Non-Invasive Biomarkers for Earlier Detection of Pancreatic Cancer—A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
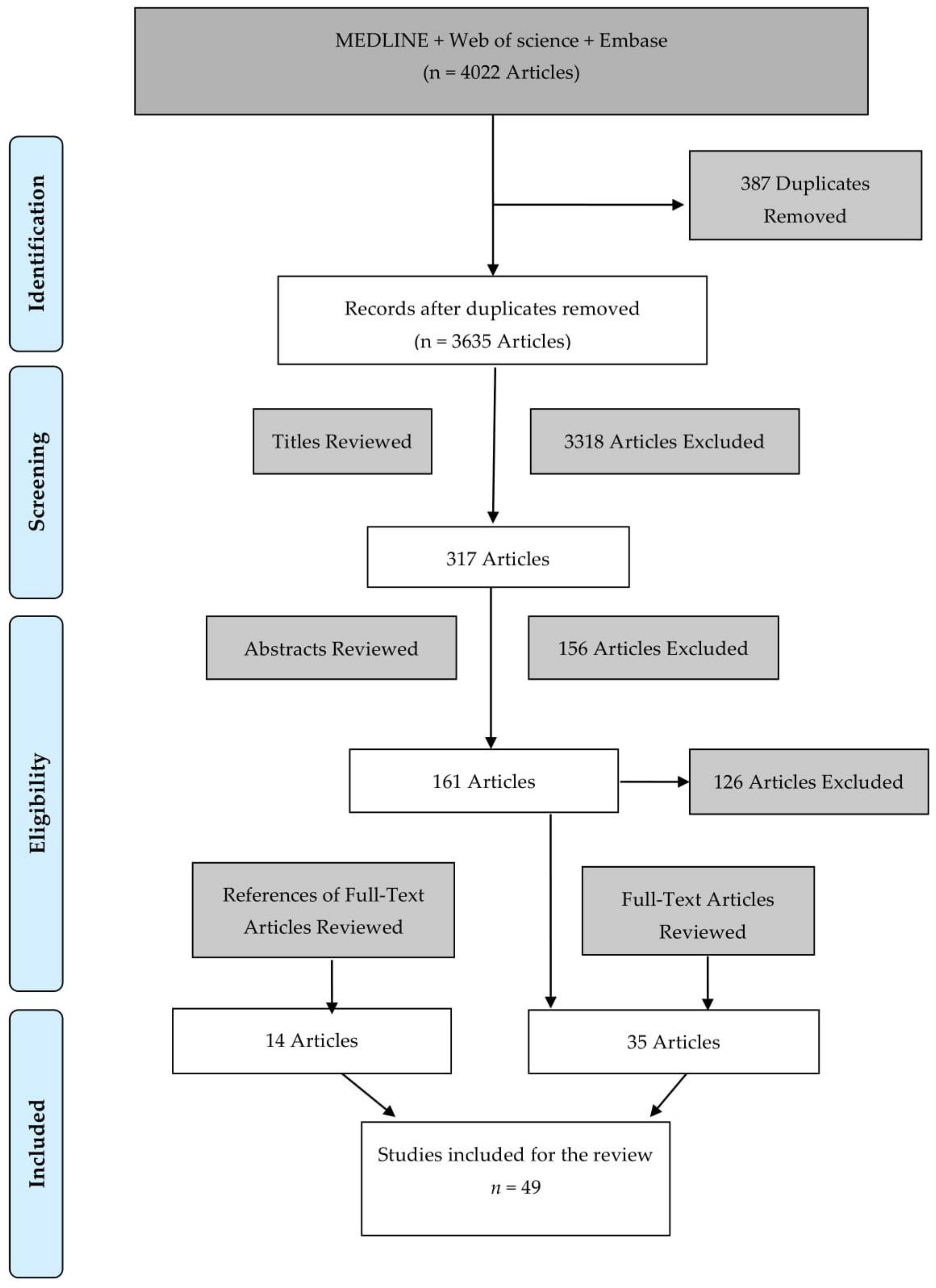
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
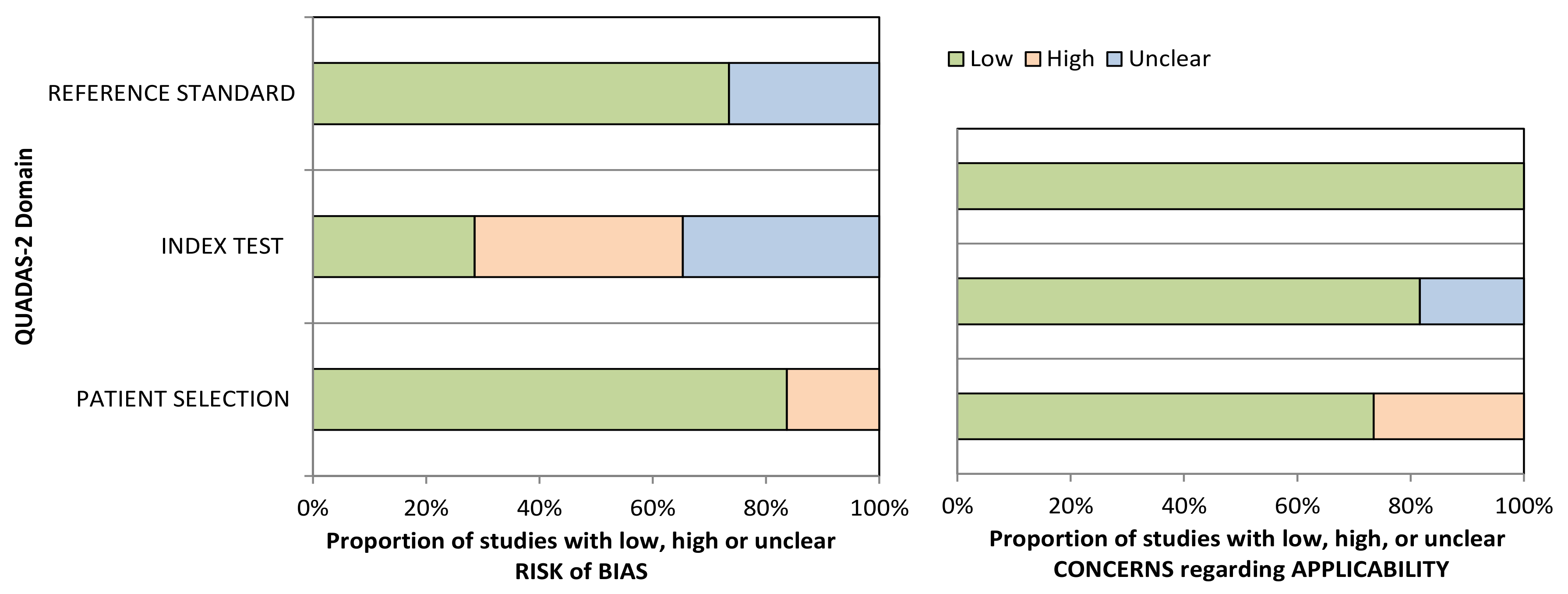
Appendix A
| Nr. | Study | Risk of Bias | Applicability Concerns | Score | ||||
|---|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Patient Selection | Index Test | Reference Standard | |||
| 1 | Gold et al., 2010 [37] | ☺ | ? | ? | ☺ | ☺ | ☺ | Low |
| 2 | Joergensen et al., 2010 [38] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 3 | Marten et al., 2010 [39] | ☹ | ? | ☺ | ☹ | ? | ☺ | High |
| 4 | Brand et al., 2011 [17] | ☺ | ☺ | ? | ☹ | ☺ | ☺ | Low |
| 5 | Park et al., 2012 [21] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 6 | Capello et al., 2013 [40] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Low |
| 7 | Gold et al., 2013 [41] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 8 | Kobayashi et al., 2013 [42] | ☺ | ☹ | ☺ | ☹ | ☺ | ☺ | Low |
| 9 | Li et al., 2013 [43] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 10 | Zhao et al., 2013 [44] | ☹ | ☹ | ? | ☺ | ☺ | ☺ | High |
| 11 | Chung et al., 2014 [45] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 12 | Lee et al., 2014 [46] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Low |
| 13 | Nolen et al., 2014 [47] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Low |
| 14 | Ren et al., 2014 [48] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 15 | Schultz et al., 2014 [49] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 16 | Yang et al., 2014 [50] | ☺ | ☹ | ? | ☹ | ? | ☺ | High |
| 17 | Zhang et al., 2014 [51] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 18 | Debernardi et al., 2015 [52] | ☹ | ? | ? | ☺ | ☺ | ☺ | High |
| 19 | Han et al., 2015 [53] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | Low |
| 20 | Melo et al., 2015 [54] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 21 | Radon et al., 2015 [55] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 22 | Ankeny et al., 2016 [56] | ☺ | ☹ | ? | ☺ | ? | ☺ | High |
| 23 | Guo et al., 2016 [57] | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ | Low |
| 24 | Henriksen et al., 2016 [58] | ☺ | ☹ | ? | ☺ | ☺ | ☺ | Low |
| 25 | Sogawa et al., 2016 [59] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | Low |
| 26 | Yoneyama et al., 2016 [60] | ☺ | ? | ☺ | ☹ | ☺ | ☺ | Low |
| 27 | Balasenthil et al., 2017 [61] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 28 | Yang et al., 2017 [62] | ☺ | ? | ☺ | ☺ | ? | ☺ | Low |
| 29 | Capello et al., 2017 [63] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 30 | Hussein et al., 2017 [64] | ☹ | ☹ | ☺ | ☹ | ☺ | ☺ | High |
| 31 | Kaur et al., 2017 [65] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 32 | Kim et al., 2017 [66] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 33 | Lai et al., 2017 [67] | ☹ | ☹ | ☺ | ☹ | ☺ | ☺ | High |
| 34 | Park et al., 2017 [68] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | Low |
| 35 | Schott et al., 2017 [69] | ☹ | ☹ | ? | ☺ | ☺ | ☺ | High |
| 36 | Arasaradnam et al., 2018 [70] | ☹ | ? | ? | ☺ | ☺ | ☺ | High |
| 37 | Dong et al., 2018 [71] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 38 | Guo et al., 2018 [72] | ☹ | ☹ | ? | ☺ | ☺ | ☺ | High |
| 39 | Mellby et al., 2018 [74] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | Low |
| 40 | Traeger et al., 2018 [75] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | Low |
| 41 | Zhou et al., 2018 [76] | ☺ | ☹ | ☺ | ☹ | ? | ☺ | High |
| 42 | Berger et al., 2019 [77] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 43 | Eissa et al., 2019 [78] | ☺ | ☹ | ? | ☺ | ☺ | ☺ | Low |
| 44 | Fahrmann et al., 2019 [79] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | Low |
| 45 | Lewis et al., 2019 [73] | ☺ | ? | ? | ☹ | ? | ☺ | High |
| 46 | Yu et al., 2019 [80] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | Low |
| 47 | Takahashi et al., 2019 [81] | ☺ | ☹ | ? | ☺ | ? | ☺ | High |
| 48 | Wei et al., 2019 [82] | ☺ | ☹ | ☺ | ☺ | ? | ☺ | Low |
| 49 | Yang et al., 2020 [83] | ☺ | ☺ | ☺ | ☺ | ? | ☺ | Low |
References
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- da Costa, W.L., Jr.; Oluyomi, A.O.; Thrift, A.P. Trends in the Incidence of Pancreatic Adenocarcinoma in All 50 United States Examined Through an Age-Period-Cohort Analysis. JNCI Cancer Spectr. 2020, 4, pkaa033. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Lin, Q.J.; Yang, F.; Jin, C.; Fu, D.L. Current status and progress of pancreatic cancer in China. World J. Gastroenterol. 2015, 21, 7988–8003. [Google Scholar] [CrossRef]
- Shen, G.Q.; Aleassa, E.M.; Walsh, R.M.; Morris-Stiff, G. Next-Generation Sequencing in Pancreatic Cancer. Pancreas 2019, 48, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Bortesi, L.; Pesci, A.; Bogina, G.; Castelli, P.; Zamboni, G. Ductal Adenocarcinoma of the Pancreas. Surg. Pathol. Clin. 2011, 4, 487–521. [Google Scholar] [CrossRef]
- CancerResearchUK. Pancreatic Cancer Statistics 2015–2017. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer (accessed on 15 July 2020).
- Pancreatic Cancer UK. Pancreatic Cancer Statistics. Available online: https://www.pancreaticcancer.org.uk/what-we-do/media-centre/pancreatic-cancer-statistics (accessed on 2 July 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jansen, L.; Balavarca, Y.; Molina-Montes, E.; Babaei, M.; van der Geest, L.; Lemmens, V.; Van Eycken, L.; De Schutter, H.; Johannesen, T.B.; et al. Resection of pancreatic cancer in Europe and USA: An international large-scale study highlighting large variations. Gut 2019, 68, 130. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yasui, K.; Matsueda, K.; Yanagisawa, A.; Yamao, K. Small carcinoma of the pancreas is curable: New computed tomography finding, pathological study and postoperative results from a single institute. J. Gastroenterol. Hepatol. 2005, 20, 1591–1594. [Google Scholar] [CrossRef]
- Ishikawa, O.; Ohigashi, H.; Imaoka, S.; Nakaizumi, A.; Uehara, H.; Kitamura, T.; Kuroda, C. Minute carcinoma of the pancreas measuring 1 cm or less in diameter—Collective review of Japanese case reports. Hepatogastroenterology 1999, 46, 8–15. [Google Scholar]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef]
- Ghaneh, P.; Costello, E.; Neoptolemos, J.P. Biology and management of pancreatic cancer. Gut 2007, 56, 1134–1152. [Google Scholar] [CrossRef]
- Brand, R.E.; Nolen, B.M.; Zeh, H.J.; Allen, P.J.; Eloubeidi, M.A.; Goldberg, M.; Elton, E.; Arnoletti, J.P.; Christein, J.D.; Vickers, S.M.; et al. Serum biomarker panels for the detection of pancreatic cancer. Clin. Cancer Res. 2011, 17, 805–816. [Google Scholar] [CrossRef]
