Impact on Extracellular Matrix and Immune Landscape Remodeling in Cancer. Fibroblasts Arbiters Matrix Remodel. 2020, 91–92, 8–18. [Google Scholar] [CrossRef]
- Jang, I.; Beningo, K.A. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers 2019, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.T. Mechanical Properties of Basement Membrane in Health and Disease. Basement Membr. Health Dis. 2017, 57–58, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.M.; Bertero, T.; Bozec, A.; Friard, J.; Bourget, I.; Pisano, S.; Lecacheur, M.; Maiel, M.; Bailleux, C.; Emelyanov, A.; et al. Matrix Stiffening and EGFR Cooperate to Promote the Collective Invasion of Cancer Cells. Cancer Res. 2018, 78, 5229. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer Associated Fibroblasts in the Reactive Stroma and Its Relation to Cancer Biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef]
- Shay, G.; Lynch, C.C.; Fingleton, B. Moving Targets: Emerging Roles for MMPs in Cancer Progression and Metastasis. Matrix Biol. 2015, 44–46, 200–206. [Google Scholar] [CrossRef]
- Fang, S.; Dai, Y.; Mei, Y.; Yang, M.; Hu, L.; Yang, H.; Guan, X.; Li, J. Clinical Significance and Biological Role of Cancer-Derived Type I Collagen in Lung and Esophageal Cancers. Thorac. Cancer 2019, 10, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zheng, K.; Liu, Y.; Li, J.; Wang, S.; Liu, K.; Song, X.; Li, N.; Xie, S.; et al. The Clinical Significance of Collagen Family Gene Expression in Esophageal Squamous Cell Carcinoma. PeerJ 2019, 7, e7705. [Google Scholar] [CrossRef] [PubMed]
- Prime, S.S.; Cirillo, N.; Hassona, Y.; Lambert, D.W.; Paterson, I.C.; Mellone, M.; Thomas, G.J.; James, E.N.L.; Parkinson, E.K. Fibroblast Activation and Senescence in Oral Cancer. J. Oral Pathol. Med. 2017, 46, 82–88. [Google Scholar] [CrossRef]
- Shoucair, I.; Weber Mello, F.; Jabalee, J.; Maleki, S.; Garnis, C. The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 6837. [Google Scholar] [CrossRef]
- Dourado, M.R.; Guerra, E.N.S.; Salo, T.; Lambert, D.W.; Coletta, R.D. Prognostic Value of the Immunohistochemical Detection of Cancer-Associated Fibroblasts in Oral Cancer: A Systematic Review and Meta-Analysis. J. Oral Pathol. Med. 2018, 47, 443–453. [Google Scholar] [CrossRef]
- Kang, S.H.; Oh, S.Y.; Lee, H.-J.; Kwon, T.-G.; Kim, J.-W.; Lee, S.-T.; Choi, S.-Y.; Hong, S.-H. Cancer-Associated Fibroblast Subgroups Showing Differential Promoting Effect on HNSCC Progression. Cancers 2021, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, W.; Kang, Y.; Yu, X.; Zhang, C.; Feng, Y. High Expression of Collagen 1A2 Promotes the Proliferation and Metastasis of Esophageal Cancer Cells. Ann. Transl. Med. 2020, 8, 1672. [Google Scholar] [CrossRef]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of Integrin Av Facilitates Proliferation and Invasion of Oral Squamous Cell Carcinoma Cells via Mek/Erk Signaling Pathway That Is Activated by Interaction of Integrin Avβ8 with TypeⅠCollagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Tan, M.L.; Hollows, R.J.; Robinson, M.; Ibrahim, M.; Margielewska, S.; Parkinson, E.K.; Ramanathan, A.; Zain, R.B.; Mehanna, H.; et al. Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1. Cancers 2019, 11, 1766. [Google Scholar] [CrossRef]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin Domain Receptor Tyrosine Kinases: New Players in Cancer Progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Sok, J.C.; Lee, J.A.; Dasari, S.; Joyce, S.; Contrucci, S.C.; Egloff, A.M.; Trevelline, B.K.; Joshi, R.; Kumari, N.; Grandis, J.R.; et al. Collagen Type XI A1 Facilitates Head and Neck Squamous Cell Cancer Growth and Invasion. Br. J. Cancer 2013, 109, 3049–3056. [Google Scholar] [CrossRef]
- Schmalbach, C.E.; Chepeha, D.B.; Giordano, T.J.; Rubin, M.A.; Teknos, T.N.; Bradford, C.R.; Wolf, G.T.; Kuick, R.; Misek, D.E.; Trask, D.K.; et al. Molecular Profiling and the Identification of Genes Associated with Metastatic Oral Cavity/Pharynx Squamous Cell Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, W.; Xu, B.; Zhu, H.; Zou, J.; Su, C.; Rong, J.; Wang, T.; Chen, Z. Expression of Fibronectin in Esophageal Squamous Cell Carcinoma and Its Role in Migration. BMC Cancer 2018, 18, 976. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-Guided Migration of Carcinoma Collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef] [PubMed]
- Jerhammar, F.; Ceder, R.; Garvin, S.; Grénman, R.; Grafström, R.C.; Roberg, K. Fibronectin 1 Is a Potential Biomarker for Radioresistance in Head and Neck Squamous Cell Carcinoma. Cancer Biol. Ther. 2010, 10, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tang, Y.-L.; Liang, X.-H. Transforming Growth Factor-β Signaling in Head and Neck Squamous Cell Carcinoma: Insights into Cellular Responses. Oncol. Lett. 2018, 16, 4799–4806. [Google Scholar] [CrossRef]
