Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging
Abstract
Simple Summary
Abstract
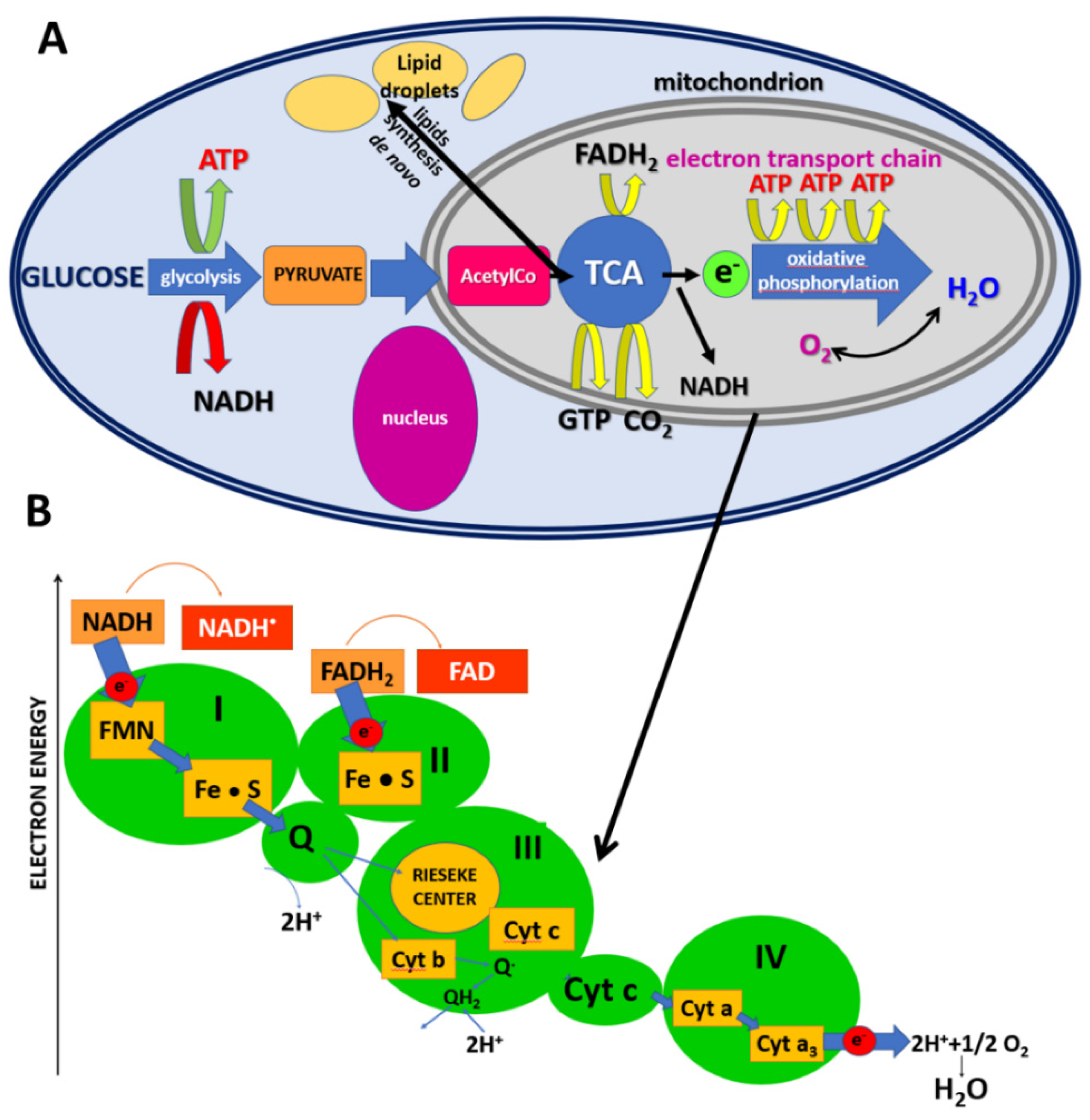
1. Introduction
2. Materials and Methods
2.1. Reference Chemicals
2.2. Patients
2.3. Tissues Samples Collection and Preparation for Raman Spectroscopy
2.4. Cell Culture and Preparation for Raman Spectroscopy
2.5. Raman Human Tissues Spectroscopic Measurements Ex Vivo
2.6. Statistical Analysis
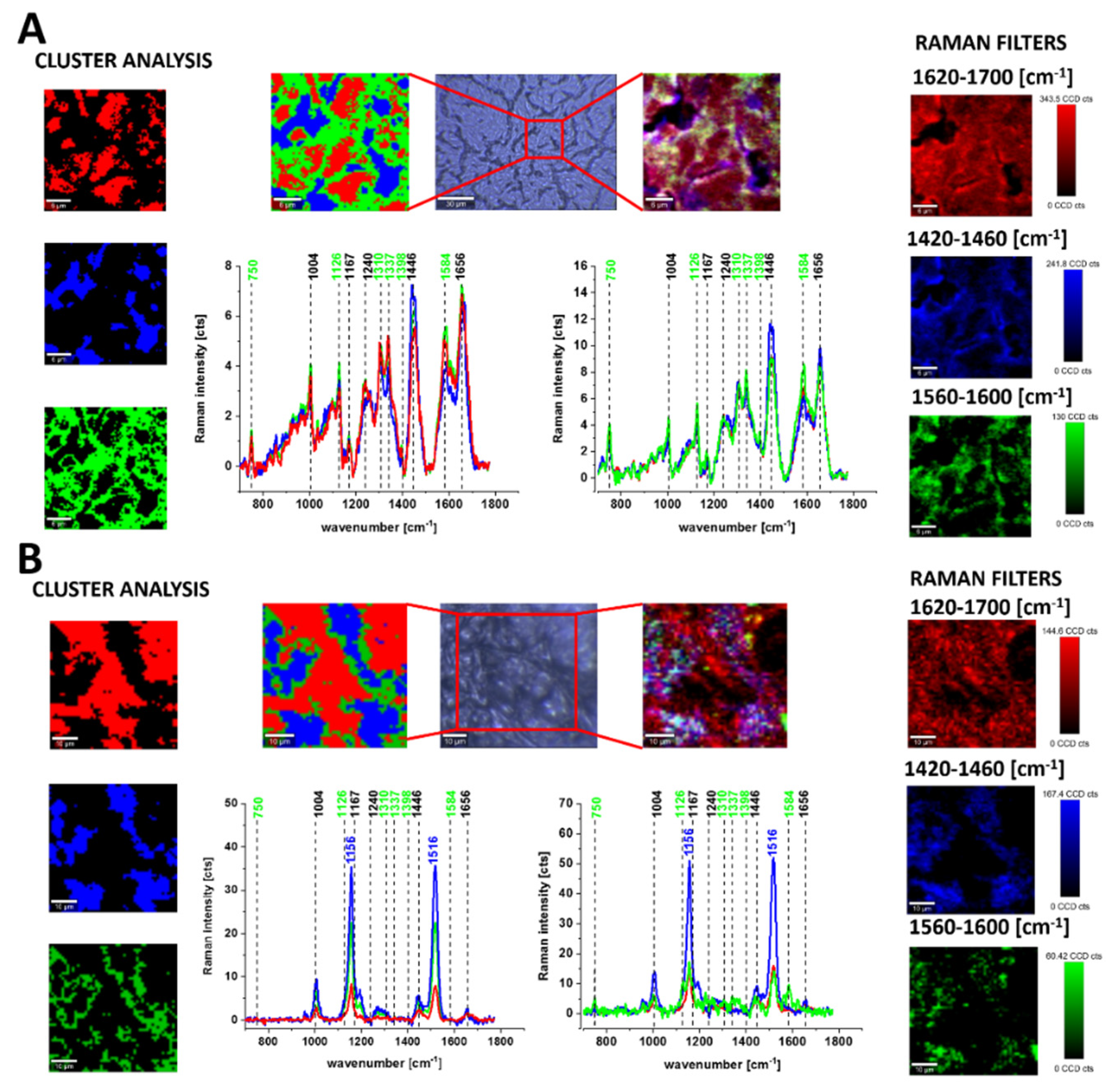
2.7. Cluster Analysis
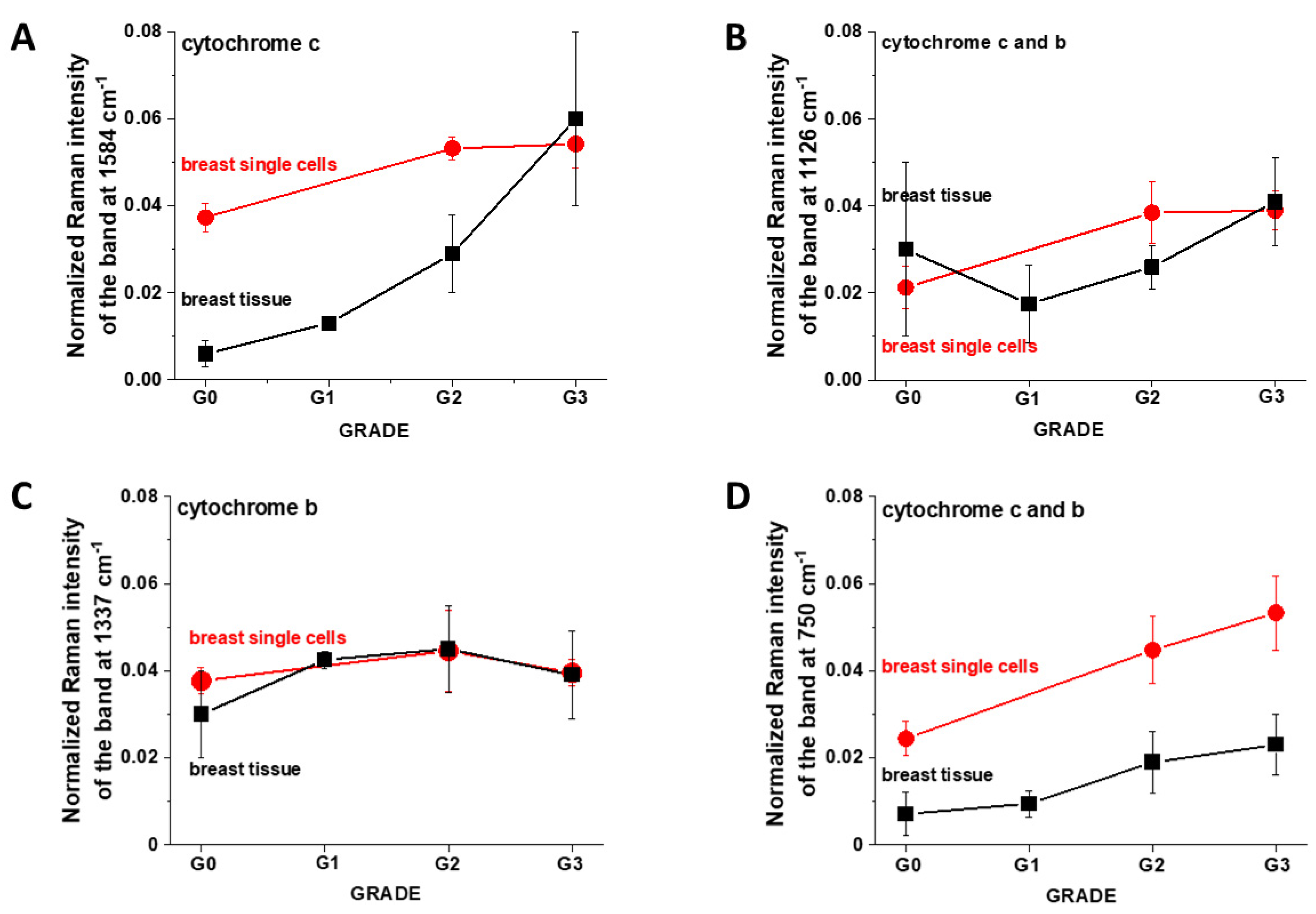
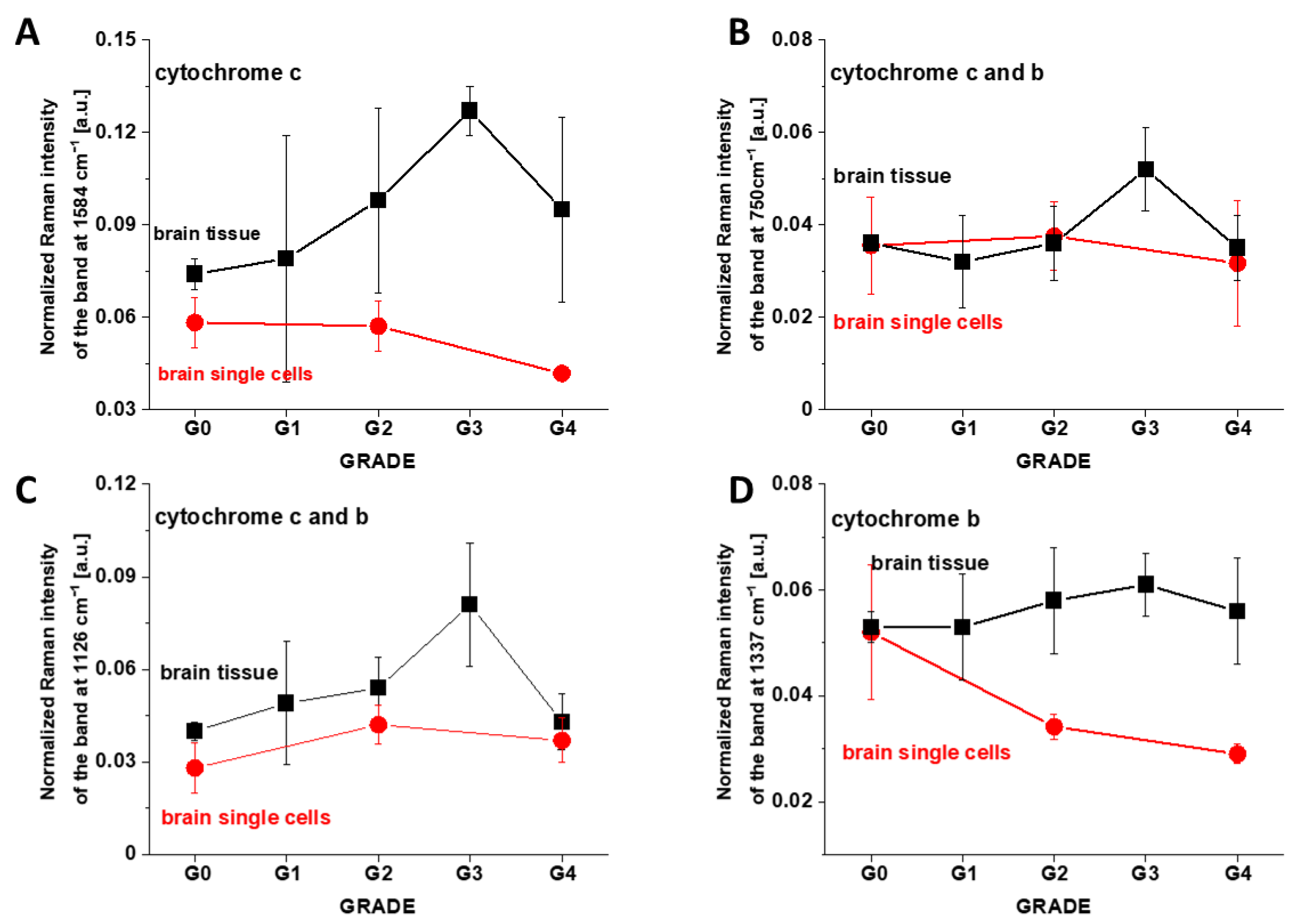
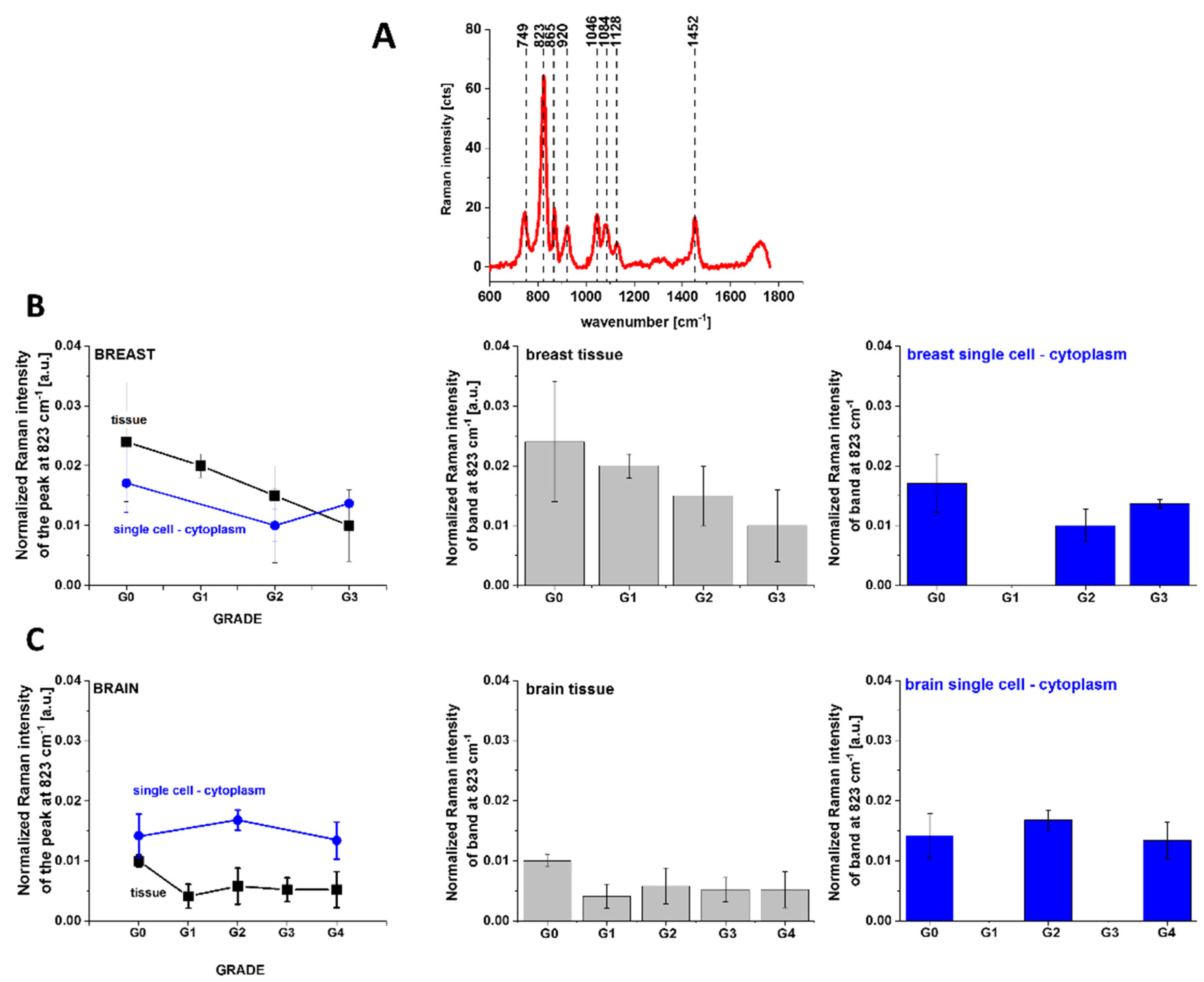
3. Results
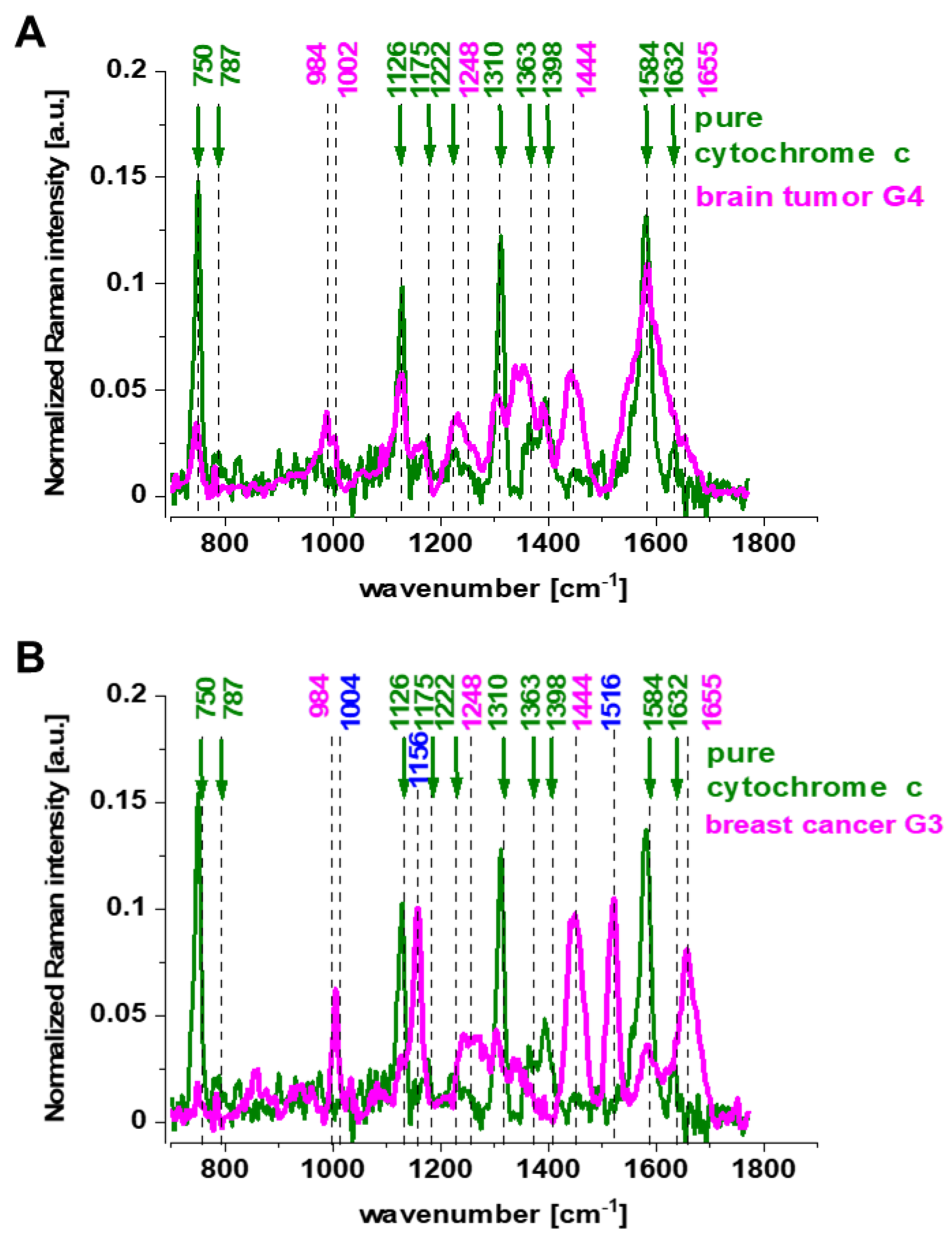
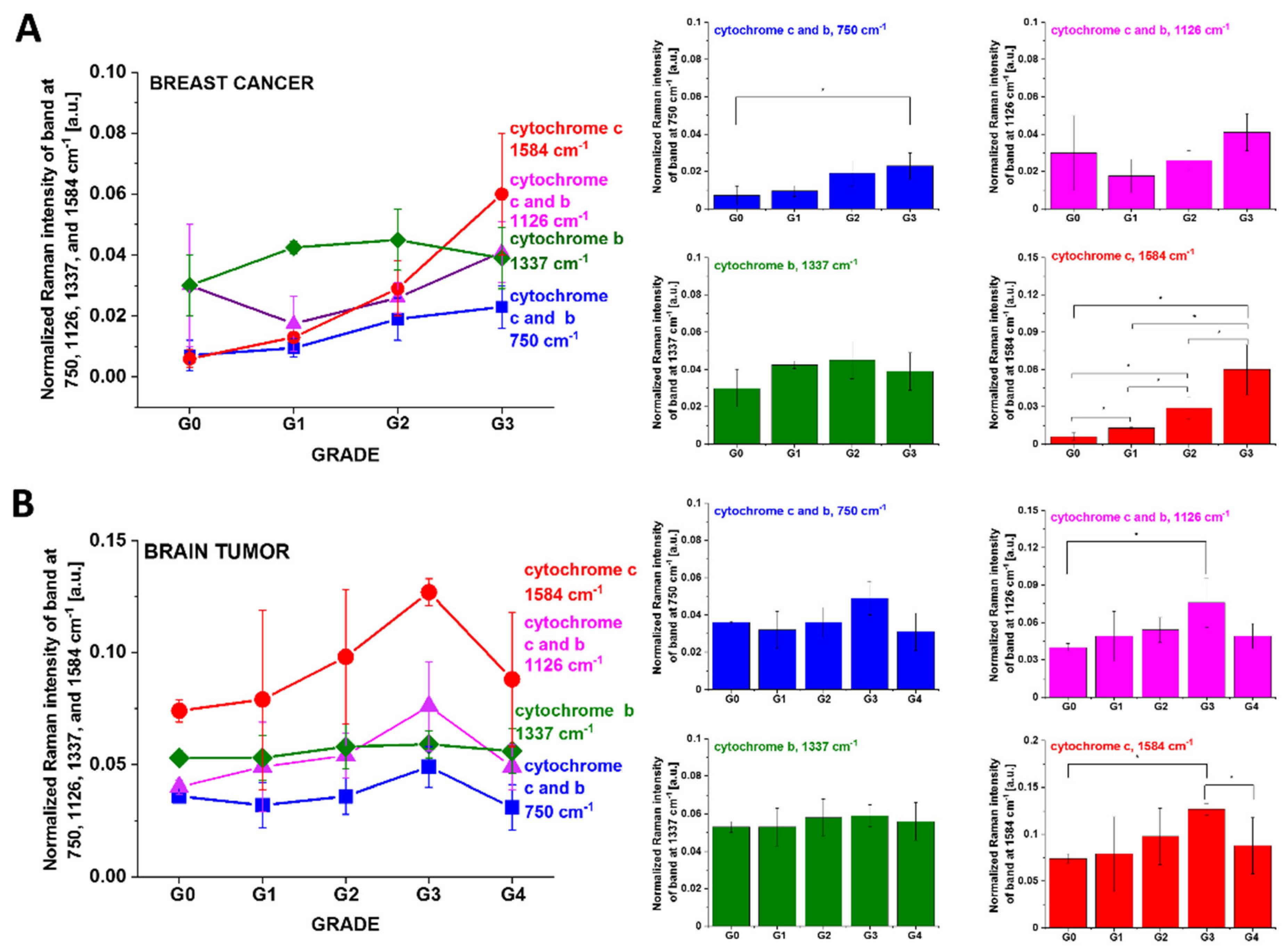
3.1. Cytochromes in Human Cancer Tissues
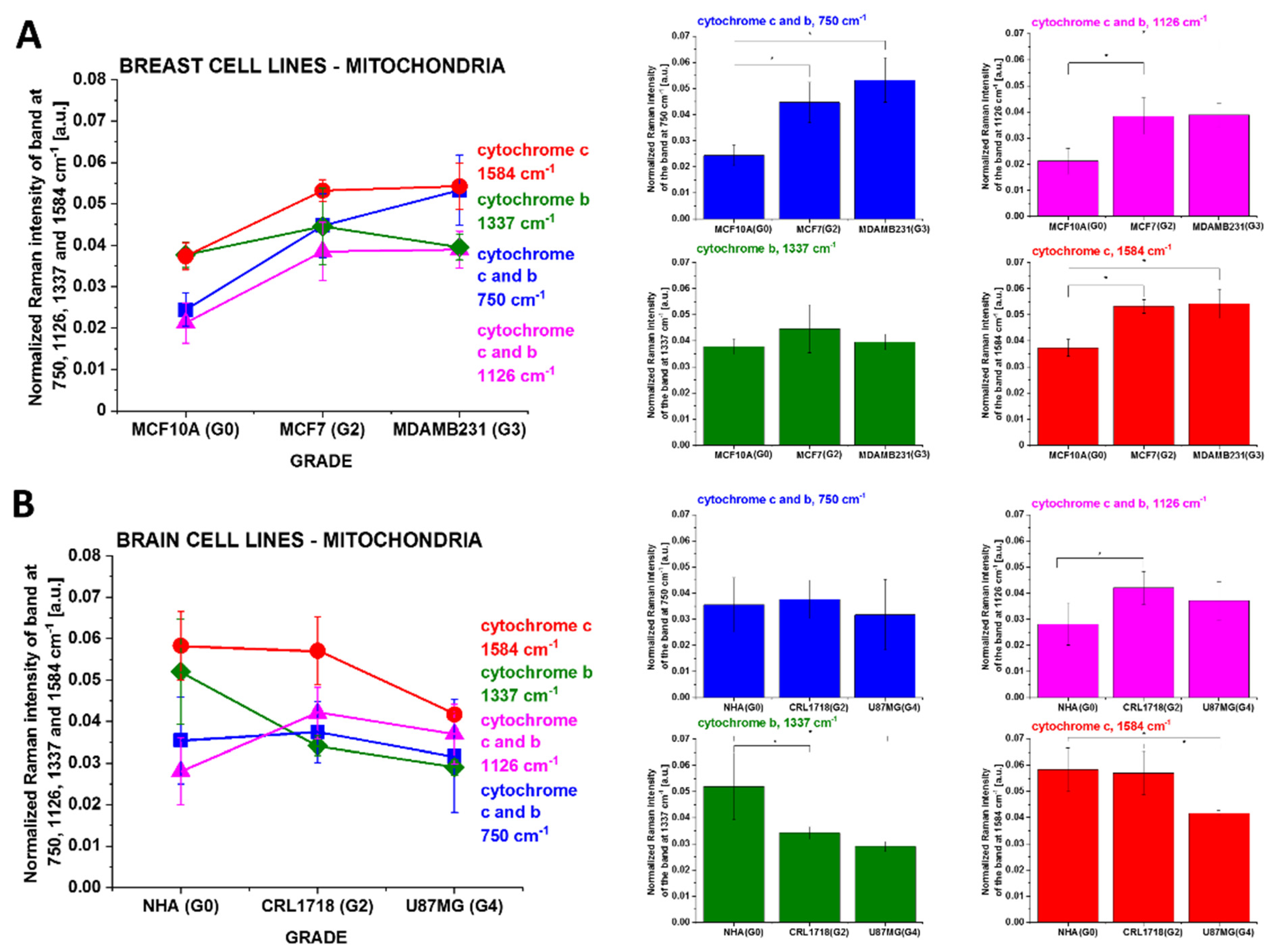
3.2. Cytochromes in Cancer Human Single Cells
4. Discussion
4.1. Discrepancies between Tissues and In Vitro Cells vs. Cancer Aggressiveness
