Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications
Abstract
Simple Summary
Abstract
1. Introduction
2. Lung Cancer
3. Uterine Cancer
4. Ovarian Cancer
5. Prostate Cancer
6. Urinary System
7. Breast Cancer
8. Neurological System
9. Hematologic Disorders
10. Bone
11. Soft Tissue Tumors
12. Limitations
13. Future Perspectives
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MRI | magnetic resonance imaging |
| CT | computed tomography |
| PET | positron emission tomography |
| DWI | diffusion weighted imaging |
| ADC | apparent diffusion imaging |
| DCE | dynamic contrast enhanced |
| DKI | diffusion kurtosis imaging |
| AUC | area under the curve |
| ROC | receiver operating characteristic |
| SVM | support vector machine |
| CNN | convolutional neural networks |
| CTTA | CT texture analysis |
| PPV | positive predictive value |
| NPV | negative predictive value |
| OS | overall survival |
| DFS | disease-free survival |
| PFS | progression-free survival |
| CRT | chemoradiation therapy |
| SUV | standard uptake values |
References
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging imaging and genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed]
- Miles, K. Radiomics for personalised medicine: The long road ahead. Br. J. Cancer 2020, 122, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Usman, Y.; Abdo, T.; Chaudry, F.; Keddissi, J.I.; Youness, H.A. Diagnosis and management of peripheral lung nodule. Ann. Transl. Med. 2019, 7, 348. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Into Imaging 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Feudis, A.; De Mattia, C.; Rizzetto, F.; Calderoni, F.; Mazzetti, S.; Torresin, A.; Vanzulli, A.; Regge, D.; Giannini, V. Standardization of CT radiomics features for multi-center analysis: Impact of software settings and parameters. Phys. Med. Biol. 2020, 65, 195012. [Google Scholar] [CrossRef]
- Beig, N.; Khorrami, M.; Alilou, M.; Prasanna, P.; Braman, N.; Orooji, M.; Rakshit, S.; Bera, K.; Rajiah, P.; Ginsberg, J.; et al. Perinodular and Intranodular Radiomic Features on Lung CT Images Distinguish Adenocarcinomas from Granulomas. Radiology 2019, 290, 783–792. [Google Scholar] [CrossRef]
- Lu, E.L.; Li, L.; Yang, L.; Schwartz, H.; Zhao, L.H. Radiomics for Classification of Lung Cancer Histological Subtypes Based on Nonenhanced Computed Tomography. Acad. Radiol. 2019, 26, 1245–1252. [Google Scholar] [CrossRef]
- Cong, M.; Feng, H.; Ren, J.-L.; Xu, Q.; Cong, L.; Hou, Z.; Wang, Y.-Y.; Shi, G. Development of a predictive radiomics model for lymph node metastases in pre-surgical CT-based stage IA non-small cell lung cancer. Lung Cancer 2020, 139, 73–79. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Zhao, Y.; Zhang, J.; Zhang, Z.; Wang, J.; Wang, Y.; Dai, M.; Han, J. Value of pre-therapy 18F-FDG PET/CT radiomics in predicting EGFR mutation status in patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1137–1146. [Google Scholar] [CrossRef]