- Ramos, G.d.O.; Bernardi, L.; Lauxen, I.; Sant’Ana Filho, M.; Horwitz, A.R.; Lamers, M.L. Fibronectin Modulates Cell Adhesion and Signaling to Promote Single Cell Migration of Highly Invasive Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0151338. [Google Scholar] [CrossRef]
- Marinkovich, M.P. Laminin 332 in Squamous-Cell Carcinoma. Nat. Rev. Cancer 2007, 7, 370–380. [Google Scholar] [CrossRef]
- Baba, Y.; Iyama, K.; Hirashima, K.; Nagai, Y.; Yoshida, N.; Hayashi, N.; Miyanari, N.; Baba, H. Laminin-332 Promotes the Invasion of Oesophageal Squamous Cell Carcinoma via PI3K Activation. Br. J. Cancer 2008, 98, 974–980. [Google Scholar] [CrossRef][Green Version]
- Marangon Junior, H.; Rocha, V.N.; Leite, C.F.; de Aguiar, M.C.F.; Souza, P.E.A.; Horta, M.C.R. Laminin-5 Gamma 2 Chain Expression Is Associated with Intensity of Tumor Budding and Density of Stromal Myofibroblasts in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2014, 43, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Pattaramalai, S.; Skubitz, A.P.N. Promotion of Human Oral Squamous Cell Carcinoma Adhesion in Vitro by the Carboxy-Terminal Globular Domain of Laminin. Arch. Oral Biol. 1994, 39, 925–933. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nohata, N.; Hanazawa, T.; Kikkawa, N.; Yamamoto, N.; Yoshino, H.; Itesako, T.; Enokida, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumour-Suppressive MicroRNA-29s Inhibit Cancer Cell Migration and Invasion by Targeting Laminin–Integrin Signalling in Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 2013, 109, 2636–2645. [Google Scholar] [CrossRef]
- Ramos, D.M.; Chen, B.; Regezi, J.; Zardi, L.; Pytela, R. Tenascin-C Matrix Assembly in Oral Squamous Cell Carcinoma. Int. J. Cancer 1998, 75, 680–687. [Google Scholar] [CrossRef]
- Sundquist, E.; Kauppila, J.H.; Veijola, J.; Mroueh, R.; Lehenkari, P.; Laitinen, S.; Risteli, J.; Soini, Y.; Kosma, V.-M.; Sawazaki-Calone, I.; et al. Tenascin-C and Fibronectin Expression Divide Early Stage Tongue Cancer into Low- and High-Risk Groups. Br. J. Cancer 2017, 116, 640–648. [Google Scholar] [CrossRef]
- Yang, Z.-T.; Yeo, S.-Y.; Yin, Y.-X.; Lin, Z.-H.; Lee, H.-M.; Xuan, Y.-H.; Cui, Y.; Kim, S.-H. Tenascin-C, a Prognostic Determinant of Esophageal Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0145807. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Borsi, L.; Hyckel, P.; Kosmehl, H. Fibrillary Co-Deposition of Laminin-5 and Large Unspliced Tenascin-C in the Invasive Front of Oral Squamous Cell Carcinoma in Vivo and in Vitro. J. Cancer Res. Clin. Oncol. 2001, 127, 286–292. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C.; Feng, Y.; Qi, W.; Cui, Y.; Xuan, Y. Tenascin-C Is Involved in Promotion of Cancer Stemness via the Akt/HIF1ɑ Axis in Esophageal Squamous Cell Carcinoma. Exp. Mol. Pathol. 2019, 109, 104239. [Google Scholar] [CrossRef]
- Spenlé, C.; Loustau, T.; Murdamoothoo, D.; Erne, W.; Beghelli-de la Forest Divonne, S.; Veber, R.; Petti, L.; Bourdely, P.; Mörgelin, M.; Brauchle, E.-M.; et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 1122. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Stieber, P.; Schmitt, U.M.; Andratschke, M.; Hoffmann, K.; Wollenberg, B. The Significance of Tenascin-C Serum Level as Tumor Marker in Squamous Cell Carcinoma of the Head and Neck. Anticancer Res. 2002, 22, 3093–3097. [Google Scholar]
- Chin, D.; Boyle, G.M.; Williams, R.M.; Ferguson, K.; Pandeya, N.; Pedley, J.; Campbell, C.M.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Novel Markers for Poor Prognosis in Head and Neck Cancer. Int. J. Cancer 2005, 113, 789–797. [Google Scholar] [CrossRef]
- Che, Y.; Luo, A.; Wang, H.; Qi, J.; Guo, J.; Liu, Z. The Differential Expression of SPARC in Esophageal Squamous Cell Carcinoma. Int. J. Mol. Med. 2006, 17, 1027–1033. [Google Scholar] [CrossRef][Green Version]
- He, Q.; Wei, J.; Zhang, J.; Jiang, H.; Wang, S.; Zhou, X.; Zhang, Z.; Huang, G.; Watanabe, H.; Su, J. Aberrant Methylation of Secreted Protein, Acidic and Rich in Cysteine in Human Laryngeal and Hypopharyngeal Carcinoma. Oncol. Lett. 2011, 2, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Tan, Y.; Liu, Z. Clinical Significance of SPARC in Esophageal Squamous Cell Carcinoma. Biochem. Biophys. Res. Commun. 2017, 492, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Cheng, J.; Maruyama, S.; Yamazaki, M.; Abé, T.; Babkair, H.; Saito, C.; Saku, T. Differential Immunohistochemical Expression Profiles of Perlecan-Binding Growth Factors in Epithelial Dysplasia, Carcinoma in Situ, and Squamous Cell Carcinoma of the Oral Mucosa. Pathol. Res. Pract. 