- Chan, A.; Diamandis, E.P.; Blasutig, I.M. Strategies for discovering novel pancreatic cancer biomarkers. J. Proteom. 2013, 81, 126–134. [Google Scholar] [CrossRef]
- Tempero, M.A.; Uchida, E.; Takasaki, H.; Burnett, D.A.; Steplewski, Z.; Pour, P.M. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987, 47, 5501–5503. [Google Scholar]
- FDA. FDA 510K Summary. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/K020566.pdf (accessed on 20 February 2021).
- Park, H.-D.; Kang, E.-S.; Kim, J.-W.; Lee, K.-T.; Lee, K.H.; Park, Y.S.; Park, J.-O.; Lee, J.; Heo, J.S.; Choi, S.H.; et al. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics 2012, 12, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Ritts, R.E.; Pitt, H.A. CA 19-9 in pancreatic cancer. Surg. Oncol. Clin. N. Am. 1998, 7, 93–101. [Google Scholar] [CrossRef]
- Duffy, M.J.; van Dalen, A.; Haglund, C.; Hansson, L.; Klapdor, R.; Lamerz, R.; Nilsson, O.; Sturgeon, C.; Topolcan, O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur. J. Cancer 2003, 39, 718–727. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Passerini, R.; Cassatella, M.C.; Boveri, S.; Salvatici, M.; Radice, D.; Zorzino, L.; Galli, C.; Sandri, M.T. The pitfalls of CA19-9: Routine testing and comparison of two automated immunoassays in a reference oncology center. Am. J. Clin. Pathol. 2012, 138, 281–287. [Google Scholar] [CrossRef]
- Duffy, M.J.; Sturgeon, C.; Lamerz, R.; Haglund, C.; Holubec, V.L.; Klapdor, R.; Nicolini, A.; Topolcan, O.; Heinemann, V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2010, 21, 441–447. [Google Scholar] [CrossRef]
- Herlyn, M.; Steplewski, Z.; Herlyn, D.; Koprowski, H. Colorectal carcinoma-specific antigen: Detection by means of monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1979, 76, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Hotakainen, K.; Tanner, P.; Alfthan, H.; Haglund, C.; Stenman, U.H. Comparison of three immunoassays for CA 19-9. Clin. Chim. Acta 2009, 400, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, W. The clinical utility of the CA 19-9 tumor-associated antigen. Am. J. Gastroenterol. 1990, 85, 350–355. [Google Scholar]
- Stern, P.; Friedecky, B.; Bartos, V.; Bezdickova, D.; Vavrova, J.; Uhrova, J.; Rozprimova, L.; Zima, T.; Palicka, V. Comparison of different immunoassays for CA 19-9. Clin. Chem. Lab. Med. 2001, 39, 1278–1282. [Google Scholar] [CrossRef]
- Goh, S.K.; Gold, G.; Christophi, C.; Muralidharan, V. Serum carbohydrate antigen 19-9 in pancreatic adenocarcinoma: A mini review for surgeons. ANZ J. Surg. 2017, 87, 987–992. [Google Scholar] [CrossRef]
- Jain, K.K. The Handbook of Biomarkers; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Young, M.R.; Wagner, P.D.; Ghosh, S.; Rinaudo, J.A.; Baker, S.G.; Zaret, K.S.; Goggins, M.; Srivastava, S. Validation of Biomarkers for Early Detection of Pancreatic Cancer: Summary of The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas 2018, 47, 135–141. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef]
- Gold, D.V.; Goggins, M.; Modrak, D.E.; Newsome, G.; Liu, M.; Shi, C.; Hruban, R.H.; Goldenberg, D.M. Detection of early-stage pancreatic adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2786–2794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joergensen, M.T.; Brunner, N.; De Muckadell, O.B.S. Comparison of Circulating MMP-9, TIMP-1 and CA19-9 in the Detection of Pancreatic Cancer. Anticancer Res. 2010, 30, 587–592. [Google Scholar] [PubMed]
- Marten, A.; Buchler, M.W.; Werft, W.; Wente, M.N.; Kirschfink, M.; Schmidt, J. Soluble iC3b as an Early Marker for Pancreatic Adenocarcinoma Is Superior to CA19.9 and Radiology. J. Immunother. 2010, 33, 219–224. [Google Scholar] [CrossRef]
- Capello, M.; Cappello, P.; Linty, F.C.; Chiarle, R.; Sperduti, I.; Novarino, A.; Salacone, P.; Mandili, G.; Naccarati, A.; Sacerdote, C.; et al. Autoantibodies to Ezrin are an early sign of pancreatic cancer in humans and in genetically engineered mouse models. J. Hematol. Oncol. 2013, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.V.; Gaedcke, J.; Ghadimi, B.M.; Goggins, M.; Hruban, R.H.; Liu, M.L.; Newsome, G.; Goldenberg, D.M. PAM4 enzyme immunoassay alone and in combination with CA 19-9 for the detection of pancreatic adenocarcinoma. Cancer 2013, 119, 522–528. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishiumi, S.; Ikeda, A.; Yoshie, T.; Sakai, A.; Matsubara, A.; Izumi, Y.; Tsumura, H.; Tsuda, M.; Nishisaki, H.; et al. A novel serum metabolomics-based diagnostic approach to pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Li, A.; Yu, J.; Kim, H.; Wolfgang, C.L.; Canto, M.I.; Hruban, R.H.; Goggins, M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin. Cancer Res. 2013, 19, 3600–3610. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; Zhang, S.; Yu, D.; Chen, Y.; Liu, Q.; Shi, M.; Ni, C.; Zhu, M. Diagnostic and biological significance of microRNA-192 in pancreatic ductal adenocarcinoma. Oncol. Rep. 2013, 30, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lim, J.B. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. J. Transl. Med. 2014, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Na, K.; Jeong, S.K.; Lim, J.S.; Kim, S.A.; Song, S.Y.; Kim, H.; Hancock, W.S.; Paik, Y.K. Identification of human complement factor B as a novel biomarker candidate for pancreatic ductal adenocarcinoma. J. Proteome Res. 2014, 13, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Brand, R.E.; Prosser, D.; Velikokhatnaya, L.; Allen, P.J.; Zeh, H.J.; Grizzle, W.E.; Huang, Y.; Lomakin, A.; Lokshin, A.E. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS ONE 2014, 9, e94928. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Chen, Y.; Han, C.; Fu, D.; Chen, H. Plasma interleukin-11 (IL-11) levels have diagnostic and prognostic roles in patients with pancreatic cancer. Tumour Biol. 2014, 35, 11467–11472. [Google Scholar] [CrossRef]
- Schultz, N.A.; Dehlendorff, C.; Jensen, B.V.; Bjerregaard, J.K.; Nielsen, K.R.; Bojesen, S.E.; Calatayud, D.; Nielsen, S.E.; Yilmaz, M.; Holländer, N.H.; et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014, 311, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Sun, Y.W.; Liu, D.J.; Zhang, J.F.; Li, J.; Hua, R. MicroRNAs in stool samples as potential screening biomarkers for pancreatic ductal adenocarcinoma cancer. Am. J. Cancer Res. 2014, 4, 663–673. [Google Scholar] [PubMed]
- Zhang, P.J.; Zou, M.; Wen, X.Y.; Gu, F.; Li, J.; Liu, G.X.; Dong, J.X.; Deng, X.X.; Gao, J.; Li, X.L.; et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int. J. Cancer 2014, 134, 2646–2655. [Google Scholar] [CrossRef]