- Zerunian, M.; Caruso, D.; Zucchelli, A.; Polici, M.; Capalbo, C.; Filetti, M.; Mazzuca, F.; Marchetti, P.; Laghi, A. CT based radiomic approach on first line pembrolizumab in lung cancer. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Khorrami, M.; Jain, P.; Bera, K.; Alilou, M.; Thawani, R.; Patil, P.; Ahmad, U.; Murthy, S.; Stephans, K.; Fu, P.; et al. Predicting pathologic response to neoadjuvant chemoradiation in resectable stage III non-small cell lung cancer patients using computed tomography radiomic features. Lung Cancer 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Feng, B.; Chen, X.; Chen, Y.; Liu, K.; Li, K.; Liu, X.; Yao, N.; Li, Z.; Li, R.; Zhang, C.; et al. Radiomics nomogram for preoperative differentiation of lung tuberculoma from adenocarcinoma in solitary pulmonary solid nodule. Eur. J. Radiol. 2020, 128, 109022. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, M.; Bera, K.; Thawani, R.; Rajiah, P.; Gupta, A.; Fu, P.; Linden, P.; Pennell, N.; Jacono, F.; Gilkeson, R.C.; et al. Distinguishing granulomas from adenocarcinomas by integrating stable and discriminating radiomic features on non-contrast computed tomography scans. Eur. J. Cancer 2021, 148, 146–158. [Google Scholar] [CrossRef]
- Fan, L.; Fang, M.; Li, Z.; Tu, W.; Wang, S.; Chen, W.; Tian, J.; Dong, D.; Liu, S. Radiomics signature: A biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur. Radiol. 2018, 29, 889–897. [Google Scholar] [CrossRef]
- D’Antonoli, T.A.; Farchione, A.; Lenkowicz, J.; Chiappetta, M.; Cicchetti, G.; Martino, A.; Ottavianelli, A.; Manfredi, R.; Margaritora, S.; Bonomo, L.; et al. CT radiomics signature of tumor and peritumoral lung parenchyma to predict nonsmall cell lung cancer postsurgical recurrence risk. Acad. Radiol. 2020, 27, 497–507. [Google Scholar] [CrossRef]
- Nardone, V.; Tini, P.; Pastina, P.; Botta, C.; Reginelli, A.; Carbone, S.; Giannicola, R.; Calabrese, G.; Tebala, C.; Guida, C.; et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol. Lett. 2020, 19, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- De Bernardi, E.; Buda, A.; Guerra, L.; Vicini, D.; Elisei, F.; Landoni, C.; Fruscio, R.; Messa, C.; Crivellaro, C. Radiomics of the primary tumour as a tool to improve 18F-FDG-PET sensitivity in detecting nodal metastases in endometrial cancer. Ejnmmi Res. 2018, 8, 86. [Google Scholar] [CrossRef]
- Kan, Y.; Dong, D.; Zhang, Y.; Jiang, W.; Zhao, N.; Han, L.; Fang, M.; Zang, Y.; Hu, C.; Tian, J.; et al. Radiomic signature as a predictive factor for lymph node metastasis in early-stage cervical cancer. J. Magn. Reson. Imaging 2019, 49, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tian, X.; Liu, Z.; Li, W.; Li, P.; Chen, J.; Zhang, W.; Fang, Z.; Du, P.; Duan, H.; et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. EBioMedicine 2019, 46, 160–169. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.-C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Yokota, H.; Nemoto, M.W.; Horikoshi, T.; Matsushima, J.; Uno, T. A multi-scanner study of MRI radiomics in uterine cervical cancer: Prediction of in-field tumor control after definitive radiotherapy based on a machine learning method including peritumoral regions. Jpn. J. Radiol. 2020, 38, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Li, Y.; Ma, F.H.; Zhang, G.F.; Feng, F.; Sun, M.H.; Lin, G.W.; Qiang, J.W. Radiologists with MRI-based radiomics aids to predict the pelvic lymph node metastasis in endometrial cancer: A multicenter study. Eur. Radiol. 2021, 31, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Li, Y.; Ma, F.H.; Feng, F.; Sun, M.H.; Lin, G.W.; Zhang, G.F.; Qiang, J.W. Preoperative assessment for high-risk endometrial cancer by developing an mri—and clinical-based radiomics nomogram: A multicenter study. J. Magn. Reson. Imaging 2020, 52, 1872–1882. [Google Scholar] [CrossRef]