2016, 212, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Macabeo-Ong, M.; Shiboski, C.H.; Silverman, S.; Ginzinger, D.G.; Dekker, N.; Wong, D.T.W.; Jordan, R.C.K. Quantitative Analysis of Cathepsin L MRNA and Protein Expression during Oral Cancer Progression. Oral Oncol. 2003, 39, 638–647. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lakshmanan, M.; Swa, H.L.F.; Chen, J.; Zhang, X.; Ong, Y.S.; Loo, L.S.; Akıncılar, S.C.; Gunaratne, J.; Tergaonkar, V.; et al. An Oncogenic Role of Agrin in Regulating Focal Adhesion Integrity in Hepatocellular Carcinoma. Nat. Commun. 2015, 6, 6184. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decoding the Matrix: Instructive Roles of Proteoglycan Receptors. Biochemistry 2015, 54, 4583–4598. [Google Scholar] [CrossRef]
- Scherbakov, N.; Knops, M.; Ebner, N.; Valentova, M.; Sandek, A.; Grittner, U.; Dahinden, P.; Hettwer, S.; Schefold, J.C.; von Haehling, S.; et al. Evaluation of C-Terminal Agrin Fragment as a Marker of Muscle Wasting in Patients after Acute Stroke during Early Rehabilitation. J. Cachexia Sarcopenia Muscle 2016, 7, 60–67. [Google Scholar] [CrossRef]
- Yu, D.; Li, H.-X.; Liu, Y.; Ying, Z.-W.; Guo, J.-J.; Cao, C.-Y.; Wang, J.; Li, Y.-F.; Yang, H.-R. The Reference Intervals for Serum C-Terminal Agrin Fragment in Healthy Individuals and as a Biomarker for Renal Function in Kidney Transplant Recipients. J. Clin. Lab. Anal. 2017, 31, e22059. [Google Scholar] [CrossRef]
- Klein-Scory, S.; Kübler, S.; Diehl, H.; Eilert-Micus, C.; Reinacher-Schick, A.; Stühler, K.; Warscheid, B.; Meyer, H.E.; Schmiegel, W.; Schwarte-Waldhoff, I. Immunoscreening of the Extracellular Proteome of Colorectal Cancer Cells. BMC Cancer 2010, 10, 70. [Google Scholar] [CrossRef]
- Twarock, S.; Freudenberger, T.; Poscher, E.; Dai, G.; Jannasch, K.; Dullin, C.; Alves, F.; Prenzel, K.; Knoefel, W.T.; Stoecklein, N.H.; et al. Inhibition of Oesophageal Squamous Cell Carcinoma Progression by in Vivo Targeting of Hyaluronan Synthesis. Mol. Cancer 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Twarock, S.; Tammi, M.I.; Savani, R.C.; Fischer, J.W. Hyaluronan Stabilizes Focal Adhesions, Filopodia, and the Proliferative Phenotype in Esophageal Squamous Carcinoma Cells. J. Biol. Chem. 2010, 285, 23276–23284. [Google Scholar] [CrossRef]
- Lu, T.; Zheng, Y.; Gong, X.; Lv, Q.; Chen, J.; Tu, Z.; Lin, S.; Pan, J.; Guo, Q.; Li, J. High Expression of Hyaluronan-Mediated Motility Receptor Predicts Adverse Outcomes: A Potential Therapeutic Target for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Shiina, M.; Bourguignon, L.Y.W. Selective Activation of Cancer Stem Cells by Size-Specific Hyaluronan in Head and Neck Cancer. Int. J. Cell Biol. 2015, 2015, 989070. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.J.; Schroeder, G.L.; Goodwin, W.J.; Weed, D.T.; Fisher, P.; Lokeshwar, V.B. Expression of Tumor Markers Hyaluronic Acid and Hyaluronidase (HYAL1) in Head and Neck Tumors. Int. J. Cancer 2003, 106, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Godin, D.A.; Fitzpatrick, P.C.; Scandurro, A.B.; Belafsky, P.C.; Woodworth, B.A.; Amedee, R.G.; Beech, D.J.; Beckman, B.S. PH-20: A Novel Tumor Marker for Laryngeal Cancer. Arch. Otolaryngol. Neck Surg. 2000, 126, 402–404. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Earle, C.; Wong, G.; Spevak, C.C.; Krueger, K. Stem Cell Marker (Nanog) and Stat-3 Signaling Promote MicroRNA-21 Expression and Chemoresistance in Hyaluronan/CD44-Activated Head and Neck Squamous Cell Carcinoma Cells. Oncogene 2012, 31, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 Interaction with Oct4-Sox2-Nanog Promotes MiR-302 Expression Leading to Self-Renewal, Clonal Formation, and Cisplatin Resistance in Cancer Stem Cells from Head and Neck Squamous Cell Carcinoma. J. Biol. Chem. 2012, 287, 32800–32824. [Google Scholar] [CrossRef]
- Wang, S.J.; Bourguignon, L.Y.W. Hyaluronan and the Interaction Between CD44 and Epidermal Growth Factor Receptor in Oncogenic Signaling and Chemotherapy Resistance in Head and Neck Cancer. Arch. Otolaryngol. Neck Surg. 2006, 132, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Coppelli, F.M.; Wells, A.; Gooding, W.E.; Song, J.; Kassis, J.; Drenning, S.D.; Grandis, J.R. Epidermal Growth Factor Receptor-Stimulated Activation of Phospholipase Cγ-1 Promotes Invasion of Head and Neck Squamous Cell Carcinoma. Cancer Res. 2003, 63, 5629. [Google Scholar]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a Glance. J. Cell Sci. 2007, 120, 1955. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Zhao, Z.; Zhao, H. Bioinformatics Analysis of Gene Expression Profiles of Esophageal Squamous Cell Carcinoma. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Astigiano, S.; Barbieri, O.; Ferrari, N.; Marchisio, S.; Ulivi, V.; Volta, C.; Manduca, P. Procollagen I COOH-Terminal Fragment Induces VEGF-A and CXCR4 Expression in Breast Carcinoma Cells. Exp. Cell Res. 2008, 314, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Ding, Y.; Liu, J.; Lu, J.; Zhan, L.; Qin, Q.; Zhang, H.; Chen, X.; Yang, Y.; et al. Recombinant Human Endostatin Enhances the Radioresponse in Esophageal Squamous Cell Carcinoma by Normalizing Tumor Vasculature and Reducing Hypoxia. Sci. Rep. 2015, 5, 14503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.; Ye, C.; Feng, C.; Riedel, F.; Liu, X.; Zeng, Q.; Grandis, J.R. Enhanced Antiangiogenic Therapy of Squamous Cell Carcinoma by Combined Endostatin and Epidermal Growth Factor Receptor-Antisense Therapy. Clin. Cancer Res. 2002, 8, 3570. [Google Scholar] [PubMed]
- Xie, R.; Wang, X.; Qi, G.; Wu, Z.; Wei, R.; Li, P.; Zhang, D. DDR1 Enhances Invasion and Metastasis of Gastric Cancer via Epithelial-Mesenchymal Transition. Tumour Biol. 2016, 37, 12049–12059. [Google Scholar] [CrossRef]
- Wierzbicka-Patynowski, I.; Schwarzbauer, J.E. The Ins and Outs of Fibronectin Matrix Assembly. J. Cell Sci. 2003, 116, 3269. [Google Scholar] [CrossRef]
- Miron-Mendoza, M.; Graham, E.; Manohar, S.; Petroll, W.M. Fibroblast-Fibronectin Patterning and Network Formation in 3D Fibrin Matrices. Matrix Biol. 2017, 64, 69–80. [Google Scholar] [CrossRef]
- Zollinger, A.J.; Smith, M.L. Fibronectin, the Extracellular Glue. Matrix Biol. 2017, 60–61, 27–37. [Google Scholar] [CrossRef]
- Rybak, J.-N.; Roesli, C.; Kaspar, M.; Villa, A.; Neri, D. The Extra-Domain A of Fibronectin Is a Vascular Marker of Solid Tumors and Metastases. Cancer Res. 2007, 67, 10948. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in Basement Membrane Assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Michopoulou, A.; Montmasson, M.; Garnier, C.; Lambert, E.; Dayan, G.; Rousselle, P. A Novel Mechanism in Wound Healing: Laminin 332 Drives MMP9/14 Activity by Recruiting Syndecan-1 and CD44. Matrix Biol. 2020, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Aldridge, K.; Ensley, J.F.; Odell, E.; Boyd, A.; Jones, J.; Gutkind, J.S.; Yeudall, W.A. Laminin-Γ2 Overexpression in Head-and-Neck Squamous Cell Carcinoma. Int. J. Cancer 2002, 99, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Yellapurkar, S.; Natarajan, S.; Boaz, K.; Manaktala, N.; Baliga, M.; Shetty, P.; Prasad, M.; Ravi, M. Expression of Laminin in Oral Squamous Cell Carcinomas. APJCP 2018, 19, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, A.C.M.; Rezaei, M.; Caliandro, M.F.; Lima, A.M.; Stehling, M.; Dhayat, S.A.; Haier, J.; Brakebusch, C.; Eble, J.A. The Interaction between Laminin-332 and A3β1 Integrin Determines Differentiation and Maintenance of CAFs, and Supports Invasion of Pancreatic Duct Adenocarcinoma Cells. Cancers 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a Glance. J. Cell Sci. 2016, 129, 4321. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. Endogenous Control of Immunity against Infection: Tenascin-C Regulates TLR4-Mediated Inflammation via MicroRNA-155. Cell Rep. 2012, 2, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Lowy, C.M.; Oskarsson, T. Tenascin C in Metastasis: A View from the Invasive Front. Cell Adhes. Migr. 2015, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in Tenascin-C Biology. Cell. Mol. Life Sci. 2011, 68, 3175. [Google Scholar] [CrossRef]
- Orend, G.; Chiquet-Ehrismann, R. Tenascin-C Induced Signaling in Cancer. Cancer Lett. 2006, 244, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Dontula, R.; El-Rayes, B.F.; Lakka, S.S. Molecular Mechanisms Underlying the Divergent Roles of SPARC in Human Carcinogenesis. Carcinogenesis 2014, 35, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Fukushima, N.; Maehara, N.; Matsubayashi, H.; Koopmann, J.; Su, G.H.; Hruban, R.H.; Goggins, M. SPARC/Osteonectin Is a Frequent Target for Aberrant Methylation in Pancreatic Adenocarcinoma and a Mediator of Tumor–Stromal Interactions. Oncogene 2003, 22, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- DiMartino, J.F.; Lacayo, N.J.; Varadi, M.; Li, L.; Saraiya, C.; Ravindranath, Y.; Yu, R.; Sikic, B.I.; Raimondi, S.C.; Dahl, G.V. Low or Absent SPARC Expression in Acute Myeloid Leukemia with MLL Rearrangements Is Associated with Sensitivity to Growth Inhibition by Exogenous SPARC Protein. Leukemia 2006, 20, 426–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Socha, M.J.; Said, N.; Dai, Y.; Kwong, J.; Ramalingam, P.; Trieu, V.; Desai, N.; Mok, S.C.; Motamed, K. Aberrant Promoter Methylation of SPARC in Ovarian Cancer. Neoplasia 2009, 11, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Kang, H.J.; Koh, K.H.; Rhee, H.; Kim, N.K.; Kim, H. Frequent Inactivation of SPARC by Promoter Hypermethylation in Colon Cancers. Int. J. Cancer 2007, 121, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Berdiaki, A.; Spyridaki, I.; Krasanakis, T.; Tsatsakis, A.; Tzanakakis, G.N. Proteoglycans-Biomarkers and Targets in Cancer Therapy. Front. Endocrinol. 2018, 9, 69. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Bang-Christensen, S.R.; Jørgensen, A.M.; Løppke, C.; Spliid, C.B.; Sand, N.T.; Clausen, T.M.; Salanti, A.; Agerbæk, M.Ø. The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis. Front. Cell Dev. Biol. 2020, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in Cancer Biology, Tumour Microenvironment and Angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef]
