1. Introduction
Hypoxia is a characteristic feature of solid animal [
1] and human [
2] that occurs because of the structural and functional abnormalities in tumor vessels [
2,
3] resulting in an insufficient blood supply of oxygen to the growing tumor mass [
4]. Generally, tumor hypoxia generates a more aggressive cancer phenotype [
5] and causes resistance to both radiation and chemotherapy [
6,
7]. Therefore, hypoxia is a marker for poor patient/treatment outcome in various cancers. Consequently, several therapeutic modalities to overcome tumor hypoxia have been investigated at both the pre-clinical [
7,
8] and clinical level [
6]. They include treatments to improve oxygen delivery, radiosensitizing hypoxic cells, hypoxic cell cytotoxins, and vascular targeting agents [
6,
7,
8]. It would be beneficial to identify which tumors are “more” or “less” hypoxic to intensify the treatment and justify potential additional side effects. Various methods to determine hypoxia have been evaluated [
9]. Exogenous nitroimidazole-based markers such as pimonidazole, identified by immunohistochemistry, and [18F]-labeled fluoroazomycin arabinose (FAZA) for positron emission tomography (PET) scans are promising strategies for the identification of tumor hypoxia [
9]. Quantification of endogenous markers expressed by hypoxic tumor cells is another approach for determining the hypoxic status of tumors [
10,
11]. These have been applied as individual markers [
9] or feature a combination of endogenous markers as gene signatures [
12]. Our own studies in vitro identified 27 genes from four human squamous cell carcinoma cell lines that were pH-independently up- or down-regulated under hypoxic conditions [
13]. Using human primary tumor biopsies from head and neck squamous cell carcinomas (HNSCC) patients with known oxygen status, that signature was refined to include a 15-gene “hypoxia gene expression classifier” [
14]. This has retrospectively been shown to have prognostic and predictive impact for the radiosensitizer nimorazole in combination with radiotherapy [
15]. The 15-gene hypoxia classifier has also been evaluated in a range of cell lines originating from head and neck, prostate, colon, or esophagus [
16], and all 15 genes were found to be up-regulated when comparing anoxic (0% oxygen) with normoxic (21% oxygen) cells in all the cancer cell lines.
Prostate tumors are known to be hypoxic [
17]. Studies in which direct pO
2 measurements in human prostate tumors were performed reported that the more hypoxic tumors were associated with biochemical relapse and local recurrence after treatment [
18,
19]. Therefore, several attempts have been made to create a clinically relevant prostate-specific hypoxia gene signature. Ragnum and colleagues found a correlation between tumor aggressiveness and pimonidazole immune score [
20]. On the basis of these findings, they constructed a 32-gene gene signature that was associated with pimonidazole staining. A validation study in two prostate cancer cohorts showed significant correlations between the gene signature and both Gleason score and survival. Yang et al. created a 28-gene hypoxia gene signature and found that 848 genes were either up- or down-regulated under hypoxic conditions [
21]. In a cross-validation study, 28 genes were finally selected, and a further validation from 11 cohorts revealed a significant increase in biochemical recurrence-free survival in radical prostatectomy patients with “low hypoxia” compared to “high hypoxia”. Furthermore, the gene signature was found to have prognostic and predictive impact on radiotherapy treatment and metastatic outcome, respectively.
Marotta et al. described a successful way of profiling hypoxic gene-markers in glioma xenografts using the exogenous hypoxia marker EF5 and a laser-capture microdissection (LCM) apparatus [
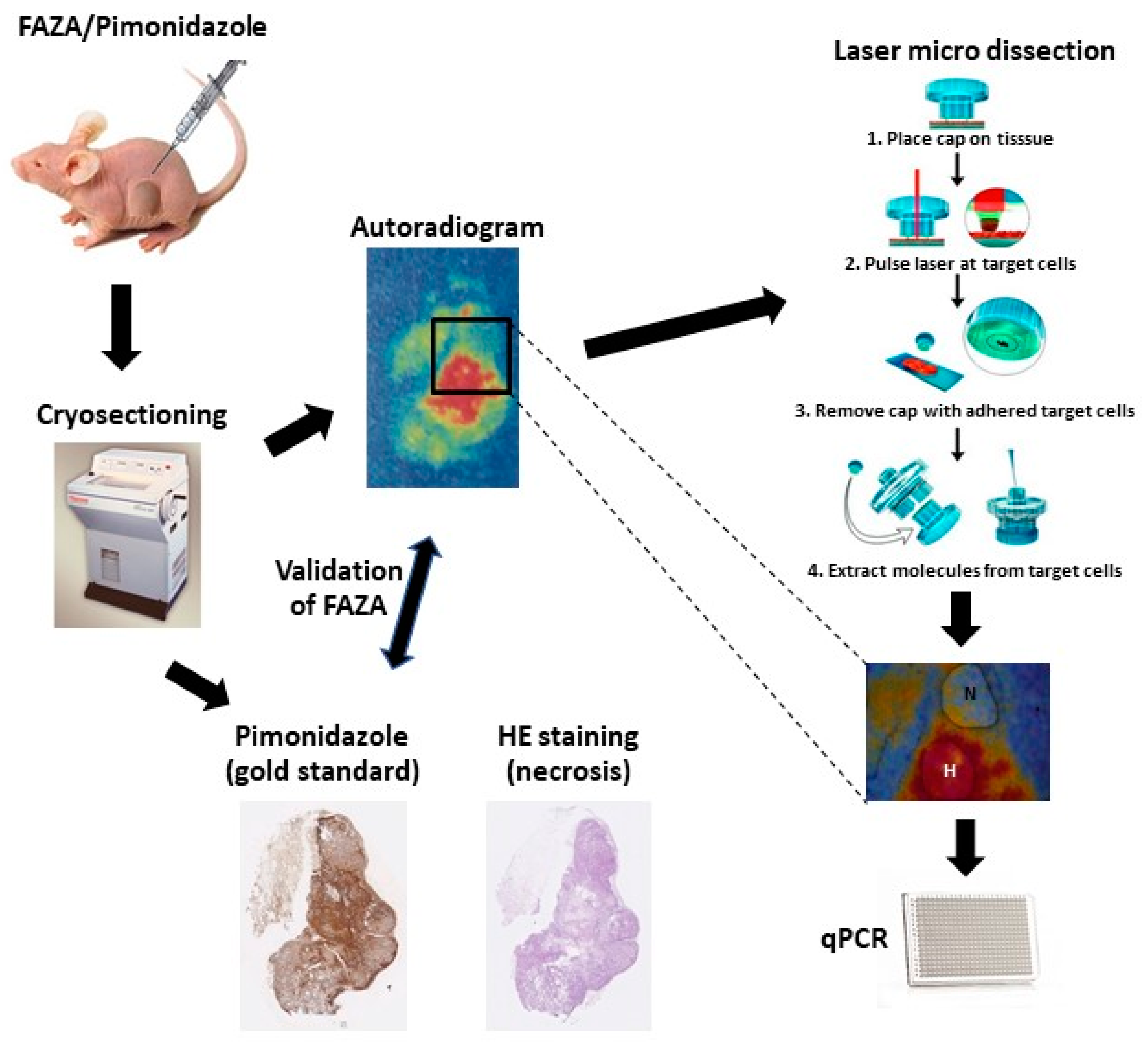
22]. The EF5 staining detected and visualized the hypoxic tumor areas, which were then dissected, along with the nonhypoxic areas, using the LCM. In our current study, we will demonstrate a refined method with broad applicability where the use of a radio-labeled tracer (in this case [18F]-FAZA) allows autoradiography-guided laser capture microdissection of unstained and unfixed cryo-preserved tumor tissue in vivo (see
Figure 1). Furthermore, we investigated the usability of the HNSCC 15-gene signature to monitor hypoxia in PC3 and DU145 prostate cancer cell lines in vitro, and using our refined in vivo method, we investigated the potential of using this hypoxia-induced gene expression in both tumor types grown as xenografts in nude mice.