- Debernardi, S.; Massat, N.J.; Radon, T.P.; Sangaralingam, A.; Banissi, A.; Ennis, D.P.; Dowe, T.; Chelala, C.; Pereira, S.P.; Kocher, H.M.; et al. Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. Am. J. Cancer Res. 2015, 5, 3455–3466. [Google Scholar]
- Han, S.X.; Zhou, X.; Sui, X.; He, C.C.; Cai, M.J.; Ma, J.L.; Zhang, Y.Y.; Zhou, C.Y.; Ma, C.X.; Varela-Ramirez, A.; et al. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget 2015, 6, 19907–19917. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Radon, T.P.; Massat, N.J.; Jones, R.; Alrawashdeh, W.; Dumartin, L.; Ennis, D.; Duffy, S.W.; Kocher, H.M.; Pereira, S.P.; Guarner, L.; et al. Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma. Clin. Cancer Res. 2015, 21, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, J.S.; Court, C.M.; Hou, S.; Li, Q.; Song, M.; Wu, D.; Chen, J.F.; Lee, T.; Lin, M.; Sho, S.; et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 2016, 114, 1367–1375. [Google Scholar] [CrossRef]
- Guo, X.; Lv, X.; Fang, C.; Wang, F.; Wang, D.; Zhao, J.; Ma, Y.; Xue, Y.; Bai, Q.; Yao, X.; et al. Dysbindin as a novel biomarker for pancreatic ductal adenocarcinoma identified by proteomic profiling. Int. J. Cancer 2016, 139, 1821–1829. [Google Scholar] [CrossRef]
- Henriksen, S.D.; Madsen, P.H.; Larsen, A.C.; Johansen, M.B.; Drewes, A.M.; Pedersen, I.S.; Krarup, H.; Thorlacius-Ussing, O. Cell-free DNA promoter hypermethylation in plasma as a diagnostic marker for pancreatic adenocarcinoma. Clin. Epigenetics 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Sogawa, K.; Takano, S.; Iida, F.; Satoh, M.; Tsuchida, S.; Kawashima, Y.; Yoshitomi, H.; Sanda, A.; Kodera, Y.; Takizawa, H.; et al. Identification of a novel serum biomarker for pancreatic cancer, C4b-binding protein α-chain (C4BPA) by quantitative proteomic analysis using tandem mass tags. Br. J. Cancer 2016, 115, 949–956. [Google Scholar] [CrossRef]
- Yoneyama, T.; Ohtsuki, S.; Honda, K.; Kobayashi, M.; Iwasaki, M.; Uchida, Y.; Okusaka, T.; Nakamori, S.; Shimahara, M.; Ueno, T.; et al. Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics. PLoS ONE 2016, 11, e0161009. [Google Scholar] [CrossRef] [PubMed]
- Balasenthil, S.; Huang, Y.; Liu, S.; Marsh, T.; Chen, J.; Stass, S.A.; KuKuruga, D.; Brand, R.; Chen, N.; Frazier, M.L.; et al. A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Im, H.; Hong, S.; Pergolini, I.; del Castillo, A.F.; Wang, R.; Clardy, S.; Huang, C.-H.; Pille, C.; Ferrone, S.; et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med. 2017, 9, eaal3226. [Google Scholar] [CrossRef] [PubMed]
- Capello, M.; Bantis, L.; Scelo, G.; Zhao, Y.; Dhillon, D.; Wang, H.; Abbruzzese, J.; Maitra, A.; Tempero, M.; Brand, R.; et al. Sequential validation of bloodbased protein biomarker candidates for early-stage pancreatic cancer. In Proceedings of the 107th Annual Meeting of the American Association for Cancer Research, AACR 2016, New Orleans, LA, USA, 16–20 April 2016; Volume 76. [Google Scholar]
- Hussein, N.A.; Kholy, Z.A.; Anwar, M.M.; Ahmad, M.A.; Ahmad, S.M. Plasma miR-22-3p, miR-642b-3p and miR-885-5p as diagnostic biomarkers for pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 83–93. [Google Scholar] [CrossRef]
- Kaur, S.; Smith, L.; Patel, A.; Menning, M.; Watley, D.; Malik, S.; Krishn, S.; Mallya, K.; Aithal, A.; Sasson, A.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am. J. Gastroenterol. 2017, 112, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bamlet, W.R.; Oberg, A.L.; Chaffee, K.G.; Donahue, G.; Cao, X.J.; Chari, S.; Garcia, B.A.; Petersen, G.M.; Zaret, K.S. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci. Transl. Med. 2017, 9, eaah5583. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.Y.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Namkung, J.; Yi, S.G.; Kim, H.; Yu, J.; Kim, Y.; Kwon, M.S.; Kwon, W.; Oh, D.Y.; et al. Diagnostic performance enhancement of pancreatic cancer using proteomic multimarker panel. Oncotarget 2017, 8, 93117–93130. [Google Scholar] [CrossRef]
- Schott, S.; Yang, R.X.; Stocker, S.; Canzian, F.; Giese, N.; Bugert, P.; Bergmann, F.; Strobel, O.; Hackert, T.; Sohn, C.; et al. HYAL2 methylation in peripheral blood as a potential marker for the detection of pancreatic cancer-a case control study. Oncotarget 2017, 8, 67614–67625. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Wicaksono, A.; O’Brien, H.; Kocher, H.M.; Covington, J.A.; Crnogorac-Jurcevic, T. Noninvasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology 2018, 154, 485–487.e481. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Jia, L.; Zhang, L.; Ma, N.; Zhang, A.; Zhou, Y.; Ren, L. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci. 2018, 109, 2841–2851. [Google Scholar] [CrossRef]
- Guo, X.B.; Yin, H.S.; Wang, J.Y. Evaluating the diagnostic and prognostic value of long non-coding RNA SNHG15 in pancreatic ductal adenocarcinoma. Eur. Rev. Med. Pharm. Sci. 2018, 22, 5892–5898. [Google Scholar] [CrossRef]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef]
- Mellby, L.D.; Nyberg, A.P.; Johansen, J.S.; Wingren, C.; Nordestgaard, B.G.; Bojesen, S.E.; Mitchell, B.L.; Sheppard, B.C.; Sears, R.C.; Borrebaeck, C.A.K. Serum Biomarker Signature-Based Liquid Biopsy for Diagnosis of Early-Stage Pancreatic Cancer. J. Clin. Oncol. 2018, 36, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Michael Traeger, M.; Rehkaemper, J.; Ullerich, H.; Steinestel, K.; Wardelmann, E.; Senninger, N.; Abdallah Dhayat, S. The ambiguous role of microRNA-205 and its clinical potential in pancreatic ductal adenocarcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2419–2431. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Dong, Y.P.; Sun, X.; Sui, X.; Zhu, H.; Zhao, Y.Q.; Zhang, Y.Y.; Mason, C.; Zhu, Q.; Han, S.X. High levels of serum glypican-1 indicate poor prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2018, 7, 5525–5533. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Reinacher-Schick, A.; Uhl, W.; Algül, H.; Friess, H.; Janssen, K.-P.; König, A.; Ghadimi, M.; Gallmeier, E.; et al. A Blood-Based Multi Marker Assay Supports the Differential Diagnosis of Early-Stage Pancreatic Cancer. Theranostics 2019, 9, 1280–1287. [Google Scholar] [CrossRef]
- Eissa, M.A.L.; Lerner, L.; Abdelfatah, E.; Shankar, N.; Canner, J.K.; Hasan, N.M.; Yaghoobi, V.; Huang, B.; Kerner, Z.; Takaesu, F.; et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin. Epigenetics 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Bantis, L.E.; Capello, M.; Scelo, G.; Dennison, J.B.; Patel, N.; Murage, E.; Vykoukal, J.; Kundnani, D.L.; Foretova, L.; et al. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2019, 111, 372–379. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Liao, Z.; Wang, Z.; Wang, Z.; Li, Y.; Qian, L.; Zhao, J.; Zong, H.; Kang, B.; et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut 2020, 69, 540–550. [Google Scholar] [CrossRef]