- Mongiat, M.; Taylor, K.; Otto, J.; Aho, S.; Uitto, J.; Whitelock, J.M.; Iozzo, R.V. The Protein Core of the Proteoglycan Perlecan Binds Specifically to Fibroblast Growth Factor-7. J. Biol. Chem. 2000, 275, 7095–7100. [Google Scholar] [CrossRef]
- Kawahara, R.; Granato, D.C.; Carnielli, C.M.; Cervigne, N.K.; Oliveria, C.E.; Martinez, C.A.R.; Yokoo, S.; Fonseca, F.P.; Lopes, M.; Santos-Silva, A.R.; et al. Agrin and Perlecan Mediate Tumorigenic Processes in Oral Squamous Cell Carcinoma. PLoS ONE 2014, 9, e115004. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, M.A.; Neill, T.; Iozzo, R.V. A Current View of Perlecan in Physiology and Pathology: A Mosaic of Functions. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 57–58, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.M.; Melrose, J.; Iozzo, R.V. Diverse Cell Signaling Events Modulated by Perlecan. Biochemistry 2008, 47, 11174–11183. [Google Scholar] [CrossRef]
- Douglass, S.; Goyal, A.; Iozzo, R.V. The Role of Perlecan and Endorepellin in the Control of Tumor Angiogenesis and Endothelial Cell Autophagy. Connect. Tissue Res. 2015, 56, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Mongiat, M.; Sweeney, S.M.; San Antonio, J.D.; Fu, J.; Iozzo, R.V. Endorepellin, a Novel Inhibitor of Angiogenesis Derived from the C Terminus of Perlecan. J. Biol. Chem. 2003, 278, 4238–4249. [Google Scholar] [CrossRef]
- Rivera, C.; Zandonadi, F.S.; Sánchez-Romero, C.; Soares, C.D.; Granato, D.C.; González-Arriagada, W.A.; Paes Leme, A.F. Agrin Has a Pathological Role in the Progression of Oral Cancer. Br. J. Cancer 2018, 118, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A Multifunctional, MegaDalton, Stealth Molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-Mediated Angiogenesis in Vascular Disease: Uncovering RHAMM and CD44 Receptor Signaling Pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1849. [Google Scholar] [CrossRef]
- Toole, B.P.; Hascall, V.C. Hyaluronan and Tumor Growth. Am. J. Pathol. 2002, 161, 745–747. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, G.; de Heer, A.-M.; Xia, W.; Bourguignon, L.Y.W. CD44 Variant Isoforms in Head and Neck Squamous Cell Carcinoma Progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Karousou, E.; Misra, S.; Ghatak, S.; Dobra, K.; Götte, M.; Vigetti, D.; Passi, A.; Karamanos, N.K.; Skandalis, S.S. Roles and Targeting of the HAS/Hyaluronan/CD44 Molecular System in Cancer. Matrix Biol. 2017, 59, 3–22. [Google Scholar] [CrossRef]
- Liu, H.; Deng, S.; Zhao, Z.; Zhang, H.; Xiao, J.; Song, W.; Gao, F.; Guan, Y. Oct4 Regulates the MiR-302 Cluster in P19 Mouse Embryonic Carcinoma Cells. Mol. Biol. Rep. 2011, 38, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Chang, D.C.; Chang-Lin, S.; Lin, C.-H.; Wu, D.T.S.; Chen, D.T.; Ying, S.-Y. Mir-302 Reprograms Human Skin Cancer Cells into a Pluripotent ES-Cell-like State. RNA 2008, 14, 2115–2124. [Google Scholar] [CrossRef]
- Lin, S.-L.; Chang, D.C.; Lin, C.-H.; Ying, S.-Y.; Leu, D.; Wu, D.T.S. Regulation of Somatic Cell Reprogramming through Inducible Mir-302 Expression. Nucleic Acids Res. 2011, 39, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Saint, A.; Van Obberghen-Schilling, E. The Role of the Tumor Matrix Environment in Progression of Head and Neck Cancer. Curr. Opin. Oncol. 2021, 33, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Xu, X.; Wu, H.; Xie, H.; Chen, L.; Lu, T.; Yang, L.; Guo, X.; Sun, G.; et al. Potential Effect of Matrix Stiffness on the Enrichment of Tumor Initiating Cells under Three-Dimensional Culture Conditions. Exp. Cell Res. 2015, 330, 123–134. [Google Scholar] [CrossRef]
