The Value of Intraoperative Ultrasound in Selective Lateral Cervical Neck Lymphadenectomy for Papillary Thyroid Cancer: A Prospective Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Technique
2.3. Measures of Outcome
- -
- The difference, between IOUS and PreUS in the overall number of patients in which each of the lateral neck compartments containing pathologically verified metastatic lymph nodes was correctly detected.
- -
- the overall mean lymph node yield per each compartment dissected, as a surrogate for completeness of the nodal compartment excision;
- -
- mean operating time in minutes;
- -
- postoperative complications rate;
- -
- local recurrence rate during follow-up.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, S.-M.; Law, S.C.K.; Chan, J.K.C.; Au, S.-K.; Yau, S.; Lau, W.-H. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer 2003, 98, 31–40. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Bocca, E. Conservative neck dissection. Laryngoscope 1975, 85, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T.; American Head and Neck Society; American Academy of Otolaryngology-Head and Neck Surgery. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Rinaldo, A.; Silver, C.E.; Shah, J.P.; Suarez, C.; Medina, J.E.; Kowalski, L.P.; Johnson, J.T.; Strome, M.; Rodrigo, J.P.; et al. Neck dissection: Then and now. Auris. Nasus. Larynx 2006, 33, 365–374. [Google Scholar] [CrossRef]
- Caron, N.R.; Clark, O.H. Papillary thyroid cancer: Surgical management of lymph node metastases. Curr. Treat. Options Oncol. 2005, 6, 311–322. [Google Scholar] [CrossRef]
- Carty, S.E.; Cooper, D.S.; Doherty, G.M.; Duh, Q.-Y.; Kloos, R.T.; Mandel, S.J.; Randolph, G.W.; Stack, B.C.; Steward, D.L.; Terris, D.J.; et al. Consensus Statement on the Terminology and Classification of Central Neck Dissection for Thyroid Cancer. Thyroid 2009, 19, 1153–1158. [Google Scholar] [CrossRef]
- Stack, B.C.J.; Ferris, R.L.; Goldenberg, D.; Haymart, M.; Shaha, A.; Sheth, S.; Sosa, J.A.; Tufano, R.P. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 2012, 22, 501–508. [Google Scholar] [CrossRef]