- Xie, H.; Hu, J.; Zhang, X.; Ma, S.; Liu, Y.; Wang, X. Preliminary utilization of radiomics in differentiating uterine sarcoma from atypical leiomyoma: Comparison on diagnostic efficacy of MRI features and radiomic features. Eur. J. Radiol. 2019, 115, 39–45. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Y.; Chen, X.; Wu, G.; Liu, X.; Zhang, P.; Bai, Y.; Lu, P.; Yao, W.; Wang, Y.; et al. Magnetic resonance imaging radiomics in categorizing ovarian masses and predicting clinical outcome: A preliminary study. Eur. Radiol. 2019, 29, 3358–3371. [Google Scholar] [CrossRef]
- Song, X.-L.; Ren, J.-L.; Zhao, D.; Wang, L.; Ren, H.; Niu, J. Radiomics derived from dynamic contrast-enhanced MRI pharmacokinetic protocol features: The value of precision diagnosis ovarian neoplasms. Eur. Radiol. 2021, 31, 368–378. [Google Scholar] [CrossRef]
- Meier, A.; Veeraraghavan, H.; Nougaret, S.; Lakhman, Y.; Sosa, R.; Soslow, R.A.; Sutton, E.J.; Hricak, H.; Sala, E.; Vargas, H.A. Association between CT-texture-derived tumor heterogeneity, outcomes, and BRCA mutation status in patients with high-grade serous ovarian cancer. Abdom. Radiol. 2019, 44, 2040–2047. [Google Scholar] [CrossRef]
- Lu, H.; Arshad, M.; Thornton, A.; Avesani, G.; Cunnea, P.; Curry, E.; Kanavati, F.; Liang, J.; Nixon, K.; Williams, S.; et al. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat. Commun. 2019, 10, 764. [Google Scholar] [CrossRef]
- Himoto, Y.; Veeraraghavan, H.; Zheng, J.; Zamarin, D.; Snyder, A.; Capanu, M.; Nougaret, S.; Vargas, H.A.; Shitano, F.; Callahan, M.; et al. Computed tomography–Derived radiomic metrics can identify responders to immunotherapy in ovarian cancer. Jco Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Danala, G.; Thai, T.; Gunderson, C.C.; Moxley, K.M.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Applying quantitative CT image feature analysis to predict response of ovarian cancer patients to chemotherapy. Acad. Radiol. 2017, 24, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Czarniecki, M.; Mehralivand, S.; Stoyanova, R.; Choyke, P.L.; Harmon, S.; Turkbey, B. Radiomics and radiogenomics of prostate cancer. Abdom. Radiol. 2018, 44, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Woźnicki, P.; Westhoff, N.; Huber, T.; Riffel, P.; Froelich, M.F.; Gresser, E.; Von Hardenberg, J.; Mühlberg, A.; Michel, M.S.; Schoenberg, S.O.; et al. Multiparametric MRI for prostate cancer characterization: Combined use of radiomics model with PI-RADS and clinical parameters. Cancers 2020, 12, 1767. [Google Scholar] [CrossRef]
- Lay, N.S.; Tsehay, Y.; Greer, M.D.; Turkbey, B.; Kwak, J.T.; Choyke, P.L.; Pinto, P.; Wood, B.J.; Summers, R.M. Detection of prostate cancer in multiparametric MRI using random forest with instance weighting. J. Med. Imaging 2017, 4, 24506. [Google Scholar] [CrossRef]
- Cuocolo, R.; Stanzione, A.; Ponsiglione, A.; Romeo, V.; Verde, F.; Creta, M.; La Rocca, R.; Longo, N.; Pace, L.; Imbriaco, M. Clinically significant prostate cancer detection on MRI: A radiomic shape features study. Eur. J. Radiol. 2019, 116, 144–149. [Google Scholar] [CrossRef]
- Wibmer, A.; Hricak, H.; Gondo, T.; Matsumoto, K.; Veeraraghavan, H.; Fehr, D.; Zheng, J.; Goldman, D.; Moskowitz, C.; Fine, S.W.; et al. Haralick texture analysis of prostate MRI: Utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur. Radiol. 2015, 25, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, S.; Wei, J.; Zhang, G.; Lei, J.; Yan, W.; Xiao, Y.; Yan, S.; Xue, H.; Feng, F.; et al. Multiparametric MRI-based radiomics for prostate cancer screening with PSA in 4–10 ng/mL to reduce unnecessary biopsies. J. Magn. Reson. Imaging 2020, 51, 1890–1899. [Google Scholar] [CrossRef]