- Matte, B.F.; Kumar, A.; Placone, J.K.; Zanella, V.G.; Martins, M.D.; Engler, A.J.; Lamers, M.L. Matrix Stiffness Mechanically Conditions EMT and Migratory Behavior of Oral Squamous Cell Carcinoma. J. Cell Sci. 2019, 132, jcs224360. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a Double-Edged Sword in Tumor Progression. Tumour Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The Role of Extracellular Matrix in Biomechanics and Its Impact on Bioengineering of Cells and 3D Tissues. Matrix Biomech. 2020, 85–86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Andreuzzi, E.; Capuano, A.; Poletto, E.; Pivetta, E.; Fejza, A.; Favero, A.; Doliana, R.; Cannizzaro, R.; Spessotto, P.; Mongiat, M. Role of Extracellular Matrix in Gastrointestinal Cancer-Associated Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3686. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix Density-Induced Mechanoregulation of Breast Cell Phenotype, Signaling and Gene Expression through a FAK-ERK Linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar] [CrossRef] [PubMed]
- Kopanska, K.S.; Alcheikh, Y.; Staneva, R.; Vignjevic, D.; Betz, T. Tensile Forces Originating from Cancer Spheroids Facilitate Tumor Invasion. PLoS ONE 2016, 11, e0156442. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Desai, R.; Solski, P.A.; Der, C.J.; Keely, P.J. ROCK-Generated Contractility Regulates Breast Epithelial Cell Differentiation in Response to the Physical Properties of a Three-Dimensional Collagen Matrix. J. Cell Biol. 2003, 163, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Runge, J.; Reichert, T.; Fritsch, A.; Käs, J.; Bertolini, J.; Remmerbach, T. Evaluation of Single-Cell Biomechanics as Potential Marker for Oral Squamous Cell Carcinomas: A Pilot Study. Oral Dis. 2014, 20, e120–e127. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting: MMPs as Potential Targets in Malignancy. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Wang, C.-Y.; Werb, Z. Matrix Metalloproteinases in Stem Cell Regulation and Cancer. Matrix Biol. 2015, 44–46, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Progress in Matrix Metalloproteinase Research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Arendt, Y.; Banci, L.; Bertini, I.; Cantini, F.; Cozzi, R.; Del Conte, R.; Gonnelli, L. Catalytic Domain of MMP20 (Enamelysin)—The NMR Structure of a New Matrix Metalloproteinase. FEBS Lett. 2007, 581, 4723–4726. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Di Leva, G.; Romano, G.; Nuovo, G.; Suh, S.-S.; Ngankeu, A.; Taccioli, C.; Pichiorri, F.; Alder, H.; Secchiero, P.; et al. MiR-221&222 Regulate TRAIL Resistance and Enhance Tumorigenicity through PTEN and TIMP3 Downregulation. Cancer Cell 2009, 16, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Overall, C.M. Missing the Target: Matrix Metalloproteinase Antitargets in Inflammation and Cancer. Trends Pharmacol. Sci. 2013, 34, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Nikitakis, N.; Aseervatham, J.; Rao, N.; Ogbureke, K. Matrix Metalloproteinases in Head and Neck Cancer: Current Perspectives. Met. Med. 2017, 4, 47–61. [Google Scholar] [CrossRef]
- Sinpitaksakul, S.N.; Pimkhaokham, A.; Sanchavanakit, N.; Pavasant, P. TGF-Β1 Induced MMP-9 Expression in HNSCC Cell Lines via Smad/MLCK Pathway. Biochem. Biophys. Res. Commun. 2008, 371, 713–718. [Google Scholar] [CrossRef]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix Metalloproteinases Participation in the Metastatic Process and Their Diagnostic and Therapeutic Applications in Cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Mukherjee, S.; Roth, M.J.; Dawsey, S.M.; Yan, W.; Rodriguez-Canales, J.; Erickson, H.S.; Hu, N.; Goldstein, A.M.; Taylor, P.R.; Richardson, A.M.; et al. Increased Matrix Metalloproteinase Activation in Esophageal Squamous Cell Carcinoma. J. Transl. Med. 2010, 8, 91. [Google Scholar] [CrossRef]
- Hauff, S.J.; Raju, S.C.; Orosco, R.K.; Gross, A.M.; Diaz-Perez, J.A.; Savariar, E.; Nashi, N.; Hasselman, J.; Whitney, M.; Myers, J.N.; et al. Matrix-Metalloproteinases in Head and Neck Carcinoma-Cancer Genome Atlas Analysis and Fluorescence Imaging in Mice. Otolaryngol. Head Neck Surg. 2014, 151, 612–618. [Google Scholar] [CrossRef]
- Hoffmann, C.; Vacher, S.; Sirven, P.; Lecerf, C.; Massenet, L.; Moreira, A.; Surun, A.; Schnitzler, A.; Klijanienko, J.; Mariani, O.; et al. MMP2 As An Independent Prognostic Stratifier In Oral Cavity Cancers. OncoImmunology 2019, 9, 1754094. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Shimada, Y.; Watanabe, G.; Uchida, S.; Makino, T.; Imamura, M. Expression of Vascular Endothelial Growth Factor, Matrix Metalloproteinase-9 and E-Cadherin in the Process of Lymph Node Metastasis in Oesophageal Cancer. Br. J. Cancer 1999, 80, 1366–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O-charoenrat, P.; Rhys-Evans, P.H.; Eccles, S.A. Expression of Matrix Metalloproteinases and Their Inhibitors Correlates With Invasion and Metastasis in Squamous Cell Carcinoma of the Head and Neck. Arch. Otolaryngol. Neck Surg. 2001, 127, 813–820. [Google Scholar]