4.2. Lipid Synthesis de Novo
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic Pathways Promoting Cancer Cell Survival and Growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Simon, M.C. Oncogenes Strike a Balance between Cellular Growth and Homeostasis. Semin. Cell Dev. Biol. 2015, 43, 3–10. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty Acid Oxidation Is Required for the Respiration and Proliferation of Malignant Glioma Cells. Neuro. Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Jarota, A.; Surmacki, J.; Imiela, A.; Kopeć, M. A Look into the Use of Raman Spectroscopy for Brain and Breast Cancer Diagnostics: Linear and Non-Linear Optics in Cancer Research as a Gateway to Tumor Cell Identity. Expert Rev. Mol. Diagn. 2020, 20, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Imiela, A.; Brozek-Pluska, B.; Kopec, M. Advances in Raman Imaging Combined with AFM and Fluorescence Microscopy Are Beneficial for Oncology and Cancer Research. Nanomedicine 2019, 14, 1873–1888. [Google Scholar] [CrossRef]
- Kopec, M.; Imiela, A.; Abramczyk, H. Monitoring Glycosylation Metabolism in Brain and Breast Cancer by Raman Imaging. Sci. Rep. 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Imiela, A.; Śliwińska, A. Novel Strategies of Raman Imaging for Exploring Cancer Lipid Reprogramming. J. Mol. Liq. 2019, 274, 52–59. [Google Scholar] [CrossRef]
- Polis, B.; Imiela, A.; Polis, L.; Abramczyk, H. Raman Spectroscopy for Medulloblastoma. Childs Nerv. Syst. 2018, 34, 2425–2430. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A. The Biochemical, Nanomechanical and Chemometric Signatures of Brain Cancer. Spectrochim Acta A Mol. Biomol. Spectrosc. 2018, 188, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Imiela, A.; Polis, B.; Polis, L.; Abramczyk, H. Novel Strategies of Raman Imaging for Brain Tumor Research. Oncotarget 2017, 8, 85290–85310. [Google Scholar] [CrossRef]