- Gugliandolo, S.G.; Pepa, M.; Isaksson, L.J.; Marvaso, G.; Raimondi, S.; Botta, F.; Gandini, S.; Ciardo, D.; Volpe, S.; Riva, G.; et al. MRI-based radiomics signature for localized prostate cancer: A new clinical tool for cancer aggressiveness prediction? Sub-study of prospective phase II trial on ultra-hypofractionated radiotherapy (AIRC IG-13218). Eur. Radiol. 2021, 31, 716–728. [Google Scholar] [CrossRef]
- Osman, S.O.; Leijenaar, R.T.; Cole, A.J.; Lyons, C.A.; Hounsell, A.R.; Prise, K.M.; O’Sullivan, J.M.; Lambin, P.; McGarry, C.K.; Jain, S. Computed Tomography-based Radiomics for Risk Stratification in Prostate Cancer. Int. J. Radiat. Oncol. 2019, 105, 448–456. [Google Scholar] [CrossRef]
- Ms, A.A.; Viswanath, S.; Shiradkar, R.; Ghose, S.; Pahwa, S.; Moses, D.; Jambor, I.; Shnier, R.; Böhm, M.; Haynes, A.-M.; et al. Radiomic features on MRI enable risk categorization of prostate cancer patients on active surveillance: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 818–828. [Google Scholar] [CrossRef]
- Bourbonne, V.; Vallières, M.; Lucia, F.; Doucet, L.; Visvikis, D.; Tissot, V.; Pradier, O.; Hatt, M.; Schick, U. MRI-Derived Radiomics to Guide Post-operative Management for High-Risk Prostate Cancer. Front. Oncol. 2019, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, L.; Zhao, L.; Mao, L.; Li, X.; Jin, Z.; Sun, H. CT-based radiomics to predict the pathological grade of bladder cancer. Eur. Radiol. 2020, 30, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Razik, A.; Kandasamy, D.; Seth, A.; Das, P.; Ganeshan, B.; Sharma, R. Role of MR texture analysis in histological subtyping and grading of renal cell carcinoma: A preliminary study. Abdom. Radiol. 2019, 44, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Basulto-Martinez, M.; Heinze, A.; Gratzke, C.; Miernik, A. Radiomics applications in renal tumor assessment: A comprehensive review of the literature. Cancers 2020, 12, 1387. [Google Scholar] [CrossRef]
- Xu, S.; Yao, Q.; Liu, G.; Jin, D.; Chen, H.; Xu, J.; Li, Z.; Wu, G. Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur. Radiol. 2020, 30, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kong, J.; Wu, S.; Li, Y.; Cai, J.; Yu, H.; Xie, W.; Qin, H.; Wu, Z.; Huang, J.; et al. Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. Cancer 2019, 125, 4388–4398. [Google Scholar] [CrossRef]
- Cha, K.H.; Hadjiiski, L.M.; Cohan, R.H.; Chan, H.-P.; Caoili, E.M.; Davenport, M.S.; Samala, R.K.; Weizer, A.Z.; Alva, A.; Kirova-Nedyalkova, G.; et al. Diagnostic accuracy of CT for prediction of bladder cancer treatment response with and without computerized decision support. Acad. Radiol. 2019, 26, 1137–1145. [Google Scholar] [CrossRef]
- Smith, A.D.; Zhang, X.; Bryan, J.; Souza, F.; Roda, M.; Sirous, R.; Zhang, H.; Vasanji, A.; Griswold, M. Vascular tumor burden as a new quantitative CT biomarker for predicting metastatic RCC response to antiangiogenic therapy. Radiology 2016, 281, 484–498. [Google Scholar] [CrossRef]