2. Materials and Methods
2.1. Cell Lines, Hypoxic Treatment, and Cell Lysis
The prostate adenocarcinoma cell lines (DU-145 and PC-3) were obtained from Dr. Bouchelouche (Department of Clinical Biochemistry, Koege Hospital, Denmark). Cells were cultured in 80 cm2 flasks (NUNC) in Dulbecco’s modified eagle medium (DMEM) with GlutaMAX I containing 4.5 g/L D-Glucose, 10% fetal-calf serum, 1% sodium pyruvate, 1% non-essential amino acids, 2% HEPES, and 1% penicillin–streptomycin, with 5% CO2 at 90% relative humidity. For hypoxia experiments, 200,000 cells were seeded into 60 mm glass Petri dishes three days prior to experiments, by which time cells were in the log phase of growth. Hypoxia was achieved by continually gassing the cells in an airtight chamber with different oxygen concentrations (0, 0.5, 1, 2, 5, and 21%), 5% CO2, and balancing with N2, at 37 °C for 24 h. To ensure the appropriateness of our gassing procedure, aerobic indicator strips were included in the chambers when doing oxygen-free incubations. These indicator strips showed that pO2 was consistently below 0.15 mmHg for the duration of the experiment. Immediately after removal from the airtight chamber, media was removed, cells were washed with Dulbecco’s phosphate-buffered saline (DPBS), and cells were lysed with Qiazol Lysis Reagent (Qiagen, Hilden, Germany). Cell lysates were stored at −80 °C. Three independent experiments were performed, with duplicate measurements in each experiment.
2.2. Animals, Tumor Model, Histology, and Laser Capture Microdissection
The human tumor xenografts, PC3 and DU-145, were grown subcutaneously in both flanks of immune-compromised male NMRI Foxn1nu/Foxn1nu mice. Mice received whole-body irradiation with 4 Gy 1–3 days before tumor cell inoculation to further suppress immunity. For the DU-145 tumor, the number of cells injected was 3 × 10
6 cells, while for the PC3 cancer, the cell number injected was 2 × 10
6 cells. Mice were used for experiments when tumors reached a size of 400 to 600 mm
3, which was typically 6–8 weeks after inoculation. Non-anaesthetized mice were administered intraperitoneally with a mixture of [18F]-FAZA ≈48 MBq (production method described in [
23] with minor modifications) in 0.2 to 0.4 mL saline and 60 mg/kg (0.02 mL/g) pimonidazole (for autoradiography and immunohistochemical detection of hypoxia) and returned to their cages. Three to four hours later, mice were sacrificed by cervical dislocation; then, tumors and reference tissue (thigh muscle) were excised and frozen immediately in pre-cooled isopentane (−40 °C, obtained with dry ice). Multiple tissue cryosections were prepared by cutting each tumor xenograft in several layers with 1 mm between each layer. In each layer, consecutive 10 μm sections were cut and either stained for hematoxylin eosin (H&E) or pimonidazole, or used for FAZA autoradiography; the autoradiogram was used as a template for laser capture microdissection (LCM). The sections for LCM were mounted onto Arcturus PEN membrane glass slides (Life Technologies, Naerum, Denmark), and the other sections were mounted on Superfrost
® White slides (Menzel-Gläser, Braunschweig, Germany). Immediately after cryosectioning, all tissue sections were processed for autoradiography by exposure to phosphor imaging plates overnight. To minimize mRNA degradation, exposure was conducted at −20 °C. Finally, the intra-tissue tracer signal distribution was extracted at a pixel size of 25 μm using a Fuji BAS 5000 scanner. Tissue sections previously analyzed using autoradiography were either immunologically stained for the distribution of pimonidazole, used to validate the reliability of [18F]-FAZA autoradiograms to visualize hypoxic areas, or H&E stained to evaluate necrosis. By demarcating areas with high [18F]-FAZA accumulation (hypoxic areas) and low [18F]-FAZA accumulation (non-hypoxic areas, defined as areas with [18F]-FAZA activity equal to muscle tissue) from the autoradiography, a printed 1:1 template was made to describe the hypoxic status within the tumor sections. Guided by H&E staining, necrotic areas were avoided, and the demarcated areas were dissected using LCM performed using the Veritas Microdissection Instrument (Arcturus Bioscience) and Capsure Macro LCM caps (Life Technologies). Tissues from LCM representing hypoxic and non-hypoxic areas were lysed immediately after dissection using RLT buffer containing 10 mL/L β-mercaptoethanol.