- Machens, A.; Hinze, R.; Thomusch, O.; Dralle, H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J. Surg. 2002, 26, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.R.; Tan, Y.Y.; Ogilvie, J.B.; Triponez, F.; Reiff, E.S.; Kebebew, E.; Duh, Q.Y.; Clark, O.H. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J. Surg. 2006, 30, 833–840. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Chow, L.; Chick, W.; King, W.; Metreweli, C. Metastatic cervical nodes in papillary carcinoma of the thyroid: Ultrasound and histological correlation. Clin. Radiol. 1995, 50, 229–231. [Google Scholar] [CrossRef]
- Rosario, P.W.S.; de Faria, S.; Bicalho, L.; Alves, M.F.G.; Borges, M.A.R.; Purisch, S.; Padrao, E.L.; Rezende, L.L.; Barroso, A.L. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J. Ultrasound Med. 2005, 24, 1385–1389. [Google Scholar] [CrossRef]
- Leboulleux, S.; Girard, E.; Rose, M.; Travagli, J.P.; Sabbah, N.; Caillou, B.; Hartl, D.M.; Lassau, N.; Baudin, E.; Schlumberger, M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2007, 92, 3590–3594. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.E.; Cruz, F.; O’Brien, A.; Goni, I.; Leon, A.; Claure, R.; Camus, M.; Dominguez, F.; Mosso, L.; Arteaga, E.; et al. Impact of preoperative ultrasonographic staging of the neck in papillary thyroid carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2007, 133, 1258–1262. [Google Scholar] [CrossRef]
- Binyousef, H.M.; Alzahrani, A.S.; Al-Sobhi, S.S.; Al, S.H.S.; Chaudhari, M.A.; Raef, H.M. Preoperative neck ultrasonographic mapping for persistent/recurrent papillary thyroid cancer. World J. Surg. 2004, 28, 1110–1114. [Google Scholar] [CrossRef]
- Agcaoglu, O.; Aliyev, S.; Taskin, H.E.; Aksoy, E.; Siperstein, A.; Berber, E. The utility of intraoperative ultrasound in modified radical neck dissection: A pilot study. Surg. Innov. 2014, 21, 166–169. [Google Scholar] [CrossRef]
- Kumbhar, S.S.; O’Malley, R.B.; Robinson, T.J.; Maximin, S.; Lalwani, N.; Byrd, D.R.; Wang, C.L. Why Thyroid Surgeons Are Frustrated with Radiologists: Lessons Learned from Pre- and Postoperative US. Radiographics 2016, 36, 2141–2153. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur. J. Radiol. 2019, 112, 14–21. [Google Scholar] [CrossRef]