- Goh, V.; Ganeshan, B.; Nathan, P.; Juttla, J.K.; Vinayan, A.; Miles, K.A. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 2011, 261, 165–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Liu, W.; Bai, J.; Zheng, J.; Yang, X.; Zhou, L. Radiomics based on multimodal MRI for the differential diagnosis of benign and malignant breast lesions. J. Magn. Reson. Imaging 2020, 52, 596–607. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Chang, K.; Lee, K.E.; Wang, O.; Li, J.; Lin, Y.; Pan, Z.; Chang, P.; Chow, D.; et al. Diagnosis of benign and malignant breast lesions on DCE-MRI by using radiomics and deep learning with consideration of peritumor tissue. J. Magn. Reson. Imaging 2020, 51, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luo, Y.; Zhao, C.; Xiao, M.; Ma, L.; Li, W.; Qin, J.; Zhu, Q.; Jiang, Y. Nomogram based on radiomics analysis of primary breast cancer ultrasound images: Prediction of axillary lymph node tumor burden in patients. Eur. Radiol. 2021, 31, 928–937. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Spuhler, K.; Gao, Y.; Ms, M.S.S.; Do, M.M.; Hussain, S.; He, X.; Liang, C.; Huang, C. Preoperative prediction of sentinel lymph node metastasis in breast cancer by radiomic signatures from dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2019, 49, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, H.; Bai, Y.; Li, J.; Lu, Q.; Chen, R.; Zhang, M.; Feng, Q.; Wang, M. Evaluating the HER-2 status of breast cancer using mammography radiomics features. Eur. J. Radiol. 2019, 121, 108718. [Google Scholar] [CrossRef]
- Braman, N.; Prasanna, P.; Whitney, J.; Singh, S.; Beig, N.; Etesami, M.; Bates, D.D.B.; Gallagher, K.; Bloch, B.N.; Vulchi, M.; et al. Association of peritumoral radiomics with tumor biology and pathologic response to preoperative targeted therapy for HER2 (ERBB2)–Positive breast cancer. JAMA Netw. Open 2019, 2, e192561. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Qu, J.; Zhang, R.; Zhou, X.; Li, L.; Sun, K.; Tang, Z.; Jiang, H.; Li, H.; et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: A multicenter study. Clin. Cancer Res. 2019, 25, 3538–3547. [Google Scholar] [CrossRef]
- Antunovic, L.; De Sanctis, R.; Cozzi, L.; Kirienko, M.; Sagona, A.; Torrisi, R.; Tinterri, C.; Santoro, A.; Chiti, A.; Zelic, R.; et al. PET/CT radiomics in breast cancer: Promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Galldiks, N.; Kocher, M.; Heinzel, A.; Filss, C.P.; Stegmayr, C.; Mottaghy, F.M.; Fink, G.R.; Shah, N.J.; Langen, K.-J. Radiomics in neuro-oncology: Basics, workflow, and applications. Methods 2021, 188, 112–121. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, L.-F.; Zhang, X.; Hu, Y.-C.; Han, Y.; Liu, Z.-C.; Nan, H.-Y.; Sun, Q.; Sun, Y.-Z.; Yang, Y.; et al. Radiomics strategy for glioma grading using texture features from multiparametric MRI. J. Magn. Reson. Imaging 2018, 48, 1518–1528. [Google Scholar] [CrossRef]
- Cho, H.-H.; Lee, S.-H.; Kim, J.; Park, H. Classification of the glioma grading using radiomics analysis. PeerJ 2018, 6, e5982. [Google Scholar] [CrossRef]
- Chang, P.; Grinband, J.; Weinberg, B.D.; Bardis, M.; Khy, M.; Cadena, G.; Su, M.-Y.; Cha, S.; Filippi, C.G.; Bota, D.; et al. Deep-learning convolutional neural networks accurately classify genetic mutations in gliomas. Am. J. Neuroradiol. 2018, 39, 1201–1207. [Google Scholar] [CrossRef]
- Li, Z.-C.; Bai, H.; Sun, Q.; Li, Q.; Liu, L.; Zou, Y.; Chen, Y.; Liang, C.; Zheng, H. Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: A multicentre study. Eur. Radiol. 2018, 28, 3640–3650. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.-E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro-Oncology 2019, 21, 404–414. [Google Scholar] [CrossRef]