2.3. RNA Extraction, Reverse Transcription, and Gene Expression Quantification
Total RNA was extracted from cell lysates or lysates from laser capture micro-dissected xenograft material using the miRNeasy Mini Kit (Qiagen) according to the manufacturer instructions. A DNase step was included, according to the manufacturer instruction. RNA was eluted in RNAse-free water, and concentrations were determined with QubitTM 3.0 Fluorometer using Qubit
® RNA Broad-Range Assay Kit (ThermoFisher Scientific, Waltham, MA, USA). Gene expression levels were quantified using Quantitative Real-Time PCR as described in [
13]. Briefly, cDNA was generated using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ABI, Foster City, CA, USA) according to the manufacturer instructions. Total RNA was reverse transcribed using random primers. Target cDNA transcripts were detected and quantified with TaqMan Gene Expression assays (ABI). For each reaction, TaqMan GeneExpression Master Mix (ABI), cDNA in TE-buffer (Ambion, Austin, TX, USA) and the TaqMan Gene Expression assays were mixed. Quantitative Real-Time PCR was performed on a 7900HT Fast Real-Time PCR System (ABI). Reference genes were ACTR, NDFIP1, and RPL37A; these were previously established as robust reference genes for the 15 gene hypoxia profile [
16].
2.4. Eppendorf Oxygen-Electrode Measurements
In a separate group of male NMRI Foxn1nu/Foxn1nu mice with the human PC3 or DU-145 xenograft inoculated in the flank, tumor pO
2 was measured using a 0.35 mm diameter computerized polarographic, oxygen-sensitive needle electrode (The KIMOC-6650 pO
2 Histograph, Eppendorf, Germany). The electrode was inserted into the tumor and moved automatically through the tissue in 0.7 mm increments, which was followed each time by a 0.3 mm backward step prior to measurement. Four to seven electrode tracks were obtained per tumor, resulting in 52–105 measurements per tumor; the size and shape of the tumor determined the length and number of tracks. Further details of the procedure are described elsewhere [
24,
25]. The pO
2 measurements were corrected for tumor temperature, which was determined by inserting a thermocouple needle probe (Ellab Instruments, Copenhagen, Denmark) into the tumor after the pO
2 measurement. Measurements with negative values were included provided that these were between 0 and −2.0 mmHg, since measurements have an uncertainty of +/− 2.0 mmHg. Values below −2.0 mmHg were discarded as invalid. Data are shown as a histogram from which we selected the median pO
2 and fraction of pO
2 values ≤5.0 mmHg; the former value likely reflecting the overall oxygenation status and the latter value radiobiological hypoxia; both parameters have been shown to correlate with radiobiological hypoxia [
26].
2.5. Immunohistochemistry
The thawed cryosections of interest (two of the 10 sections from each layer) were fixed in a neutral buffered formalin solution (NBF), and endogenous peroxidases were blocked with H2O2. Then, the sections were rinsed in PBS and slides transferred to LabVision Autostainer 480 (LabVision, Femint, CA, USA). To prevent non-specific background staining, sections were treated with a protein blocking solution (DAKO X0909). Afterwards, the slides were incubated for 40 min with the primary antibody, rabbit polyclonal antibody against pimonidazole (Pab2627 from Hypoxyprobe, Burlington, MA, USA). The primary antibody was detected using the anti-rabbit IgG–horseradish peroxidase-conjugated polymers with a 30 min incubation time (EnVision rabbit reagent, K4003; DakoCytomation, Glostrup, Denmark). Lastly, sections were counterstained with Mayers hematoxylin.