- Testini, M.; Nacchiero, M.; Piccinni, G.; Portincasa, P.; Di Venere, B.; Lissidini, G.; Bonomo, G.M. Total thyroidectomy is improved by loupe magnification. Microsurgery 2004, 24, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Moo, T.-A.S.; Fahey, T.J., 3rd. Lymph node dissection in papillary thyroid carcinoma. Semin. Nucl. Med. 2011, 41, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Lim, J.W.; Dickey, W.; Tanyeri, H.; Kirshenbaum, G.L.; Phadke, D.M.; Caldarelli, D. Quantification of lymph nodes in selective neck dissection. Laryngoscope 1999, 109, 368–370. [Google Scholar] [CrossRef]
- Won, H.-R.; Chang, J.W.; Kang, Y.E.; Kang, J.Y.; Koo, B.S. Optimal extent of lateral neck dissection for well-differentiated thyroid carcinoma with metastatic lateral neck lymph nodes: A systematic review and meta-analysis. Oral Oncol. 2018, 87, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sung, T.-Y.; Nam, K.-H.; Chung, W.Y.; Soh, E.-Y.; Park, C.S. Is level IIb lymph node dissection always necessary in N1b papillary thyroid carcinoma patients? World J. Surg. 2008, 32, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Merdad, M.; Eskander, A.; Kroeker, T.; Freeman, J.L. Predictors of Level II and Vb Neck Disease in Metastatic Papillary Thyroid Cancer. Arch. Otolaryngol. Neck Surg. 2012, 138, 1030–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Lei, J.; Liu, Y.; Fan, Y.; Wang, X.; Lu, X. Preoperative predictors of lateral neck lymph node metastasis in papillary thyroid microcarcinoma. Medicine 2017, 96, 6240. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Wang, S.-G.; Lee, J.-C.; Son, S.-M.; Kim, I.-J.; Kim, Y.-K. Level IIb Lymph Node Metastasis in Neck Dissection for Papillary Thyroid Carcinoma. Arch. Otolaryngol. Neck Surg. 2007, 133, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Lee, J.H.; Yi, J.S.; Shong, Y.K.; Hong, S.J.; Lee, D.H.; Choi, C.G.; Kim, S.J. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J. Surg. 2008, 32, 1552–1558. [Google Scholar] [CrossRef]
- Lombardi, D.; Paderno, A.; Giordano, D.; Barbieri, D.; Taboni, S.; Piazza, C.; Cappelli, C.; Bertagna, F.; Barbieri, V.; Piana, S.; et al. Therapeutic lateral neck dissection in well-differentiated thyroid cancer: Analysis on factors predicting distribution of positive nodes and prognosis. Head Neck 2018, 40, 242–250. [Google Scholar] [CrossRef]
- McGarvey, A.C.; Hoffman, G.R.; Osmotherly, P.G.; Chiarelli, P.E. Intra-operative monitoring of the spinal accessory nerve: A systematic review. J. Laryngol. Otol. 2014, 128, 746–751. [Google Scholar] [CrossRef]