- Takahashi, K.; Ota, Y.; Kogure, T.; Suzuki, Y.; Iwamoto, H.; Yamakita, K.; Kitano, Y.; Fujii, S.; Haneda, M.; Patel, T.; et al. Circulating extracellular vesicle-encapsulated HULC is a potential biomarker for human pancreatic cancer. Cancer Sci. 2020, 111, 98–111. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, X.; Zhang, Q.; Yang, J.; Chen, Q.; Wang, J.; Li, X.; Chen, J.; Ma, T.; Li, G.; et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 2019, 452, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; LaRiviere, M.J.; Ko, J.; Till, J.E.; Christensen, T.; Yee, S.S.; Black, T.A.; Tien, K.; Lin, A.; Shen, H.; et al. A Multianalyte Panel Consisting of Extracellular Vesicle miRNAs and mRNAs, cfDNA, and CA19-9 Shows Utility for Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 3248–3258. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Holmes, M.V.; Chen, Z.; Kartsonaki, C. A review of lifestyle, metabolic risk factors, and blood-based biomarkers for early diagnosis of pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2019, 34, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014, 20, 11182–11198. [Google Scholar] [CrossRef]
- Ghatnekar, O.; Andersson, R.; Svensson, M.; Persson, U.; Ringdahl, U.; Zeilon, P.; Borrebaeck, C.A. Modelling the benefits of early diagnosis of pancreatic cancer using a biomarker signature. Int. J. Cancer 2013, 133, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Frampton, A.E.; Prado, M.M.; López-Jiménez, E.; Fajardo-Puerta, A.B.; Jawad, Z.A.R.; Lawton, P.; Giovannetti, E.; Habib, N.A.; Castellano, L.; Stebbing, J.; et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 2018, 9, 19006–19013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Wang, W.Q.; Han, X.; Gao, H.L.; Li, T.J.; Xu, S.S.; Li, S.; Xu, H.X.; Li, H.; Ye, L.Y.; et al. Advances on diagnostic biomarkers of pancreatic ductal adenocarcinoma: A systems biology perspective. Comput. Struct. Biotechnol. J. 2020, 18, 3606–3614. [Google Scholar] [CrossRef]
- Almeida, P.P.; Cardoso, C.P.; de Freitas, L.M. PDAC-ANN: An artificial neural network to predict pancreatic ductal adenocarcinoma based on gene expression. BMC Cancer 2020, 20, 82. [Google Scholar] [CrossRef]
- Muhammad, W.; Hart, G.R.; Nartowt, B.; Farrell, J.J.; Johung, K.; Liang, Y.; Deng, J. Pancreatic Cancer Prediction Through an Artificial Neural Network. Front. Artif. Intell. 2019, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Marya, N.B.; Powers, P.D.; Chari, S.T.; Gleeson, F.C.; Leggett, C.L.; Abu Dayyeh, B.K.; Chandrasekhara, V.; Iyer, P.G.; Majumder, S.; Pearson, R.K.; et al. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Bhasin, M.K. A Transcriptomics-Based Meta-Analysis Combined With Machine Learning Identifies a Secretory Biomarker Panel for Diagnosis of Pancreatic Adenocarcinoma. Front. Genet. 2020, 11, 572284. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.Y.; Correa, E.; Yoshimura, K.; Chang, M.C.; Dennison, A.; Takeda, S.; Chang, Y.T. Using probe electrospray ionization mass spectrometry and machine learning for detecting pancreatic cancer with high performance. Am. J. Transl. Res. 2020, 12, 171–179. [Google Scholar] [PubMed]
- Sollie, S.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Santaolalla, A.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer 2019, 19, 858. [Google Scholar] [CrossRef]
- de la Fuente, J.; Sharma, A.; Chari, S.; Majumder, S. Peripheral blood monocyte counts are elevated in the pre-diagnostic phase of pancreatic cancer: A population based study. Pancreatology 2019, 19, 1043–1048. [Google Scholar] [CrossRef]
- Cui, Y.; Shu, X.O.; Li, H.L.; Yang, G.; Wen, W.; Gao, Y.T.; Cai, Q.; Rothman, N.; Yin, H.Y.; Lan, Q.; et al. Prospective study of urinary prostaglandin E2 metabolite and pancreatic cancer risk. Int. J. Cancer 2017, 141, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Elliott, V.L.; Evans, A.; Oldfield, L.; Jenkins, R.E.; O’Brien, D.P.; Apostolidou, S.; Gentry-Maharaj, A.; Fourkala, E.O.; Jacobs, I.J.; et al. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin. Cancer Res. 2016, 22, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Sandanayake, N.S.; Jenkinson, C.; Gentry-Maharaj, A.; Apostolidou, S.; Fourkala, E.O.; Camuzeaux, S.; Blyuss, O.; Gunu, R.; Dawnay, A.; et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: Implications for early disease detection. Clin. Cancer Res. 2015, 21, 622–631. [Google Scholar] [CrossRef]
- Capello, M.; Bantis, L.E.; Scelo, G.; Zhao, Y.; Li, P.; Dhillon, D.S.; Patel, N.J.; Kundnani, D.L.; Wang, H.; Abbruzzese, J.L.; et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109, djw266. [Google Scholar] [CrossRef] [PubMed]
- Mirus, J.E.; Zhang, Y.; Li, C.I.; Lokshin, A.E.; Prentice, R.L.; Hingorani, S.R.; Lampe, P.D. Cross-species antibody microarray interrogation identifies a 3-protein panel of plasma biomarkers for early diagnosis of pancreas cancer. Clin. Cancer Res. 2015, 21, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Magis, A.T.; Rappaport, N.; Conomos, M.P.; Omenn, G.S.; Lovejoy, J.C.; Hood, L.; Price, N.D. Untargeted longitudinal analysis of a wellness cohort identifies markers of metastatic cancer years prior to diagnosis. Sci. Rep. 2020, 10, 16275. [Google Scholar] [CrossRef]
- Moore, H.M.; Kelly, A.B.; Jewell, S.D.; McShane, L.M.; Clark, D.P.; Greenspan, R.; Hayes, D.F.; Hainaut, P.; Kim, P.; Mansfield, E.A.; et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011, 119, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and elaboration. PLoS Med. 2012, 9, e1001216. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Crosby, D.; Lyons, N.; Greenwood, E.; Harrison, S.; Hiom, S.; Moffat, J.; Quallo, T.; Samuel, E.; Walker, I. A roadmap for the early detection and diagnosis of cancer. Lancet Oncol. 2020, 21, 1397–1399. [Google Scholar] [CrossRef]
- Kunovsky, L.; Tesarikova, P.; Kala, Z.; Kroupa, R.; Kysela, P.; Dolina, J.; Trna, J. The Use of Biomarkers in Early Diagnostics of Pancreatic Cancer. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5389820. [Google Scholar] [CrossRef] [PubMed]
- Paulovich, A.G.; Whiteaker, J.R.; Hoofnagle, A.N.; Wang, P. The interface between biomarker discovery and clinical validation: The tar pit of the protein biomarker pipeline. Proteom. Clin. Appl. 2008, 2, 1386–1402. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Root, A.; Allen, P.; Tempst, P.; Yu, K. Protein Biomarkers for Early Detection of Pancreatic Ductal Adenocarcinoma: Progress and Challenges. Cancers 2018, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Immunovia. Available online: https://immunovia.com/about-us (accessed on 25 August 2020).