- Surmacki, J.; Brozek-Pluska, B.; Kordek, R.; Abramczyk, H. The Lipid-Reactive Oxygen Species Phenotype of Breast Cancer. Raman Spectroscopy and Mapping, PCA and PLSDA for Invasive Ductal Carcinoma and Invasive Lobular Carcinoma. Molecular Tumorigenic Mechanisms beyond Warburg Effect. Analyst 2015, 140, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, R.G.; Weis, S.; Mayr, J.A.; Zimmermann, F.; Geilberger, R.; Sperl, W.; Kofler, B. Alterations of Oxidative Phosphorylation Complexes in Astrocytomas. Glia 2014, 62, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Deighton, R.F.; Le Bihan, T.; Martin, S.F.; Gerth, A.M.J.; McCulloch, M.; Edgar, J.M.; Kerr, L.E.; Whittle, I.R.; McCulloch, J. Interactions among Mitochondrial Proteins Altered in Glioblastoma. J. Neurooncol. 2014, 118, 247–256. [Google Scholar] [CrossRef]
- DeHaan, C.; Habibi-Nazhad, B.; Yan, E.; Salloum, N.; Parliament, M.; Allalunis-Turner, J. Mutation in Mitochondrial Complex I ND6 Subunit Is Associated with Defective Response to Hypoxia in Human Glioma Cells. Mol. Cancer 2004, 3, 19. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Chishiki, M.; Takagi, K.; Sato, A.; Miki, Y.; Yamamoto, Y.; Ebata, A.; Shibahara, Y.; Watanabe, M.; Ishida, T.; Sasano, H.; et al. Cytochrome C1 in Ductal Carcinoma in Situ of Breast Associated with Proliferation and Comedo Necrosis. Cancer Sci. 2017, 108, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Perna, G.; Scrima, R.; Cela, O.; Rinaldi, R.; Boffoli, D.; Capozzi, V.; Capitanio, N. A Novel Redox State Heme a Marker in Cytochrome c Oxidase Revealed by Raman Spectroscopy. Phys. Scr. 2005, 2005, 199. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.