- Mehanna, H.; Kong, A.; Ahmed, S.K. Recurrent head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S181–S190. [Google Scholar] [CrossRef]
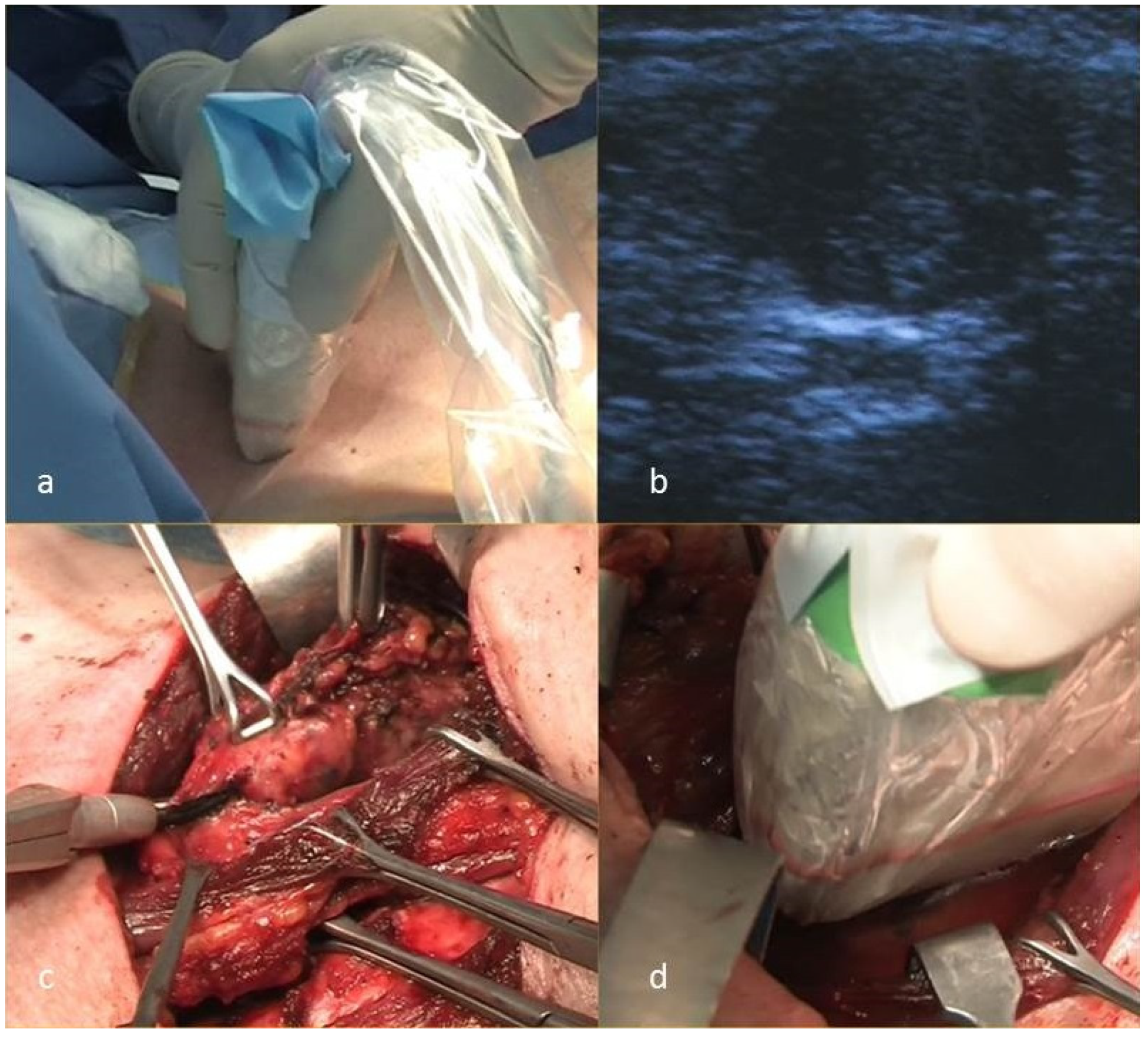
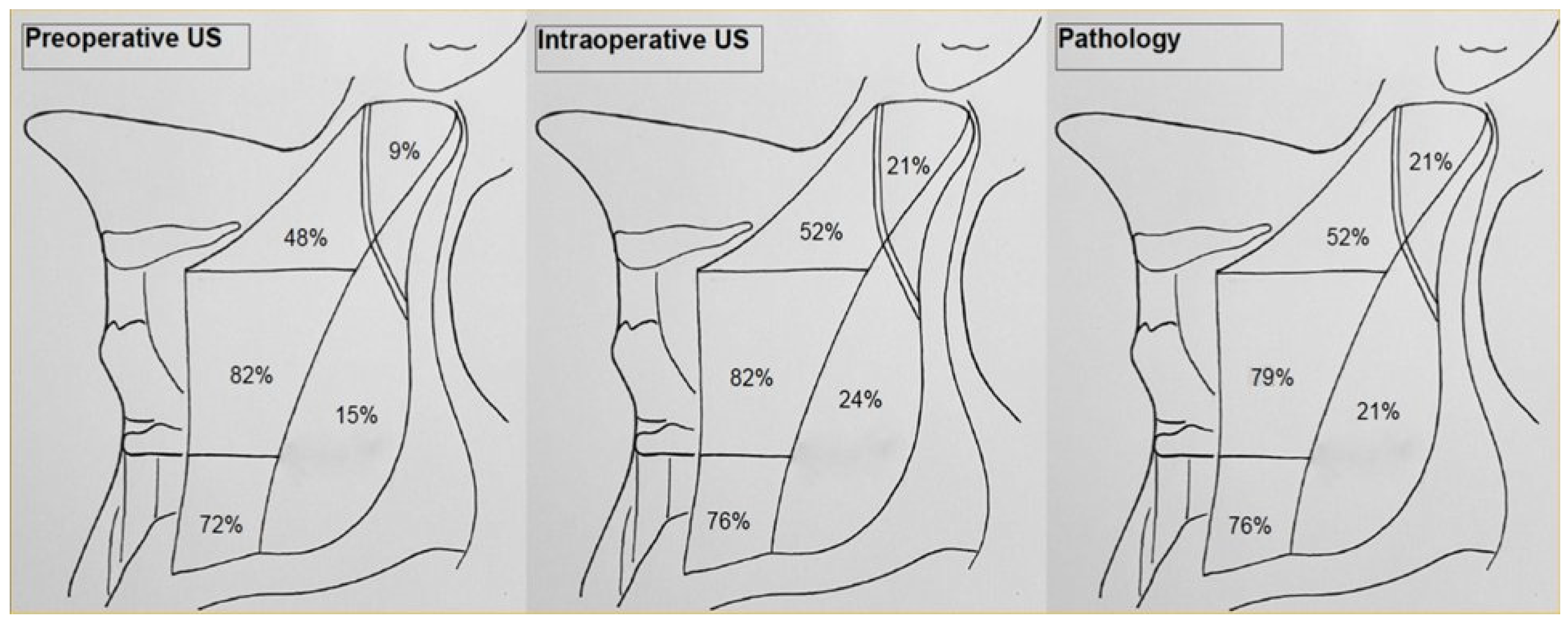
| Demographics and Data | N (%) | |
|---|---|---|
| Total cases | 33 (100) | |
| Gender | ||
| F | 24 (73) | |
| Mean BMI (range) | 26.6 (17–36) | |
| Mean age (range), years | 46 (20–77) | |
| <55 | 21 (64) | |
| ≥55 | 12 (36) | |
| Selective Neck Dissection | ||
| with TT | 24 (73) | |
| without TT | 9 (27) | |
| Mean operating time (range), min | 226 (120–330) | |
| Pathology | ||
| Classic papillary | 26 (79) | |
| Follicular variant | 7 (21) | |
| Papillary thyroid microcarcinoma | 4 (12) | |
| Unifocal PTC | 21 (64) | |
| Multifocal PTC | 8 (24) | |
| T1a | 9 (27) | |
| T1b | 5 (15) | |
| T2 | 3 (9) | |
| T3 | 16 (49) | |
| N1b | 33 (100) | |
| Mean number of nodes retrieved in SND (range) * | 10.4 (4–24) | |
| IIa | 3.9 (2–7) | |
| IIb | 4.3 (2–6) | |
| III | 4 (2–7) | |
| IV | 5 (2–10) | |
| V | 2.2 (1–3) | |
| Mean number of nodes positive at pathology (range) * | 2.61 (1–5) | |
| Mean Follow-up (range), years | 59 (17–100) | |
| Recurrence | 2 (6) |
| Positive Compartment Nodes Detected, by Modality, n. pts (%) | p * (PreUS vs. IOUS) | Additional Compartments Detected at IOUS Confirmed at Pathology N. pts (%) | Mean Number of LN Retrieved in Additional Compartments Detected by IOUS | Sensitivity % (95% CI) | Specificity | Accuracy | PPV | NPV | FP Rate | FN Rate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All cases | 33 | 8 (24.2) | 4.3 | |||||||||
| N level | ||||||||||||
| IIA | PreUS | 16 (48) | p < 0.0001 | 94.1 (0.69–1) | 100 (0.77–1) | 96.9 | 100 (0.76–1) | 94.1 (0.70–1) | 0 (0–0.24) | 5.9 (0.71–1) | ||
| IOUS | 17 (52) | 1/33 (3) | 4 | 100 (0.77–1) | 100 (0.76–1) | 100 | 100 (0.77–1) | 100 (0.76–1) | 0 (0–0.23) | 0 (0–0.24) | ||
| Pathol | 17 (52) | |||||||||||
| IIB | PreUS | 3 (9) | p = 0.006 | 42.9 (0.11–0.8) | 100 (0.84–1) | 87.8 | 100 (0.31–1) | 86.7 (0.68–0.96) | 0 (0–0.69) | 13.3 (0.04–0.31) | ||
| IOUS | 7 (21) | 4/33 (12.1) | 5.3 | 100 (0.56–1) | 100 (0.84–1) | 100 | 100 (0.56–1) | 100 (0.84–1) | 0 (0–0.43) | 0 (0–0.16) | ||
| Pathol | 7 (21) | |||||||||||
| III | PreUS | 27 (82) | p < 0.0001 | 100 (0.84–1) | 85.7 (0.42–1) | 96.9 | 96.3 (0.79–1) | 100 (0.52–1) | 0.03 (0.01–0.21) | 0 (0–0.48) | ||