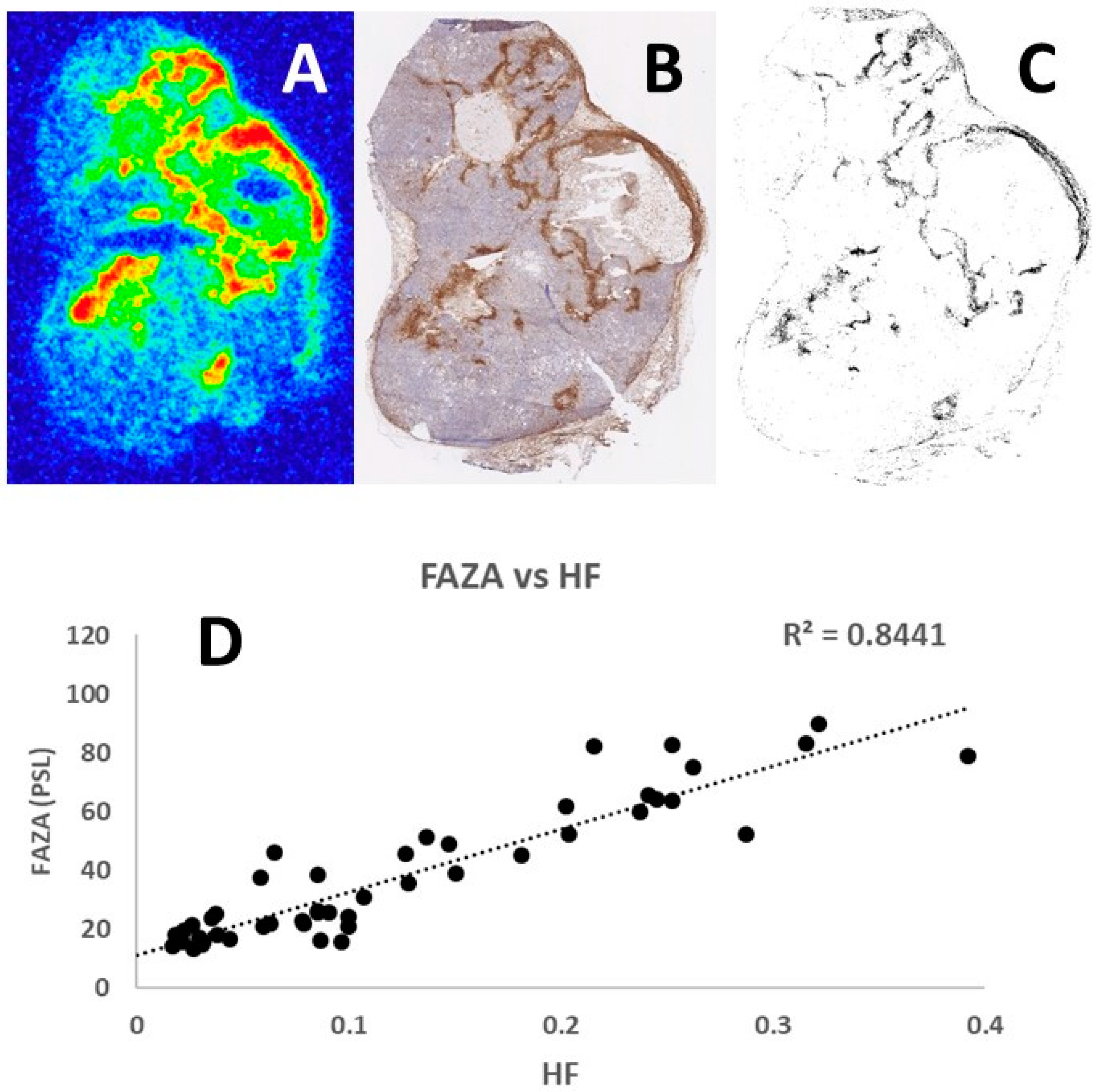
2.6. Comparison of [18F]-FAZA Autoradiography and Pimonidazole Images
Pimonidazole and [18F]-FAZA are closely related tracers with a similar binding mechanism. However, since unbound (hypoxia unrelated) pimonidazole is removed during the staining protocol, and binding can be resolved at a cellular level, pimonidazole provides highly reliable information on the distribution of chronically hypoxic cells (“ground truth”). Since autoradiograms contain an unknown amount of unbound [18F]-FAZA, we validated the hypoxia-specificity of [18F]-FAZA by a direct comparison of total tracer signal and density of hypoxic cells. In short, pimonidazole-stained tumor sections were digitalized at a high resolution by the Hamamatsu NanoZoomer 2.0 HT slide scanner (Hamamatsu Photonics, Hamamatsu City, Japan) images were thresholded in ImageJ (NIH, National Institutes of Health, Bethesda, MD, USA), resulting in maps displaying the distribution of viable hypoxic (pO
2 < 10 mmHg) cells (for further details, see reference [
27]). [18F]-FAZA autoradiograms and pimonidazole hypoxia maps were manually co-registered using different landmarks such as tissue section periphery or tissue holes/folds. Following co-registration, the spatial relationship between [18F]-FAZA signal and hypoxic fraction were analyzed by covering the sections with a grid consisting of 1 mm
2 squares. Only squares fully within the tumor periphery and largely free of necrosis were included.
2.7. Data Analysis and Statistics
Correlations between [18F]-FAZA signal intensities and pimonidazole-estimated hypoxic fractions were expressed as Pearson regression coefficients. For the gene expression analysis, thresholds were set manually in the SDS2.1 software. The threshold cycle (CT) values above 35 were regarded as below the detection limit. The gene expression levels of hypoxia-induced genes were calculated using the comparative CT method [
28]. ΔCT values were generated by normalizing to the geometric mean of the reference genes ACTR3, NDFIP1, and RPL37A. The fold up-regulation of hypoxic areas compared to normoxic areas was calculated by 2
-ΔCT Hypoxia/2
-ΔCT Normoxia. Results from the in vitro gene expression represent data from duplicates of three independent experiments. The results from in vivo gene expression were compiled of 43 samples from non-hypoxic areas and 33 samples from hypoxic areas for the PC3 xenografts, while the results from the in vivo DU-145 xenografts were compiled of 34 samples from non-hypoxic areas and 43 samples from hypoxic areas. Eppendorf oxygen electrode measurements were conducted in single flank tumors in both the PC3 and the DU-145 xenografts. All values are listed as Mean ± 1 Standard Error. Gene expression in hypoxic and non-hypoxic areas of both the DU-145 and the PC3 tumor xenografts were also compared with a Student’s T-test with a
p-value < 0.05 being considered significant.