- Debernardi, S.; O’Brien, H.; Algahmdi, A.S.; Malats, N.; Stewart, G.D.; Plješa-Ercegovac, M.; Costello, E.; Greenhalf, W.; Saad, A.; Roberts, R.; et al. A combination of urinary biomarker panel and PancRISK score for earlier detection of pancreatic cancer: A case–control study. PLoS Med. 2020, 17, e1003489. [Google Scholar] [CrossRef] [PubMed]
- Blyuss, O.; Zaikin, A.; Cherepanova, V.; Munblit, D.; Kiseleva, E.M.; Prytomanova, O.M.; Duffy, S.W.; Crnogorac-Jurcevic, T. Development of PancRISK, a urine biomarker-based risk score for stratified screening of pancreatic cancer patients. Br. J. Cancer 2020, 122, 692–696. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Early Detection of Pancreatic Adenocarcinoma (PDAC) Using a Panel of Biomarkers (UroPanc). Available online: https://clinicaltrials.gov/ct2/show/NCT04449406 (accessed on 3 March 2021).
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Consortium, C. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Killock, D. CancerSEEK and destroy—A blood test for early cancer detection. Nat. Rev. Clin. Oncol. 2018, 15, 133. [Google Scholar] [CrossRef]
| Inclusion Criteria |
|---|
| 1. Pancreatic Ductal Adenocarcinoma |
| 2. Non-invasive method of obtaining a liquid biopsy e.g., plasma, serum, urine, saliva, stool |
| 3. Original data with reported AUC, SN and SP of a proposed biomarker |
| 4. Human studies |
| 5. Manuscripts from January 2010 until August 2020 |
| Exclusion Criteria |
| 1. No specification of what type of pancreatic cancer it was |
| 2. Invasive procedures to obtain the biomarker e.g., tissue biopsy |
| 3. No recorded data either of SN, SP and/or AUC for the tested biomarker |
| 4. Biomarker used for a purpose other than detection e.g., prognostic biomarkers |
| 5. Abstracts, Conference reports/writings, NHS reports, Review Articles |
| Reference | Specimen Type | Biomarker | Clinical Setting | Subjects | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|---|---|---|
| Gold et al., 2010 [37] | Serum | PAM4 | 68 PDAC, 19 HC | PDAC vs. HC | 82.0 | 95.0 | 0.92 (0.84–0.97) |
| Joergensen et al., 2010 [38] | Serum | CA19-9 MMP-9 TIMP1 | 51 PDAC, 52 HC | PDAC vs. HC | 86.0 58.8 47.1 | 73.0 34.6 69.2 | 0.84 (0.77–0.92) 0.50 (0.39–0.61) 0.64 (0.53–0.74) |
| Marten et al., 2010 [39] | Plasma | siC3b | 157 PDAC, 38 HC | 2–4mo prior radiologically defined recurrence | 54.0 | 94.0 | 0.85 |
| siC3b | 0–2mo prior radiologically defined recurrence | 62.0 | 94.0 | 0.84 | |||
| Brand et al., 2011 [17] | Serum | CA19-9 CA19-9 + ICAM-1 + OPG CA19-9 + CEA +TIMP-1 | 160 PDAC, 74 BPD, 107 HC | TS: PDAC vs. HC PDAC vs. BPD PDAC vs. HC | 57,2 88.0 76.0 | 90.0 90.0 90.0 | 0.83 (0.81–0.86) 0.93 (0.91–0.95) 0.86 |
| CA19-9 CA19-9 + ICAM-1 + OPG CA19-9 + CEA +TIMP-1 CA19-9 | 173 PDAC, 70 BPD, 120 HC | VS: PDAC vs. BPD PDAC vs. HC PDAC vs. BPC PDAC vs. BPC | 56.4 78.0 71.2 52.1 | 90.0 94.1 88.6 90.2 | 0.82 (0.78–0.86) 0.91 (0.88–0.95) 0.83 (0.88–0.89) 0.78 (0.74–0.83) | ||
| Park et al., 2012 [21] | Serum | Cathepsin D MMP-7 CA19-9 CA19-9 + Cathepsin D + MMP-7 | 109 PDAC, 40 HC, 30 CP | TS: PDAC vs. HC + CP | 54.0 72.0 74.0 88.0 | 80.0 80.0 80.0 80.0 | 0.67 0.81 0.84 0.90 (p = 0.002) |
| Cathepsin D MMP-7 CA19-9 CA19-9 + Cathepsin D + MMP-7 | 129 PDAC, 74 HC, 72 CP | VS: PDAC vs. HC + CP | 53.0 65.0 78.0 89.0 | 79.0 79.0 84.0 77.0 | 0.65 0.77 0.88 0.91 (p = 0.002) | ||
| Capello et al., 2013 [40] | Serum | EZR-autoantibody | 69 PDAC, 46 CP, 60 HC, 12 Aim, 50 Non-PDAC | PDAC vs. HC + CP + Aim | 93.2 | 75.5 | 0.90 |
| PDAC vs. non-PDAC cancer | 94.9 | 96.4 | 0.99 | ||||
| Gold et al., 2013 [41] | Serum | PAM4 CA19-9 PAM4 + CA19-9 | 298 PDAC, 120 BPD, 50 CP | PDAC vs. BPD | 74.0 77.0 84.0 | 85.0 73.0 83.0 | 0.87 (p = 0.0001) 0.85 (p = 0.0257) 0.91 |
| PAM4 CA19-9 PAM4 + CA19-9 | PDAC vs. CP | 74.0 77.0 84.0 | 86.0 68.0 82.0 | 0.87 (p = 0.0001) 0.84 (p = 0.0073) 0.91 | |||
| Kobayashi et al., 2013 [42] | Serum | Diagnostic Model * CA19-9 CEA Diagnostic Model * CA19-9 CEA | 43 PDAC, 42 HC | TS: PDAC vs. HC | 86.0 62.8 44.2 | 88.1 100.0 97.6 | 0.93 (0.86–0.97) 0.82 (0.70–0.90) 0.80 (0.69–0.88) |
| 9 PDAC (stage 0-IIB), 41 HC, 23 CP | VS: PDAC stage 0-IIB vs. HC + CP | 77.8 55.6 44.4 | 78.1 85.9 79.7 | 0.76 (0.66–0.86) 0.79 (0.68–0.88) 0.67 (0.55–0.76) | |||
| Li et al., 2013 [43] | Serum | CA19-9 miR-1290 miR-146a miR-484 | 41 PDAC, 19 HC, 35 CP | PC vs. HC | 71.0 88.0 78.0 76.0 | 90.0 84.0 79.0 63.0 | 0.86 0.96 (0.91–1.00) 0.82 (0.71–0.92) 0.78 |
| CA19-9 miR-1290 miR-146a miR-484 | PDAC vs. CP | 71.0 83.0 73.0 75.0 | 63.0 69.0 80.0 69.0 | 0.71 0.81 (0.71–0.91) 0.78 (0.68–0.89) 0.75 | |||
| Zhao et al., 2013 [44] | Serum | miR -192 | 70 PDAC, 40 HC | PDAC vs. HC | 76.0 | 55.0 | 0.63 (0.51–0.75) |
| Chung et al., 2014 [45] | Serum | sCD40L CA19-9 CEA | 55 PDAC, 30 CP, 30 HC | VS: PDAC vs. CP vs. HC | 80 80 68.9 | 85.5 72.7 60 | 0.88 0.78 0.70 |
| Lee et al., 2014 [46] | Serum | CFB CA19-9 CFB + CA19-9 | 41 PDAC, 44 HC, 12 CP, 31 HCC, 22 CC, 35 GC | PDAC vs. non-PDAC | 73.1 80.4 90.1 | 97.9 70.0 97.2 | 0.958 (0.956–0.959) 0.833 (0.829–0.837) 0.986 (p < 0.001) |
| Nolen et al., 2014 [47] | Serum | CA19-9 CA19-9 + CEA CA19-9 + CEA + Cyfra 21-1 | 343 PDAC, 227 HC | 1–12 months Pre-diagnostic PDAC vs. HC | 25.7 26.7 32.4 | 95.0 95.0 95.0 | 0.680 0.67 0.69 |