- Bani-Sadr, A.; Eker, O.F.; Berner, L.-P.; Ameli, R.; Hermier, M.; Barritault, M.; Meyronet, D.; Guyotat, J.; Jouanneau, E.; Honnorat, J.; et al. Conventional MRI radiomics in patients with suspected early- or pseudo-progression. Neuro-Oncol. Adv. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kong, Z.; Jiang, C.; Zhu, R.; Feng, S.; Wang, Y.; Li, J.; Chen, W.; Liu, P.; Zhao, D.; Ma, W.; et al. 18F-FDG-PET-based radiomics features to distinguish primary central nervous system lymphoma from glioblastoma. Neuroimage Clin. 2019, 23, 101912. [Google Scholar] [CrossRef]
- Ma, Z.; Fang, M.; Huang, Y.; He, L.; Chen, X.; Liang, C.; Huang, X.; Cheng, Z.; Dong, D.; Liang, C.; et al. CT-based radiomics signature for differentiating Borrmann type IV gastric cancer from primary gastric lymphoma. Eur. J. Radiol. 2017, 91, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Fruchart, C.; Nganoa, C.; Gac, A.-C.; Lasnon, C. Baseline 18F-FDG PET radiomic features as predictors of 2-year event-free survival in diffuse large B cell lymphomas treated with immunochemotherapy. Eur. Radiol. 2020, 30, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Riedl, C.C.; Kumar, A.; Gibbs, P.; Weber, M.; Tal, I.; Schilksy, J.; Schöder, H. Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2760–2769. [Google Scholar] [CrossRef]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; Van Hoof, A.; Kluin-Nelemans, H.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H.; et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008, 111, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Wörtler, K.; Tagliafico, A.; Papakonstantinou, O.; Åström, G.; Isaac, A.; Weber, M.-A.; Lalam, R.; Bloem, J.; Noebauer-Huhmann, I.; Vanhoenacker, F.; et al. ESSR consensus document for detection, characterization, and referral pathway for tumors and tumorlike lesions of bone. Semin. Musculoskelet. Radiol. 2017, 21, 630–647. [Google Scholar]
- Yin, P.; Mao, N.; Zhao, C.; Wu, J.; Sun, C.; Chen, L.; Hong, N. Comparison of radiomics machine-learning classifiers and feature selection for differentiation of sacral chordoma and sacral giant cell tumour based on 3D computed tomography features. Eur. Radiol. 2019, 29, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kido, S.; Suga, K.; Hirano, Y.; Tachibana, R.; Muramatsu, K.; Chagawa, K.; Tanaka, S. Texture analysis on 18F-FDG PET/CT images to differentiate malignant and benign bone and soft-tissue lesions. Ann. Nucl. Med. 2014, 28, 926–935. [Google Scholar] [CrossRef]
- Lin, P.; Yang, P.-F.; Chen, S.; Shao, Y.-Y.; Xu, L.; Wu, Y.; Teng, W.; Zhou, X.-Z.; Li, B.-H.; Luo, C.; et al. A Delta-radiomics model for preoperative evaluation of Neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imaging 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, L.; Yang, P.; Lin, N.; Huang, X.; Pan, W.; Li, H.; Lin, P.; Li, B.; Bunpetch, V.; et al. Survival prediction in high-grade osteosarcoma using radiomics of diagnostic computed tomography. EBioMedicine 2018, 34, 27–34. [Google Scholar] [CrossRef]
- Lang, N.; Zhang, Y.; Zhang, E.; Zhang, J.; Chow, D.; Chang, P.; Yu, H.J.; Yuan, H.; Su, M.-Y. Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn. Reson. Imaging 2019, 64, 4–12. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, N.; Wang, Y.; Xie, H.; Duan, S.; Zhang, X.; Wang, B. A Radiomics nomogram for predicting bone metastasis in newly diagnosed prostate cancer patients. Eur. J. Radiol. 2020, 128, 109020. [Google Scholar] [CrossRef]