3. Results
Representative examples of the tumor section images obtained following [18F]-FAZA autoradiography and pimonidazole immunohistochemistry are shown in
Figure 2. Comparison of the [18F]-FAZA signal and density of pimonidazole positive cells in the same grid areas increased in a similar fashion, resulting in a linear regression coefficient of 0.8441. Analysis of other tumor sections showed similar positive results with the regression coefficient always around 0.9, thus confirming the potential of using this refined autoradiography and laser-guided microdissection approach to identify hypoxic tumor areas.
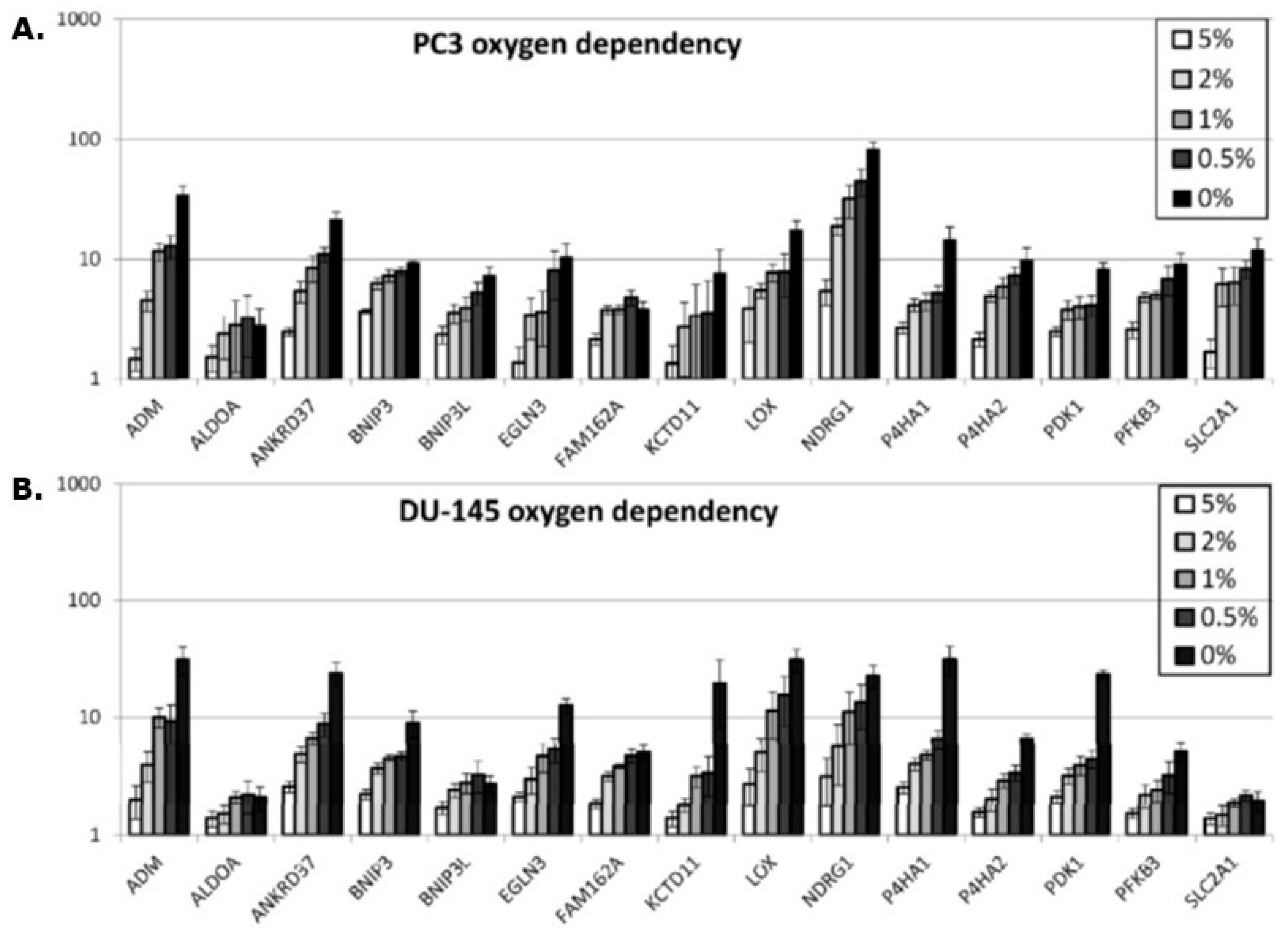
In vitro, a hypoxia-induced gene expression in all 15 genes was observed in both the PC3 and DU145 prostate cancer cell lines (
Figure 3A,B). It is worth noticing that the fold up-regulation increases as oxygen tension is lowered, but not with the similar intensity in the two cell lines. In the DU-145 cell line, the increase is steeper for the genes EGLN3, KCTD11, P4HA1, and PDK1 when the oxygen tension is decreased from 0.5% to 0% compared to the same genes in the PC3 cell line. The NDRG1 gene expression is much higher in the PC3 cells compared to the DU-145.