| IOUS | 27 (82) | 0/33 (0) | 100 (0.84–1) | 85.7 (0.42–1) | 96.9 | 96.3 (0.79–1) | 100 (0.52–1) | 0.03 (0.01–0.21) | 0 (0–0.48) | |||
| Pathol | 26 (79) | |||||||||||
| IV | PreUS | 24 (72) | p < 0.0001 | 96 (0.78–1) | 100 (0.6–1) | 96.9 | 100 (0.83–1) | 89 (0.51–1) | 0 (0–0.17) | 0.11 (0.01–0.5) | ||
| IOUS | 25 (76) | 1/33 (3) | 7 | 100 (0.83–1) | 89 (0.6–1) | 100 | 100 (0.83–1) | 100 (0.6–1) | 0 (0–0.16) | 0 (0–0.4) | ||
| Pathol | 25 (76) | |||||||||||
| V | PreUS | 5 (15) | p < 0.0001 | 71.4 (0.3–0.95) | 100 (0.84–1) | 93.9 | 100 (0.46–1) | 92.9 (0.75–0.99) | 0 (0–0.54) | 0.07 (0.01–0.25) | ||
| IOUS | 8 (24) | 2/33 (6) | 2.5 | 100 (0.56–1) | 96.1 (0.78–1) | 100 | 87.5 (0.47–1) | 100 (0.83–1) | 0.125 (0.01–0.53) | 0 (0–0.17) | ||
| Pathol | 7 (21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meo, G.; Prete, F.P.; De Luca, G.M.; Pasculli, A.; Sgaramella, L.I.; Minerva, F.; Logoluso, F.A.; Calculli, G.; Gurrado, A.; Testini, M. The Value of Intraoperative Ultrasound in Selective Lateral Cervical Neck Lymphadenectomy for Papillary Thyroid Cancer: A Prospective Pilot Study. Cancers 2021, 13, 2737. https://doi.org/10.3390/cancers13112737
Di Meo G, Prete FP, De Luca GM, Pasculli A, Sgaramella LI, Minerva F, Logoluso FA, Calculli G, Gurrado A, Testini M. The Value of Intraoperative Ultrasound in Selective Lateral Cervical Neck Lymphadenectomy for Papillary Thyroid Cancer: A Prospective Pilot Study. Cancers. 2021; 13(11):2737. https://doi.org/10.3390/cancers13112737
Chicago/Turabian StyleDi Meo, Giovanna, Francesco Paolo Prete, Giuseppe Massimiliano De Luca, Alessandro Pasculli, Lucia Ilaria Sgaramella, Francesco Minerva, Francesco Antonio Logoluso, Giovanna Calculli, Angela Gurrado, and Mario Testini. 2021. "The Value of Intraoperative Ultrasound in Selective Lateral Cervical Neck Lymphadenectomy for Papillary Thyroid Cancer: A Prospective Pilot Study" Cancers 13, no. 11: 2737. https://doi.org/10.3390/cancers13112737
APA StyleDi Meo, G., Prete, F. P., De Luca, G. M., Pasculli, A., Sgaramella, L. I., Minerva, F., Logoluso, F. A., Calculli, G., Gurrado, A., & Testini, M. (2021). The Value of Intraoperative Ultrasound in Selective Lateral Cervical Neck Lymphadenectomy for Papillary Thyroid Cancer: A Prospective Pilot Study. Cancers, 13(11), 2737. https://doi.org/10.3390/cancers13112737