- Ohashi, K.; Nemoto, T.; Nakamura, K.; Nemori, R. Increased Expression of Matrix Metalloproteinase 7 and 9 and Membrane Type 1-Matrix Metalloproteinase in Esophageal Squamous Cell Carcinomas. Cancer 2000, 88, 2201–2209. [Google Scholar] [CrossRef]
- Yamamoto, H.; Adachi, Y.; Itoh, F.; Iku, S.; Matsuno, K.; Kusano, M.; Arimura, Y.; Endo, T.; Hinoda, Y.; Hosokawa, M.; et al. Association of Matrilysin Expression with Recurrence and Poor Prognosis in Human Esophageal Squamous Cell Carcinoma. Cancer Res. 1999, 59, 3313–3316. [Google Scholar] [PubMed]
- Yamashita, K.; Mori, M.; Kataoka, A.; Inoue, H.; Sugimachi, K. The Clinical Significance of MMP-1 Expression in Oesophageal Carcinoma. Br. J. Cancer 2001, 84, 276–282. [Google Scholar] [CrossRef][Green Version]
- Bai, X.; Li, Y.; Zhang, H.; Wang, F.; He, H.; Yao, J.; Liu, L.; Li, S. Role of Matrix Metalloproteinase-9 in Transforming Growth Factor-β1-Induced Epithelial–Mesenchymal Transition in Esophageal Squamous Cell Carcinoma. OncoTargets Ther. 2017, 10, 2837–2847. [Google Scholar] [CrossRef]
- Zeng, Z.-S.; Cohen, A.M.; Guillem, J.G. Loss of Basement Membrane Type IV Collagen Is Associated with Increased Expression of Metalloproteinases 2 and 9 (MMP-2 and MMP-9) during Human Colorectal Tumorigenesis. Carcinogenesis 1999, 20, 749–755. [Google Scholar] [CrossRef]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 Activity Facilitates Invasive Behavior in Heterogeneous Human BMSC and HNSCC 3D Spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef]
- Ruokolainen, H.; Pääkkö, P.; Turpeenniemi-Hujanen, T. Expression of Matrix Metalloproteinase-9 in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2004, 10, 3110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-K.; Tung, C.-W.; Lee, J.-Y.; Hung, Y.-C.; Lee, C.-H.; Chou, S.-H.; Lin, H.-S.; Wu, M.-T.; Wu, I.-C. Plasma Matrix Metalloproteinase 1 Improves the Detection and Survival Prediction of Esophageal Squamous Cell Carcinoma. Sci. Rep. 2016, 6, 30057. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, H.; Zhou, W.; Ye, T.; Geng, C.; Li, X.; Yuan, L.; Du, M.; Xu, H.; Wang, Q. Lower Serum Matrix Metalloproteinase-9 in Metastatic Patients with Esophageal Squamous Cell Carcinoma After Concurrent Radiotherapy Was Significant for Prognosis. OncoTargets Ther. 2020, 13, 12857–12866. [Google Scholar] [CrossRef]
- Virós, D.; Camacho, M.; Zarraonandia, I.; García, J.; Quer, M.; Vila, L.; León, X. Prognostic Role of MMP-9 Expression in Head and Neck Carcinoma Patients Treated with Radiotherapy or Chemoradiotherapy. Oral Oncol. 2013, 49, 322–325. [Google Scholar] [CrossRef]
- Murphy, G. The ADAMs: Signalling Scissors in the Tumour Microenvironment. Nat. Rev. Cancer 2008, 8, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Uehara, E.; Shiiba, M.; Shinozuka, K.; Saito, K.; Kouzu, Y.; Koike, H.; Kasamatsu, A.; Sakamoto, Y.; Ogawara, K.; Uzawa, K.; et al. Upregulated Expression of ADAM12 Is Associated with Progression of Oral Squamous Cell Carcinoma. Int. J. Oncol. 2012, 40, 1414–1422. [Google Scholar] [CrossRef]
- Walker, J.L.; Fournier, A.K.; Assoian, R.K. Regulation of Growth Factor Signaling and Cell Cycle Progression by Cell Adhesion and Adhesion-Dependent Changes in Cellular Tension. Cytokine Growth Factor Rev. 2005, 16, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Coppari, R.; De Franco, R.; Cho, J.-Y.; Libermann, T.A.; Pinzani, M.; Toker, A. A Secreted Form of ADAM9 Promotes Carcinoma Invasion through Tumor-Stromal Interactions. Cancer Res. 2005, 65, 4728–4738. [Google Scholar] [CrossRef]
- Mochizuki, S.; Okada, Y. ADAMs in Cancer Cell Proliferation and Progression. Cancer Sci. 2007, 98, 621–628. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Active Metalloproteases of the A Disintegrin And Metalloprotease (ADAM) Family: Biological Function and Structure. J. Proteome Res. 2011, 10, 17–33. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.; Kakkar, V. ADAM Proteins- Therapeutic Potential in Cancer. Curr. Cancer Drug Targets 2008, 8, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Lin, S.-C.; Wong, Y.-K.; Liu, C.-J.; Chang, K.-W.; Liu, T.-Y. Increase of Disintergin Metalloprotease 10 (ADAM10) Expression in Oral Squamous Cell Carcinoma. Cancer Lett. 2007, 245, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, L.J.; Villaret, D.; Popp, M.; Lui, L.; McLaren, R.; Brown, H.; Cohen, D.; Yun, J.; McFadden, M. Gene Expression Profiling in Squamous Cell Carcinoma of the Oral Cavity Shows Abnormalities in Several Signaling Pathways. Laryngoscope 2005, 115, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.; Joutsa, J.; Ala-aho, R.; Pitchers, M.; Pennington, C.J.; Martin, C.; Premachandra, D.J.; Okada, Y.; Peltonen, J.; Grénman, R.; et al. Expression Profiles and Clinical Correlations of Degradome Components in the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2010, 16, 2022–2035. [Google Scholar] [CrossRef]