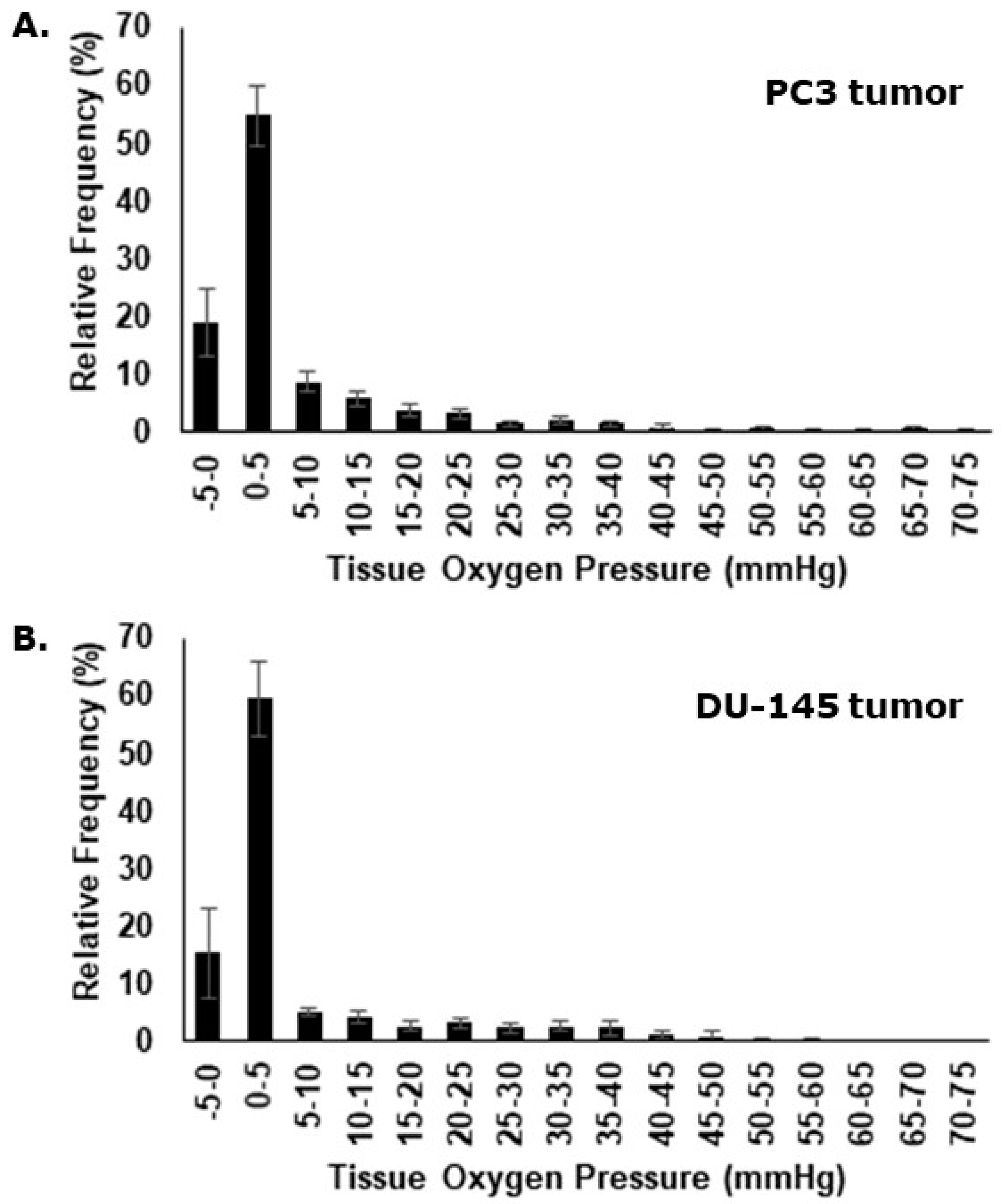
Eppendorf oxygen electrode measurements were assessed individually and as a compiled dataset. Oxygenation measurements performed in the PC3 tumor ranged from 0 to 70 mmHg with relative frequencies peaking in the very hypoxic spectrum (
Figure 4A). Median values and the fraction of pO
2 values ≤ 5.0 mmHg, were determined for each individual animal. The mean median value for all animals was 1.7 mmHg (± 0.3 mmHg) and the mean fraction of pO
2 values ≤ 5.0 mmHg was 73.7% (± 3.6%). For the oxygenation measurements in the DU-145 xenografts, pO
2 ranged from 0 to 60 mmHg (
Figure 4B), with a mean median value of 2.1 mmHg (± 0.6 mmHg), and a mean fraction of pO
2 values ≤ 5.0 mmHg of 73.0% (±5.6%).