- Peeken, J.C.; Spraker, M.B.; Knebel, C.; Dapper, H.; Pfeiffer, D.; Devecka, M.; Thamer, A.; Shouman, M.A.; Ott, A.; von Eisenhart-Rothe, R.; et al. Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine 2019, 48, 332–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Shi, X.; Tao, J.; Cui, J.; Dai, Y.; Zheng, M.; Wang, S. Soft tissue sarcomas: Preoperative predictive histopathological grading based on radiomics of MRI. Acad. Radiol. 2019, 26, 1262–1268. [Google Scholar] [CrossRef]
- Vos, M.; Starmans, M.P.A.; Timbergen, M.J.M.; van der Voort, S.; Padmos, G.A.; Kessels, W.; Niessen, W.J.; Van Leenders, G.J.L.H.; Grünhagen, D.J.; Sleijfer, S.; et al. Radiomics approach to distinguish between well differentiated liposarcomas and lipomas on MRI. BJS 2019, 106, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Ms, C.P.; Kind, M.; De Senneville, B.D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. T2 -based MRI Delta-radiomics improve response prediction in soft-tissue sarcomas treated by neoadjuvant chemotherapy. J. Magn. Reson. Imaging 2019, 50, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Le Loarer, F.; Sitbon, M.; Italiano, A.; Stoeckle, E.; Buy, X.; Kind, M. Can radiomics improve the prediction of metastatic relapse of myxoid/round cell liposarcomas? Eur. Radiol. 2020, 30, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
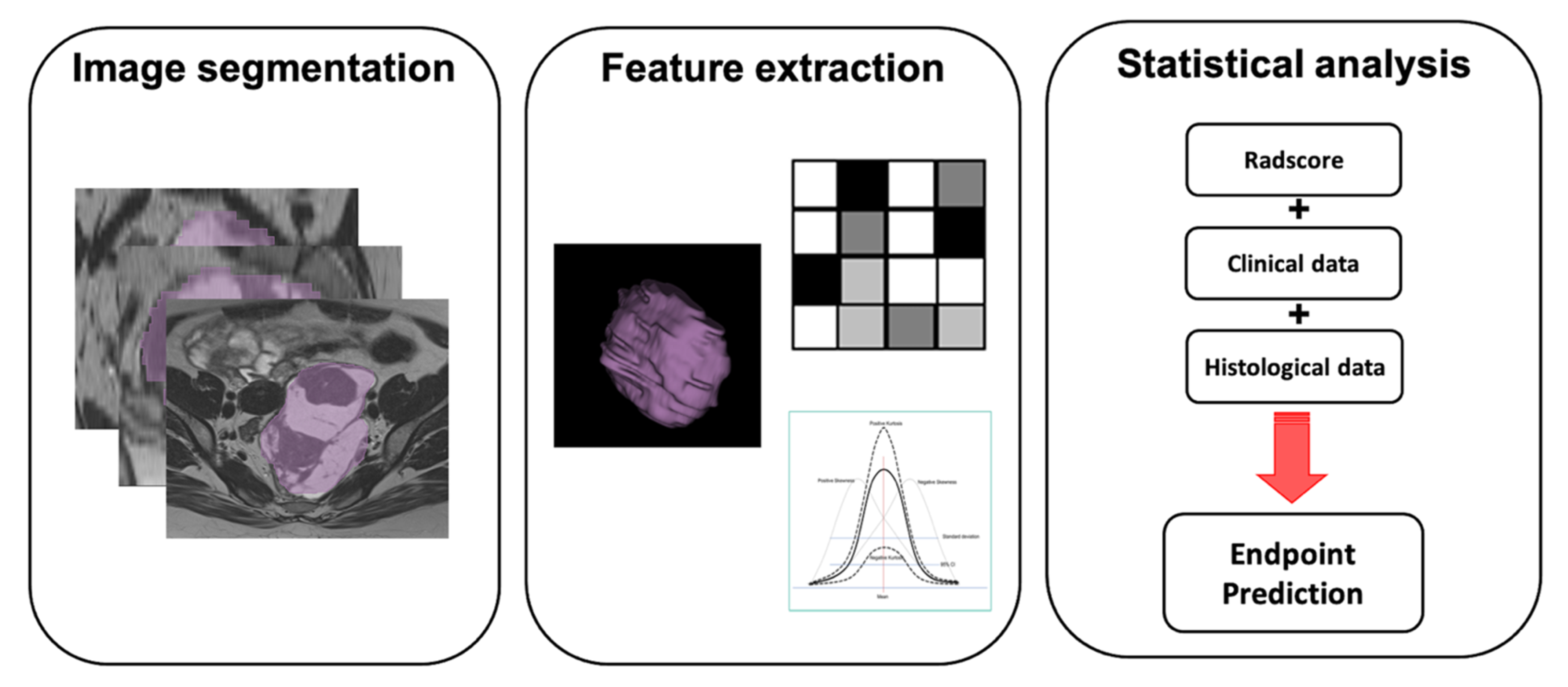
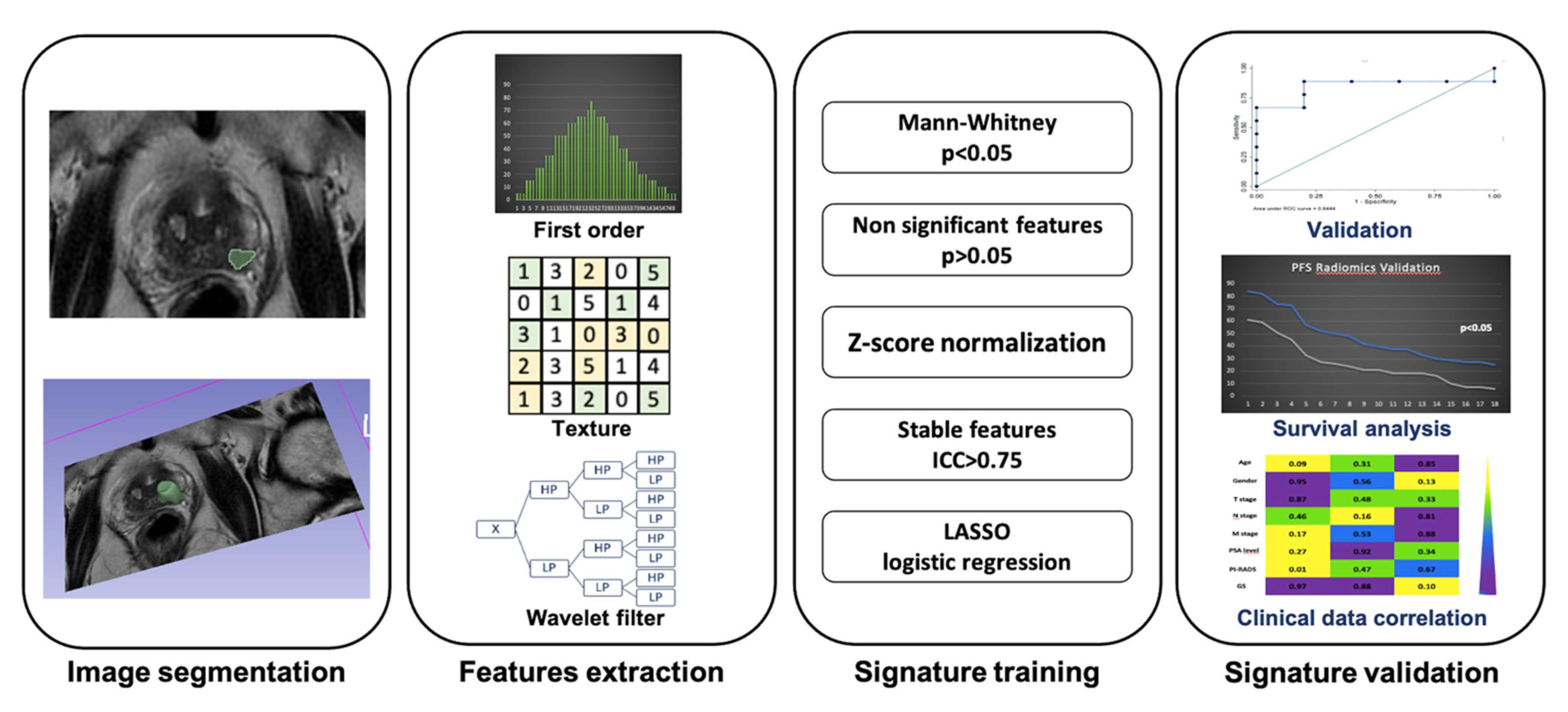
| Study | N Patients | Objective | Types of Evaluation | Model Performance | Imaging Modality | Features Selected | Nature of Study |
|---|---|---|---|---|---|---|---|
| Beig N. et al., Radiol. 2019 [7] | Total 290 | AD vs. Granulomas | Intranodular and Perinodular radiomic analysis | 0.80 0.76 0.60–0.61 | CT | 12 | Multicentric Restrospective |
| Linning E. et al., Acad. Radiol. 2019 [8] | Total 278 | SCLC vs. NSCLC SCLC vs. AD SCLC vs. SCC | Primary lesion radiomic analysis | End. 1 AUC: 0.74 End. 2 AUC: 0.82 End. 3 AUC: 0.66 | CT | 20 | Monocentric Restrospective |
| Cong M. et al., Lung Cancer 2020 [9] | Training 455 Validation 194 | Assessment nodal metastases | Predictive Radiomics on primary lesion | AUC: 0.91 AUC: 0.86 | CT | 7 | Monocentric Restrospective |
| Zhang J. et al., Eur. J. Nucl. Med Mol. Imaging 2020 [10] | Training 175 Validation 73 | EGFR status | Radiomic signature Fusion models | AUC: 0.86 AUC: 0.87 | 18F-FDG PET/CT | 10 | Monocentric Restrospective |
| Zerunian M. et al., Sci. Rep 2021 [11] | Total 21 | OS PFS | Volumetric Textural analysis | End. 1 AUC: 0.72 End. 2 AUC: 0.74 | CT | 6 | Monocentric Prospective |
| Khorrami M. et al., Lung Cancer 2019 [12] | Training 45 Validation 45 | Pathological response | Intranodular and perinodular radiomic analysis | AUC: 0.90 AUC: 0.86 | CT | 13 | Monocentric Restrospective |
| Study | N Patients | Endpoint | Types of Evaluation | Model Performance | Imaging Modality | Features Selected | Nature of Study |
|---|---|---|---|---|---|---|---|
| Zhang H. et al., Eur. Rad. 2019 [26] | Validation 195 Testing 85 | Benign vs. Malignant OEC type I vs. type II | LOO cross-validation Indipendent testing | End. 1 AUC: 0.97 End 1 AUC: 0.85 End 2 AUC: 0.96 End 2 AUC: 0.82 | MRI | End. 1: 84 End. 2: 56 | Monocentric Restrospective |
| Song X.L. et al., Eur. Rad. 2021 [27] | Training 72 Validation 32 | Benign vs. Borderline Benign vs. Malignant Borderline vs. Malignant | 2-class classification | End 1 AUC: 0.89 End 2 AUC: 0.86 End 3 AUC: 0.89 | MRI | End. 1: 51 End. 2: 23 End. 3: 18 | Monocentric Prospective |