| CA19-9 CA19-9 + CEA CA19-9 + CEA + Cyfra 21-1 | 12–35 months Pre-diagnostic PDAC vs. HC | 17.2 28.1 29.7 | 95.0 95.0 95.0 | 0.63 0.66 0.66 | |||
| Ren et al., 2014 [48] | Serum | IL-11p | 44 PDAC, 30 HC | PDAC vs. HC | 97.7 | 70.0 | 0.901 (p < 0.001) |
| Schultz et al., 2014 [49] | Serum | Index 1 (miR-145, -150, -223, -636) Index 2 (miR-26b, -34a, -122, -126, -145, -150, -223, -505, -636, -885.5p) CA19-9 Index 1 + CA19-9 Index 2 + CA19-9 | 143 PDAC, 18 CP, 69 HC | DC: PDAC vs. HC + CP | 85.0 | 73.0 | 0.88 (0.85–0.92) |
| 85.0 88.0 85.0 85.0 | 86.0 92.0 93.0 97.0 | 0.93 (0.89–0.96) 0.87 (0.82–0.92) 0.88 (0.83–0.93) 0.95 (0.92–0.98) | |||||
| Index 1 Index 2 CA19-9 Index 1 + CA19-9 Index 2 + CA19-9 | 180 PDAC, 199 HC | TS: PDAC vs. HC | 85.0 85.0 86.0 85.0 85.0 | 64.0 85.0 99.0 95.0 98.0 | 0.86 (0.82–0.90) 0.93 (0.89–0.96) 0.90 (0.87–0.94) 0.93 (0.90–0.96) 0.97 (0.95–0.98) | ||
| Index 1 Index 2 CA19-9 Index 1 + CA19-9 Index 2 + CA19-9 | 86 PDAC, 7 CP, 44 HC | VS: PDAC vs. HC + CP | 85.0 85.0 79.0 85.0 85.0 | 45.0 51.0 88.0 88.0 86.0 | 0.83 (0.76–0.90) 0.81 (0.73–0.87) 0.89 (0.83–0.95) 0.93 (0.88–0.97) 0.92 (0.87–0.96) | ||
| Yang et al., 2014 [50] | Stool | miR-21 miR-155 miR -216 miR-216 + miR-21+ miR-155 | 30 PDAC, 15 HC | PDAC vs. HC | 90.0 76.7 86.7 83.3 | 66.7 73.3 60.0 83.3 | 0.80 (0.68–0.92) 0.72 (0.58–0.86) 0.73 (0.60–0.86) 0.87 (0.77–0.96) |
| Zhang et al., 2014 [51] | Serum | CA19-9 + Albumin + CRP + IL-8 CA19-9 CA19-9 + Albumin + CRP + IL-8 CA19-9 | 163 PDAC (77 Early stage I-II), 109 BC, 200 HC | All stage PDAC vs. HC All stage PDAC vs. HC Early stage PDAC vs. HC Early stage PDAC vs. HC | 99.4 80.6 96.1 72.7 | 90.0 90.0 90.0 90.0 | 0.98 (0.97–1.00) 0.85 (0.80–0.90) 0.97 (0.95–1.00) 0.83 (0.75–0.90) |
| CA19-9 + CO2 +CRP + IL-6 CA19-9 CA19-9 + CO2 +CRP + IL-6 CA19-9 | All stage PDAC vs. BC All stage PDAC vs. BC Early stage PDAC vs. BC Early stage PDAC vs. BC | 74.2 53.4 75.3 40.3 | 90.0 90.0 90.0 90.0 | 0.89 (0.86–0.93) 0.75 (0.69–0.81) 0.87 (0.82–0.93) 0.69 (0.61–0.78) | |||
| Debernardi et al., 2015 [52] | Urine | miR-143 miR-223 miR-30e miR-143 + miR-30e | 6 PDAC (stage I), 26 HC | Stage I PDAC vs. HC | 83.3 83.3 83.3 83.3 | 88.5 76.9 80.8 96.2 | 0.86 (0.70–1.00) 0.80 (0.59–1.00) 0.85 (0.67–1.00) 0.92 (0.79–1.00) |
| Han et al., 2015 [53] | Serum | Dickkopf-1 (DKK1) CA19-9 | 140 PDAC, (62 Early stage I-II), 48 HC, 18 BPT, 26 CP | PDAC vs. HC + BPT + CP | 89.3 73.6 | 79.4 83.7 | 0.92 (0.88–0.95) 0.85 (0.80–0.90) |
| Dickkopf-1 (DKK1) CA19-9 | PDAC vs. BPT + CP | 89.3 73.6 | 72.7 81.8 | 0.89 (0.83–0.95) 0.83 (0.77–0.89) | |||
| Dickkopf-1 (DKK1) CA19-9 | Early-PDAC vs. HC + BPT + CP | 85.5 64.5 | 79.3 83.7 | 0.89 (0.84–0.94) 0.81 (0.74–0.89) | |||
| Dickkopf-1 (DKK1) CA19-9 | Early-PDAC vs. BPT + CP | 85.5 64.5 | 72.7 81.8 | 0.85 (0.78–0.93) 0.78 (0.70–0.87) | |||
| Melo et al., 2015 [54] | Serum | GPC1 + crExos CA19-9 | 190 PDAC, 100 HC, 26 BPD | DS: PDAC vs. HC + BPD | 100.0 76.8 | 100.0 64.3 | 1.00 (0.99–1.00) 0.74 (0.70–0.83) |
| GPC1 + crExos GPC1 (ELISA) | 56 PDAC, 20 HC, 6 BPD | VS: PDAC vs. HC + BPD | 100.0 82.1 | 100.0 75.0 | 1.00 (0.96–1.00) 0.78 (0.68–0.87) | ||
| Radon et al., 2015 [55] | Urine | LYVE1 REG1A TFF1 LYVE1 + REG1A + TFF1 + (Creatinine +Age) | 143 PDAC (stage I-IV), 59 HC | TS: PDAC vs. HC | 76.9 62.2 72.7 76.9 | 88.1 94.9 59.3 89.8 | 0.85 (0.80–0.90) 0.82 (0.77–0.88) 0.69 (0.61–0.77) 0.89 (0.85–0.94) |
| LYVE1 REG1A TFF1 LYVE1 + REG1A + TFF1 + (Creatinine +Age) | 56 PDAC (stage I-II), 61 HC | 67.9 75.0 78.6 82.1 | 91.8 68.9 52.5 88.5 | 0.84 (0.77–0.91) 0.75 (0.66–0.84) 0.70 (0.60–0.79) 0.90 (0.84–0.96) | |||
| LYVE1 + REG1A + TFF1 + (Creatinine +Age) | 49 PDAC (stage I-IV), 28 HC | VS: PDAC vs. HC | 75.5 | 100 | 0.92 (0.84–1.00) | ||
| LYVE1 + REG1A + TFF1 + (Creatinine +Age) | 56 PDAC (stage I-II), 61 HC | 80.0 | 76.9 | 0.93 (0.84–1.00) | |||
| Plasma CA19-9 Panel (LYVE1 + REG1A + TFF1) Panel + Plasma CA19-9 | 71 PDAC (stage I-II), 28 HC | Exploratory Comparison | 83.1 93.0 94.4 | 92.9 92.9 100 | 0.88 (0.81–0.95) 0.97 (0.95–1.00) 0.99 (0.98–1.00) | ||
| Ankeny et al., 2016 [56] | Serum | CTCs and KRAS mutation analysis | 72 PDAC, 28 non-adenocarcinoma | PDAC vs. non-adenocarcinoma | 78.0 | 96.4 | 0.87 (0.80–0.94) |
| Guo et al., 2016 [57] | Serum | DTNBP1 CA19-9 | 250 PDAC, 70 CP, 80 BBO, 150 HC | PDAC vs. BBO + CP + HC | 81.9 76.3 | 84.7 52.5 | 0.85 (0.81–0.89) 0.74 (0.70–0.78) |
| DTNBP1 CA19-9 | PDAC vs. CP | 73.9 66.3 | 78.9 73.2 | 0.80 (0.75–0.86) 0.69 (0.63–0.75) | |||
| DTNBP1 CA19-9 | PDAC vs. BBO | 82.3 53.8 | 84.0 49.4 | 0.85 (0.80–0.89) 0.59 (0.53–0.65) | |||
| Henriksen et al., 2016 [58] | Plasma | (Model13): age >65+ BMP3+ RASSF1A+ BNC1+ MESTv2+ TFPI2+ APC+ SFRP1 + SFRP2 | 95 PDAC, 97 CP, 27 “screened negative” | PDAC vs. screened negative + CP | 73.0 | 83.0 | 0.86 (0.81–0.91) |
| Sogawa et al., 2016 [59] | Serum | C4BPA CA19-9 CEA C4BPA + CA19-9 | 66 PDAC, 40 HC, 20 CP | PDAC vs. HC + CP | 67.3 71.2 34.6 86.4 | 95.4 95.4 95.4 95.4 | 0.86 (p < 0.001) 0.85 0.77 0.93 |
| C4BPA CA19-9 CEA | 18 PDAC (stage I-II), 40 HC, 20 CP | PDAC stage I-II vs. HC + CP | 50.0 22.2 22.2 | 95.4 95.4 95.4 | 0.91 (p < 0.001) 0.74 0.87 | ||
| Yoneyama et al., 2016 [60] | Serum | CA19-9 IGFBP2 IGFBP3 | 38 PDAC (stage I-II), 65 HC | Stage I-II PDAC vs. HC | 60.5 68.4 76.3 | 92.3 67.7 70.7 | 0.84 (0.75–0.93) 0.71 (0.60–0.81) 0.77 (0.67–0.86) |