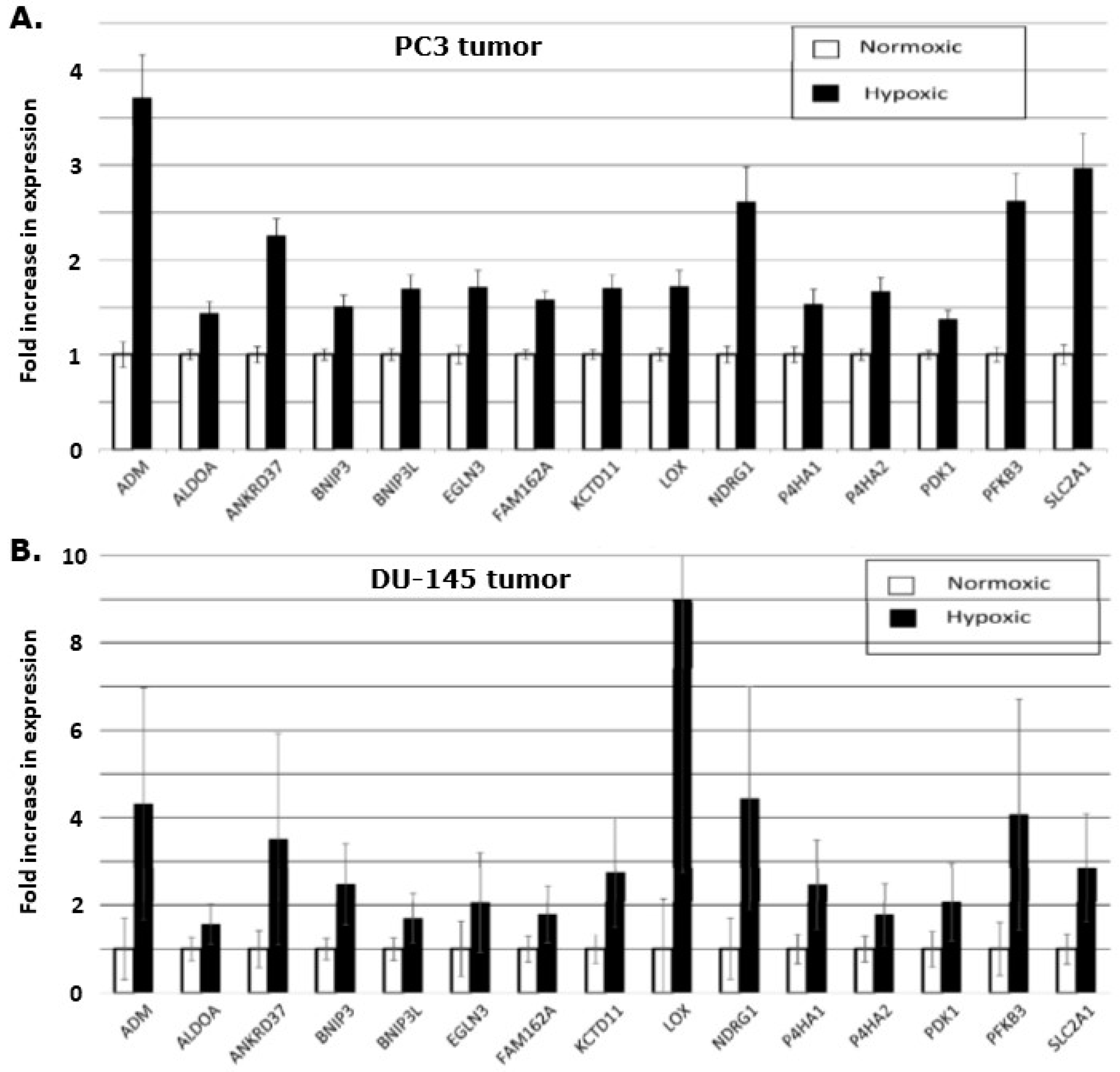
The gene expression levels were quantified in the different fractions of tissue from the sectioning procedure, and the level of the hypoxia inducible genes were compared between the hypoxic and the normoxic areas of the tumor sections. This demonstrated that all 15 genes were significantly up-regulated when comparing hypoxic to non-hypoxic regions in the tumor (
Figure 5). A comparison of the genes in the two xenografts revealed an uneven up-regulation. In the DU-145 xenograft, the LOX-gene is nearly 9-fold increased, but it only increased by 1.75-fold in the PC3 xenograft. The size of the standard error of the mean is also larger in the DU-145 LOX-gene expression compared to the LOX-gene expression in the PC3 xenograft, reflecting larger variation in the hypoxic level in the DU-145 xenografts.
4. Discussion
We have developed a method that applies laser-guided microdissection in unfixed cryo-preserved tissue sections based on autoradiograms displaying hypoxic and non-hypoxic regions and validated it using a hypoxia gene signature. The method is broadly applicable and can be applied for establishing intra-tumoral spatial links between gene expression and tracers that provides information on microenvironmental (i.e., hypoxia in the present study), metabolism (i.e., FDG/14C-2DG), proliferation (i.e., FLT), and other biological features of interest.
Pimonidazole is a well-known and consistent hypoxic tracer that binds at a partial oxygen pressure <10 mmHg [
29]. The pimonidazole adducts can subsequently be visualized using immunohistochemical techniques and is often considered a gold standard. A few studies have reported staining in non-hypoxic tissue such as areas of keratinization in head and neck cancers [
30]. In addition, inappropriate antibody levels may reduce the dynamic range of the pimonidazole assay dramatically [
31]. Regardless, we, and others, have shown pimonidazole staining patterns typical of chronic hypoxia, with distinct accumulation at a distance from vessels, and in peri-necrotic tissue in a large number of preclinical tumor models [
32,
33]. Pimonidazole has also been used as a hypoxia marker in prostate cancer patients [
34,
35,
36]. A comparison of [18F]-FAZA autoradiography and pimonidazole images has been done before, revealing a linear relationship between the [18F]-FAZA signal and the density of pimonidazole-positive hypoxic cells [
37]. In our experiments, we have made several comparisons of [18F]-FAZA autoradiograms and pimonidazole images, similar to the example shown in
Figure 2, and all analyses showed a regression coefficient at approximately 0.90. Since pimonidazole is one of the most verified and validated hypoxic markers, the consistent correlation with [18F]-FAZA autoradiograms reveals a strong association and correspondence between the two methods.
By using fresh frozen tumor material, the RNA degradation is minimal when compared to formalin-fixed and paraffin-embedded (FFPE) tumor samples [
38]. The fact that FFPE samples often are exposed to watery solution procedures during immunohistochemical staining protocols worsens the RNA degradation [
38]. Marotta et al. stated that their previously developed EF5 fixation method led to RNA degradation [
22]. They managed to change the “staining protocol” in order to obtain reliable results, but some degree of RNA degradation was still expected. Ragnum and colleagues described how the index tumor biopsies were FFPE before immunohistochemistry was performed [
20]. The validation cohort was from two prostate cancer gene expression datasets, and the tissue fixation protocol of these tumor samples was not described. In the study by Yang et al., the biopsies from seven of the 11 validation cohorts were from FFPE samples, the remainder being fresh frozen tumor samples [
21]. Our study, using [18F]-FAZA-autoradiography, enabled us to keep the tumors in an unfixed cryopreserved condition during the whole experiment; from tumor excision until the final steps of dissection and lysing of the hypoxic and non-hypoxic areas of interest.