- Jones, A.V.; Lambert, D.W.; Speight, P.M.; Whawell, S.A. ADAM 10 Is over Expressed in Oral Squamous Cell Carcinoma and Contributes to Invasive Behaviour through a Functional Association with Avβ6 Integrin. FEBS Lett. 2013, 587, 3529–3534. [Google Scholar] [CrossRef]
- Takamune, Y.; Ikebe, T.; Nagano, O.; Nakayama, H.; Ota, K.; Obayashi, T.; Saya, H.; Shinohara, M. ADAM-17 Associated with CD44 Cleavage and Metastasis in Oral Squamous Cell Carcinoma. Virchows Arch. 2007, 450, 169–177. [Google Scholar] [CrossRef]
| ECM Molecule | Receptors | Intracellular Signaling | Clinical Relevance | Sample Type | Expression | References |
|---|---|---|---|---|---|---|
| Type I collagen | αvβ8 integrin | FAK-MEK/ERK | Increased tumor aggressiveness (biomarker) | RNA levels in tumor tissue | Increased | [101,102,107,108] |
| Collagen A1 (XI) | DDR1 | Shp-2, Src, MAPK | Lymph nodes metastasis (biomarker) | RNA and protein levels in tumor tissue | Increased | [109,110,111,112] |
| Fibronectin (FN) | αvβ6 and α9β1 integrins | TGF-β | Poor patient prognosis, resistance to radiotherapy (biomarker) | RNA and protein levels in tumor tissue | Increased | [113,114,115,116,117,118] |
| Laminin 5 | α3β1 and α6β4 integrins | PI3K/AKT/mTOR | Increased tumor invasiveness (biomarker) | Protein levels in tumor tissue | Increased | [78,119,120,121,122,123] |
| Tenascin-C | Integrins | Akt/HIF1α, CCL21/CCR7 | Poor clinical outcomes (biomarker) | Protein levels in tumor tissue and plasma | Increased | [124,125,126,127,128,129,130] |
| SPARC | Integrins | MAPK, PI3K/AKT | Poor clinical outcome and metastatic disease (biomarker) | Protein and RNA levels in tumor tissue | Increased | [131,132,133,134] |
| Perlecan | Growth factors | MAPK, VEGF-VEGFR | Increased tumor Invasiveness (biomarker) | Protein levels in tumor tissue | Increased | [135,136] |
| Agrin | Lrp4, MuSK | FAK/ERK/cyclin D1 | Poor prognosis and chemotherapy resistance (biomarker) | Protein levelsin tumor tissue | Increased | [137,138,139,140,141] |
| Hyaluronan | CD44 | Nanog-STAT3 MAPK/ERK | Chemotherapy resistance, increased tumor invasiveness (biomarker and potential therapeutic target) | Protein levels in saliva and RNA levels in tumor tissue | Increased | [142,143,144,145,146,147,148,149,150,151] |
| Biomarker | Profile | Clinical Relevance | References |
|---|---|---|---|
| MMP -2,-3,-7,-9 | Up-regulated in UADT SCC patients | Depth of invasion, lymph node metastasis and vessel permeation | [87,222,223,224,225,226] |
| MMP-7 | Up-regulated at the invasive front of the tumor | Short disease-free and overall survival | [227] |
| MMP-1 | Up-regulated in tumors and responsible for collagen degradation | Local invasion | [228,233] |
| MMP-9 | Up-regulated in tumors and association with vimentin and SNAI1 levels | Shortened relapse-free survival and poor prognosis of patients | [229,230,231,232,234,235] |
| VEGF-A | Increased release upon ECM remodeling | Advanced disease and poor prognosis | [86] |
| VEGF-C, -D | Increased release upon ECM remodeling | Lymph node metastasis and poor prognosis | [84,86] |
| TGF-β1 | Increased expression in UADT SCC patients | Distant lymph nodes metastasis, low rate of survival and poor prognosis | [218,229] |
| FGF, HGF, EGF | Increased release upon ECM remodeling | Poor prognosis, advanced tumor stage | [86,204] |
| Endostatin | Induces VEGF-A and MMP-2,-9 expression promoting ECM remodeling and angiogenesis | Important role in tumor dissemination influencing the efficacy of targeted therapies | [156,157] |
| ADAM 12 | Over-expressed in OSCC | Increased tumor progression | [237] |
| ADAM 17 | Over-expressed in OSCC | Nodal metastasis, local recurrence and OSCC invasion | [247] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fejza, A.; Camicia, L.; Poletto, E.; Carobolante, G.; Mongiat, M.; Andreuzzi, E. ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers. Cancers 2021, 13, 2759. https://doi.org/10.3390/cancers13112759
Fejza A, Camicia L, Poletto E, Carobolante G, Mongiat M, Andreuzzi E. ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers. Cancers. 2021; 13(11):2759. https://doi.org/10.3390/cancers13112759
Chicago/Turabian StyleFejza, Albina, Lucrezia Camicia, Evelina Poletto, Greta Carobolante, Maurizio Mongiat, and Eva Andreuzzi. 2021. "ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers" Cancers 13, no. 11: 2759. https://doi.org/10.3390/cancers13112759
APA StyleFejza, A., Camicia, L., Poletto, E., Carobolante, G., Mongiat, M., & Andreuzzi, E. (2021). ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers. Cancers, 13(11), 2759. https://doi.org/10.3390/cancers13112759