| Balasenthil et al., 2017 [61] | Plasma | CA19-9 | 55 PDAC (stage IA/ IB-IIA), 61HC | Stage IA/ IB-IIA vs. HC | 71.0 | 61.0 | 0.74 (0.64–0.84) |
| TNC + TFP1 + CA19-9 | 55 PDAC (stage IA/ IB-IIA), 62 CP | Stage IA/ IB-IIA vs. CP | 73.0 | 82.0 | 0.79 (0.70–0.87) | ||
| CA19-9 TNC + TFP1 + CA19-9 | 71.0 73.0 | 44.0 71.0 | 0.69 (0.58–0.79) 0.75 (0.65–0.84) | ||||
| Yang et al., 2017 [62] | Plasma | EGFR EPCAM HER2 MUC1 GPC1 WNT2 GRP94 B7-H3 EGFR + EPCAM + HER2 + MUC1 EGFR + EPCAM + GPC1 + WNT2 EGFR + EPCAM + MUC1 + GPC1 + WNT2 EGFR + EPCAM + HER2 + MUC1 + GPC1 + WNT2 | 22 PDAC, 10 HC | TS: PDAC vs. HC | 73 73 59 36 55 77 73 50 91 100 100 100 | 100 100 90 100 60 90 70 100 100 100 100 100 | 0.90 (0.79–1) 0.88 (0.77–0.99) 0.72 (0.55–0.89) 0.66 (0.48–0.84) 0.48 (0.28–0.67) 0.84 (0.71–0.96) 0.73 (0.55–0.90) 0.75 (0.58–0.93) 0.99 (0.97–1) 1.0 1.0 1.0 |
| EGFR EPCAM HER2 MUC1 GPC1 WNT2 GRP94 EGFR + EPCAM + HER2 + MUC1 EGFR + EPCAM + GPC1 + WNT2 EGFR + EPCAM + MUC1 + GPC1 + WNT2 EGFR + EPCAM + HER2 + MUC1 + GPC1 + WNT2 | 22 PDAC, 8 CP, 5 BPD, 8 other abdominal indications | VS: PDAC vs. CP vs. BPD vs. other abdominal indications | 59 45 59 36 82 64 55 86 82 86 95 | 76 95 85 90 52 76 71 86 90 81 81 | 67 (51–81) 70 (54–83) 72 (56–85) 63 (47–77) 67 (51–81) 70 (54–83) 63 (47–77) 86 (72–95) 86 (72–95) 84 (69–93) 88 (75–96) | ||
| Capello et al., 2017 [63] | Serum/ Plasma | CA19-9 TIMP1 + LRG1 + CA19-9 TIMP1 + LRG1 + CA19-9 (“OR” Rule) CA19-9 | 39 early stage PDAC, 82 HC | TS: early stage PDAC vs. HC | 53.8 66.7 72.6 84.9 | 95.0 95.0 95.0 95.0 | 0.82 (0.74–0.91) 0.89 (0.82–0.96) 0.88 (0.81–0.96) 0.95 (0.92–0.98) |
| CA19-9 TIMP1 + LRG1 + CA19-9 | 73 early stage PDAC, 60 HC | VS: early stage PDAC vs. HC | 84.9 28.8 | 95.0 95.0 | 0.96 (0.89–1.00) 0.82 (0.74–0.91) | ||
| Hussein et al., 2017 [64] | Serum | miR-22-3p miR-642b-3p miR-885-5p CA19-9 | 35 PDAC (33 early stage, 2 late stage) 15 HC | PDAC vs. HC | 97.1 100 100 91.4 | 93.3 100 100 100 | 0.94 (p < 0.001) 1.00 (p < 0.001) 1.00 (p < 0.001) 0.92 (p < 0.001) |
| Kaur et al., 2017 [65] | Serum | MUC5AC CA19-9 | 70 PDAC (stage I or II), 43 CP, 35 HC, 30 BC | Early PDAC vs. HC | 83.0 56.0 | 80.0 95.0 | 0.87 (0.79–0.95) 0.72 (0.59–0.84) |
| MUC5AC CA19-9 | Early PDAC vs. BC | 67.0 48.0 | 87.0 89.0 | 0.85 (0.76–0.93) 0.71 (0.59–0.83) | |||
| MUC5AC CA19-9 | Early PDAC vs. CP | 83.0 48.0 | 77.0 86.0 | 0.84 (0.76–0.92) 0.62 (0.50–0.74) | |||
| MUC5AC CA19-9 MUC5AC + CA19-9 | PDAC vs. HC + BC + CP | 89.0 79.0 83.0 | 70.0 43.0 83.0 | 0.88 (0.83–0.93) 0.61 (0.54–0.68) 0.91 (0.86–0.95) | |||
| Kim et al., 2017 [66] | Plasma | CA19-9 (≥55) THBS2 (36ng/mL cut-off) CA19-9 + THBS2 | 58 (stage I-II, phase 2a), 80 HC | PDAC stage I or II vs. HC | 69.0 33.0 74.1 | 100 96.0 96.3 | 0.85 (0.80–0.89) 0.83 (0.78–0.89) 0.95 (0.92–0.98) |
| CA19-9 THBS2 CA19-9 + THBS2 | 88 (stage I-II, phase 2b), 140 HC | 77.7 58.4 88.3 | 98.6 93.6 92.9 | 0.83 (0.79–0.97) 0.89 (0.85–0.92) 0.96 (0.94–0.98) | |||
| Lai et al., 2017 [67] | Plasma | CA19-9 miR-10b miR-21 miR-30c miR-106b miR-20a miR-181a miR-483 miR-let7a miR-122 | 29 PDAC, 6 HC | PDAC vs. HC | 86.0 100.0 86.0 100.0 97.0 93.0 97.0 66.0 93.0 100.0 | 100.0 100.0 100.0 100.0 100.0 100.0 100.0 67.0 100.0 67.0 | 0.92 (p < 0.001) 1.00 (p < 0.001) 0.95 (p < 0.001) 1.00 (p < 0.001) 0.98 (p < 0.001) 0.99 (p < 0.001) 0.97 (p < 0.001) 0.67 (p = 0.20) 0.99 (p < 0.001) 0.89 (p = 0.003) |
| Park et al., 2017 [68] | Serum | LRG1 + TTR + CA19-9 CA19-9 LRG1 + TTR + CA19-9 CA19-9 LRG1 + TTR + CA19-9 CA19-9 LRG1 + TTR + CA19-9 CA19-9 LRG1 + TTR + CA19-9 CA19-9 | 80 PDAC (50 stage I-II) (29 CA19-9 negative PDAC), 68 HC, 21 BPD, 52 Thyroid Ca, 52 Breast Ca, 45 Colorectal Ca) | PDAC vs. HC + BPC (n = 89) PDAC stage I-II vs. HC + BPC (n = 89) PDAC vs. Other Cancers (n = 149) PDAC vs. BPD CA19-9 negative PDAC (n = 29) vs. HC + BPC | 82.5 72.5 76.0 64.0 82.5 72.5 82.5 72.5 51.7 24.1 | 92.1 88.8 92.1 88.8 83.9 87.9 85.7 81.0 92.1 88.8 | 0.93 (p < 0.01) 0.83 0.91 (p < 0.01) 0.79 0.90 (p < 0.001) 0.80 0.90 0.81 0.83 (p < 0.001) 0.52 |
| Schott et al., 2017 [69] | Serum | HYAL2 Methylation | 82 PDAC, 191 HC 60 PDAC (stage I-II), 191 HC | PDAC vs. HC PDAC stage I-II vs. HC | 75.6 66.7 | 93.7 95.3 | 0.92 (0.88–0.96) 0.93 (0.89–0.98) |
| Arasaradnam et al., 2018 [70] | Urine | Volatile organic compounds | 4 PDAC stage I, 5 stage IIA, 35 stage IIB, 24 stage III, 12 stage IV, 81 HC | TS: PDAC vs. HC VS: PDAC vs. HC PDAC stage I -II vs. HC PDAC stage I -II vs. PDAC stage III-IV | 0.91 0.90 0.91 0.82 | 0.83 0.81 0.78 0.89 | 0.92 (0.88–0.96) 0.92 (0.85–0.98) 0.89 (0.83–0.94) 0.92 (0.86–0.97) |
| Dong et al., 2018 [71] | Serum | CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | 30 PDAC (early stage), 68 PDAC (late stage), 32 BPC, 37 HC, 27 PDAC (CA19-9 negative) | TS: HC vs. early PDAC | 96.7 70.0 81.1 93.3 90.0 83.3 96.7 | 83.8 75.7 81.1 94.6 94.6 86.5 94.6 | 0.94 (0.86–0.99) 0.78 (0.66–0.87) 0.89 (0.79–0.95) 0.97 (0.90–1.00) 0.96 (0.88–0.99) 0.90 (0.80–0.96) 0.98 (0.90–1.00) |
| CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | BPC vs. all PDAC | 85.7 64.3 58.1 84.7 75.5 67.4 84.7 | 81.3 87.5 87.5 90.6 90.6 96.9 90.6 | 0.88 (0.82–0.93) 0.81 (0.74–0.88) 0.78 (0.70–0.85) 0.93 (0.88–0.97) 0.89 (0.83–0.94) 0.87 (0.80–0.92) 0.94 (0.88–0.97) | |||
| CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | 38 PDAC (early stage), 77 PDAC (late stage), 43 BPC; 37 HC, 29 PDAC (CA19-9 negative) | BPC vs. early PDAC | 86.7 53.3 83.3 96.7 90.0 56.7 96.7 | 81.3 87.5 62.5 75.0 78.1 96.9 75.0 | 0.88 (0.77–0.95) 0.74 (0.61–0.84) 0.78 (0.66–0.88) 0.90 (0.80–0.96) 0.90 (0.79–0.96) 0.80 (0.68–0.89) 0.92 (0.82–0.97) | ||
| CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | VS: HC vs. early PDAC | 86.8 63.2 57.9 86.8 86.8 79.0 92.1 | 94.6 78.4 100 97.3 97.3 94.6 97.3 | 0.94 (0.86–0.98) 0.78 (0.66–0.86) 0.83 (0.73–0.91) 0.95 (0.88–0.99) 0.97 (0.90–1.00) 0.92 (0.84–0.97) 0.98 (0.92–1.00) | |||
| CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | BPC vs. all PDAC | 84.4 77.4 60.0 83.5 77.4 84.4 80.0 | 81.4 79.1 93.0 93.0 88.4 83.7 97.7 | 0.88 (0.82–0.93) 0.82 (0.76–0.88) 0.79 (0.72–0.85) 0.92 (0.87–0.96) 0.90 (0.84–0.94) 0.89 (0.83–0.93) 0.93 (0.88–0.96) | |||
| CA19-9 POSTN CA242 CA19-9 + POSTN CA19-9 + CA242 POSTN+ CA242 CA19-9 + POSTN+ CA242 | BPC vs. early PDAC | 86.8 65.8 57.9 65.8 76.3 76.3 83.6 | 79.1 79.1 93.0 93.0 93.0 86.1 88.4 | 0.87 (0.78–0.93) 0.72 (0.61–0.82) 0.77 (0.66–0.85) 0.84 (0.75–0.92) 0.92 (0.83–0.97) 0.84 (0.74–0.91) 0.90 (0.81–0.96) | |||
| Guo et al., 2018 [72] | Serum | SNHG15 | 171 PDAC, 59 HC | PDAC vs. HC | 68.3 | 89.6 | 0.73 (p < 0.01) |
| Lewis et al., 2018 [73] | Whole blood, plasma, serum | GPC1 + CD63 | 20 PDAC 6 BPD | PDAC vs. BPD | 81 | 70 | 0.79 (0.99–1.00) |
| Mellby et al., 2018 [74] | Plasma | Panel of 29 biomarkers | 15 PDAC stage I, 75 stage II, 15 stage III, 38 stage IV 57 CP, 20 IPMN, 219 HC | TS 2: PDAC stage I-II vs. HC | 95 | 94 | 0.96 (0.94–0.98) |
| VS (USA cohort): PDAC stage I-II vs. HC | 93 | 95 | 0.96 (0.94–0.98) | ||||
| Traeger et al., 2018 [75] | Serum | miRNA-205 CA19-9 miRNA-205 +CA19-9 | 47 PDAC, 16 CP, 5 IPMN, 17 BPC, 17 HC | PDAC vs. non-PDAC | 0.643 0.810 0.867 | 0.684 0.768 0.933 | 0.70 (0.548–0.789) 0.79 (0.698–0.887) 0.89 (0.782–0.995) |
| Zhou et al., 2018 [76] | Serum | GPC1 CA19-9 | 156 PDAC, 20 BPT, 16 CP, 163 HC | PDAC vs. HC + BPT + CP | 76.92 82.69 | 70.85 93.97 | 0.80 (0.749–0.841) 0.91 (0.868–0.947) |
| GPC1 CA19-9 | PDAC vs. HC | 76.92 82.69 | 70.55 97.55 | 0.81 (0.763–0.856) 0.91 (0.875–0.953) | |||
| GPC1 CA19-9 | Early PDAC vs. HC + BPT + CP | 68.06 79.17 | 70.85 93.97 | 0.76 (0.695–0.816) 0.88 (0.816–0.946) | |||
| GPC1 CA19-9 | Early PDAC vs. HC | 68.06 79.17 | 70.55 97.55 | 0.77 (0.705–0.830) 0.89 (0.824–0.953) | |||
| Berger et al., 2019 [77] | Plasma | CA19-9 THBS2 cfDNA CA19-9 + THBS2 THBS2 + CA19-9 + cfDNA | 52 PDAC, 15 IPMN, 32 CP | TS: PDAC vs. IPMN vs. CP | 55 41 32–86 1 73 41–86 1 | 91 96 70–100 1 91 78–96 1 | 0.80 0.73 0.90 0.87 0.94 |
| CA19-9 THBS2 cfDNA CA19-9 + THBS2 THBS2 + CA19-9 + cfDNA | VS: PDAC vs. IPMN vs. CP | 63 50 43–80 1 80 50–93 1 | 96 96 79–96 1 96 92–96 1 | 0.70 0.63 0.81 0.78 0.88 | |||
| Eissa et al., 2019 [78] | Plasma | ADAMTS1 | 39 PDAC, 95 HC | PDAC vs. HC | 87.2 | 95.8 | 0.91 (0.77–0.90) |
| BNC1 | 64.1 | 93.7 | 0.79 (0.63–0.78) | ||||
| ADAMTS1 and/or BNC1 | 97.4 | 91.6 | 0.95 (0.71–0.86) | ||||
| Fahrmann et al., 2019 [79] | Plasma | AcSperm+ DAS + indole-derivative+ LysoPC(18:0) + LysoPC(20:3) | 29 PDAC, 10 HC | TS: PDAC vs. HC | 69.0 | 99.0 | 0.90 (0.818–0.989) |
| AcSperm+ DAS + indole-derivative+ LysoPC(18:0) + LysoPC(20:3) | 39 Resectable PDAC, 82 HC | VS: PDAC vs. HC | 66.7 | 43.3 | 0.89 (0.828–0.996) | ||
| Indole-derivative LysoPC(18:0) LysoPC(20:3) AcSperm DAS | 23.1 51.3 48.7 33.3 51.3 | 11.3 26.3 11.3 27.5 27.5 | 0.73 (0.631–0.822) 0.84 (0.764–0.920) 0.84 0.757–0.925) 0.76 (0.659–0.852) 0.80 (0.712–0.890) | ||||
| Yu et al., 2019 [80] | Plasma | d-signature: EV long RNA (FGA, KRT19, HIST1H2BK, ITIH2, MARCH2, CLDN1, MAL2 and TIMP1) | 284 PDAC, 100 CP, 117 HC | PDAC vs. CP vs. HC d-signature with CA19-9 | 93.68 | 91.57 | 0.936 (0.889–0.983) |
| Takahashi et al., 2019 [81] | Serum | Circulating EV-encapsulated HULC | 20 PDAC, 22 IPMN, 21 HC | PDAC vs. IPMN vs. HC | 80 | 92.1 | 0.92 |
| Wei et al., 2019 [82] | Plasma | Vimetin-positive CTCs | 100 PDAC, 16 IPMN, 30 HC | PDAC vs. IPMN vs. HC | 65 | 100 | 0.968 |
| Yang et al., 2020 [83] | Plasma | EV miRNAs and mRNAs, cfDNA, ccfDNA KRAS G12D/V/R mutations and CA19-9 | 30 CP + BPC, 49 PDAC, 57 HC | PDAC vs. non-PDAC vs. HC | 88 | 95 | 0.95 (p= 0.103) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezgyte, G.; Shah, V.; Jach, D.; Crnogorac-Jurcevic, T. Non-Invasive Biomarkers for Earlier Detection of Pancreatic Cancer—A Comprehensive Review. Cancers 2021, 13, 2722. https://doi.org/10.3390/cancers13112722
Brezgyte G, Shah V, Jach D, Crnogorac-Jurcevic T. Non-Invasive Biomarkers for Earlier Detection of Pancreatic Cancer—A Comprehensive Review. Cancers. 2021; 13(11):2722. https://doi.org/10.3390/cancers13112722
Chicago/Turabian StyleBrezgyte, Greta, Vinay Shah, Daria Jach, and Tatjana Crnogorac-Jurcevic. 2021. "Non-Invasive Biomarkers for Earlier Detection of Pancreatic Cancer—A Comprehensive Review" Cancers 13, no. 11: 2722. https://doi.org/10.3390/cancers13112722
APA StyleBrezgyte, G., Shah, V., Jach, D., & Crnogorac-Jurcevic, T. (2021). Non-Invasive Biomarkers for Earlier Detection of Pancreatic Cancer—A Comprehensive Review. Cancers, 13(11), 2722. https://doi.org/10.3390/cancers13112722