One study attempted to compare the location of [18F]-FAZA-PET with immunohistochemical staining of hypoxia markers hypoxia inducible factor-1α (HIF-1α) and carbon anhydrase XI (CAXI) to validate [18F]-FAZA-PET as a useful hypoxia identification tool in prostate cancer patients [
39]. In none of the 14 patients involved in the study were the tumor nodules visualized by [18F]-FAZA-PET. Nor were the nodules positive for CAIX staining, while the HIF-1α staining was inconsistent. The authors suggested that there might not be any correlation between hypoxia and HIF-1α in prostate cancer, but they could not explain the absence of [18F]-FAZA-PET visualization. Supiot and colleagues used another nitroimidazole-derived PET hypoxia-tracer, namely [18F]-FMISO [
40]. In their pilot study, they found that only about 75% of patients with prostate cancer, at any stage, would have [18F]-FMISO-detectable tumors. The small sample size in the Garcia-Parra study [
39] could have played a role in their failure to detect [18F]-FAZA-positive tumors. Another factor that could help explain this lack of detection has been suggested [
40] and one that involves an underestimation of [18F]-MISO positive areas because of small volumes of hypoxic areas within the prostate tumors; with current PET-scanning techniques, one cannot map volumes less than a diameter of 3 mm. The use of autoradiography in our study allowed us to detect even small [18F]-FAZA-positive areas within the tumors. The inconsistencies in detecting hypoxia in prostate cancer using PET-based approaches does not mean that hypoxia does not exist at meaningful levels in this tumor type or that it is not relevant. Clinical studies in prostate cancer patients using oxygen electrodes [
18,
19] and the more relevant hypoxic tracer pimonidazole [
34,
35,
36] clearly demonstrated significant levels of hypoxia. Furthermore, these measurements correlated with response to radiation treatment [
18,
19] and with tumor aggressiveness [
34].
A comprehensive analysis of eight published hypoxia signatures in 8006 tumors across 19 cancer types demonstrated a significant correlation between the hypoxia scores generated using the different signatures. Furthermore, the gene expression level of the hypoxia-induced genes were highest in squamous cell carcinomas of the head and neck, cervix and lung, whereas adenocarcinomas of the prostate and thyroid had the lowest level of hypoxic inducible gene expression [
41]. This can be interpreted as squamous cell carcinomas of the head and neck being more hypoxic than prostate cancer. However, this must be considered with the reservation that the expression level of some of the hypoxia-inducible genes may be differentially regulated in the different cancer types, even though the genes in the 15-gene hypoxia classifier in vitro have demonstrated to be similarly expressed across different cancer types [
16].
Our current study found that all the 15 genes in the hypoxia gene signature were up-regulated in vitro under hypoxic conditions and that the degree of expression increased as the oxygen concentration decreased (Figure 3A,B). This validated the use of the genes for testing the refined microdissection procedure, and applying this approach to the two prostate tumor types, we found similar increased expression of the hypoxia inducible genes (Figure 5A,B), although here, we do not know the exact oxygen concentration. The Eppendorf pO
2 measurements in the PC3 and the DU-145 xenografts demonstrated that these tumor types are generally hypoxic with the percentage of pO
2 values ≤ 5.0 mmHg being 74% and 73%, respectively. Oxygen electrode measurements have clearly shown that low tumor pO
2 values correlate with poor clinical outcome for a review, see [
42]. In fact, this has even been seen in prostate cancer [
18,
19]. The use of oxygen electrodes is generally considered to be a reliable tool to determine hypoxia in tumors, but the method cannot distinguish between acute and chronic hypoxia or low pO
2 readings due to tumor necrosis. In the study by Milosevic and colleagues [
18], direct pO
2 measurements were made in 247 prostate cancer patients. They found pO
2 values ≤ 5.0 mmHg in 35% of the measurements and pO
2 values ≤ 10.0 mmHg in 63% of the readings. The xenografts in our study were grown ectopically (in the flank) compared to an orthotopic location in the prostate gland as in patients in the Milosevic study. Tumor micro-environmental differences due to the location and geno-/pheno-topical dissimilarities may explain the variation in the oxygen partial pressures measured between the clinical study and our pre-clinical evaluation. One study has suggested that pO
2 values obtained with the Eppendorf histograph were substantially lower in tumors grown in mice compared to human tumors [
43], so our findings are not unexpected. What is important is that all 15 genes up-regulated in the in vitro study were also up-regulated in the hypoxic tumor areas, compared to the normoxic areas, in both tumor types. This 15-gene hypoxia classifier was developed in squamous cell carcinoma cell lines and refined in head and neck squamous cell carcinoma patients [
13,
14]. In the review by Harris and colleagues [
12] a total of 32 different hypoxia gene classifiers, in various cancer types, were found following a comprehensive literature search in the most common databases. Nine of the 15 genes in the Toustrup-15-gene hypoxia classifier appeared in the top 20 most frequently appearing genes from all 32 reviewed classifiers. The Toustrup hypoxic gene classifier is also the only classifier that has been directly linked to direct pO
2 measurements [
14]. One study, in which a 28-gene hypoxia gene classifier was created, tried to further validate and compare their findings by testing the Toustrup gene signature on their prostate cancer patient cohorts and found it to be prognostic [
21]. Furthermore, the results revealed a strong correlation between their 28-gene scores and the Toustrup signature scores. These findings show that the Toustrup gene-signature is applicable in many tumor types, including prostate cancer.