| Meier A. et al., Abdom. Radiol. 2019 [28] | Total 88 | Assosiation Survival and texture heterogeneity | Inter-site texture heterogeneity | p < 0.05 | CT | 3 | Monocentric Restrospective |
| Lu H. et al., Nat. Commun 2019 [29] | Total 364 | Survival | Radiomic prognostic vector | HR > 3.83 | CT | 4 | Multicentric Restrospective |
| Himoto Y. et al., JCO Precis. Oncol. 2019 [30] | Total 75 | Time to off-treatment | Intra-site texture heterogeneity Inter-site texture heterogeneity | p < 0.05 HR: 0.88 HR: 1.19 | CT | 7 | Monocentric Restrospective |
| Danala. et al., Acad. Radiol 2017 [31] | Total 91 | Early prediction treatment response | Delta Radiomics Fusion models | AUC: 0.77 AUC: 0.81–0.82 | CT | 24 | Monocentric Restrospective |
| Study | N Patients | Endpoint | Types of Evaluation | Model Performance | Imaging Modality | Features Selected | Nature of Study |
|---|---|---|---|---|---|---|---|
| Tian Q. et al., J. Magn. Reson. Imaging 2018 [59] | Total 153 | LGG vs. HGG Grade II vs. III | Volumetric radiomic analysis | End 1 AUC: 0.98 End 1 Acc: 96.8% End 2 AUC: 0.99 End 2 Acc: 98.1% | MRI | End. 1: 30 End. 2: 28 | Monocentric Restrospective |
| Cho H.H. et al., PeerJ 2018 [60] | Total 285 | LGG vs. HGG | Multi-regional radiomic features | AUC: 0.91 AUC: 0.88 AUC: 0.92 | MRI | 5 | Multicentric Restrospective |
| Chang P. et al., Am. J. Neuroradiol. 2018 [61] | Total 259 | IDH1 status 1p/19q codelation MGMT status | Volumetric deep learning CNN | End 1 Acc: 94% End 2 Acc: 92% End 3 Acc: 83% | MRI | 64 | Multicentric Restrospective |
| Li Z.C. et al., Eur. Radiol. 2018 [62] | Training 133 Validation 60 | MGMT status | Multi-regional radiomic features Fusion models | AUC: 0.95 Acc: 87% AUC: 0.88 Acc: 80% | MRI | 6 | Multicentric Restrospective |
| Kim J.Y. et al., Neuro Oncol. 2019 [63] | Total 61 | Pseudoprogression vs. Progression | Multiparametric radiomic models | AUC: 0.90 | MRI | 12 | Monocentric Restrospective |
| Bani-Sadr. et al., Neurooncol. Adv. 2019 [64] | Training 55 Validation 21 | Pseudoprogression vs. Progression | Multi-regional radiomic features | AUC: 0.82 Acc: 83% AUC: 0.85 Acc: 79.2% | MRI | 11 | Monocentric Restrospective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Landolfi, F.; Nicolai, M.; Lucertini, E.; Tarallo, M.; et al. Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications. Cancers 2021, 13, 2681. https://doi.org/10.3390/cancers13112681
Caruso D, Polici M, Zerunian M, Pucciarelli F, Guido G, Polidori T, Landolfi F, Nicolai M, Lucertini E, Tarallo M, et al. Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications. Cancers. 2021; 13(11):2681. https://doi.org/10.3390/cancers13112681
Chicago/Turabian StyleCaruso, Damiano, Michela Polici, Marta Zerunian, Francesco Pucciarelli, Gisella Guido, Tiziano Polidori, Federica Landolfi, Matteo Nicolai, Elena Lucertini, Mariarita Tarallo, and et al. 2021. "Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications" Cancers 13, no. 11: 2681. https://doi.org/10.3390/cancers13112681
APA StyleCaruso, D., Polici, M., Zerunian, M., Pucciarelli, F., Guido, G., Polidori, T., Landolfi, F., Nicolai, M., Lucertini, E., Tarallo, M., Bracci, B., Nacci, I., Rucci, C., Eid, M., Iannicelli, E., & Laghi, A. (2021). Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications. Cancers, 13(11), 2681. https://doi.org/10.3390/cancers13112681