-C.; Petrecca, K.; Leblond, F. Intraoperative Brain Cancer Detection with Raman Spectroscopy in Humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; So, J.B.Y.; Shabbir, A.; Huang, Z. Fiber-Optic Raman Spectroscopy Probes Gastric Carcinogenesis In Vivo at Endoscopy. J. Biophotonics 2013, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Diagnosing Breast Cancer by Using Raman Spectroscopy. Prac. Natl. Aaad. Sci. USA 2005, 102, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Chiu, L.; Kanda, H.; Kawagoe, H.; Ozawa, T.; Nakamura, M.; Nishida, K.; Fujita, K.; Fujikado, T. Using Redox-Sensitive Mitochondrial Cytochrome Raman Bands for Label-Free Detection of Mitochondrial Dysfunction. Analyst 2019, 144, 2531–2540. [Google Scholar] [CrossRef]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A Brain Tumor Molecular Imaging Strategy Using A New Triple-Modality MRI-Photoacoustic-Raman Nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Brozek-Pluska, B.; Kopec, M.; Błaszczyk, M.; Radek, M. Redox Imbalance and Biochemical Changes in Cancer by Probing Redox-Sensitive Mitochondrial Cytochromes in Label-Free Visible Resonance Raman Imaging. bioRxiv 2020. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.M.; Brozek-Pluska, B. Redox State Changes of Mitochondrial Cytochromes in Brain and Breast Cancers by Raman Spectroscopy and Imaging. bioRxiv 2020. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A.; Brożek-Płuska, B.; Kopeć, M.; Surmacki, J.; Śliwińska, A. Aberrant Protein Phosphorylation in Cancer by Using Raman Biomarkers. Cancers 2019, 11, 2017. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A.; Surmacki, J. Novel Strategies of Raman Imaging for Monitoring Intracellular Retinoid Metabolism in Cancer Cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Strekas, T.C.; Spiro, T.G. Cytochrome c: Resonance Raman Spectra. Biochim. Biophys. Acta (BBA) Protein Struct. 1972, 278, 188–192. [Google Scholar] [CrossRef]
- Hu, S.; Morris, I.K.; Singh, J.P.; Smith, K.M.; Spiro, T.G. Complete Assignment of Cytochrome c Resonance Raman Spectra via Enzymic Reconstitution with Isotopically Labeled Hemes. J. Am. Chem. Soc. 1993, 115, 12446–12458. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B. New Look inside Human Breast Ducts with Raman Imaging. Raman Candidates as Diagnostic Markers for Breast Cancer Prognosis: Mammaglobin, Palmitic Acid and Sphingomyelin. Anal. Chim. Acta 2016, 909, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Brozek-Pluska, B. Apical-Basal Polarity of Epithelial Cells Imaged by Raman Microscopy and Raman Imaging: Capabilities and Challenges for Cancer Research. J. Mol. Liq. 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Jablonska-Gajewicz, J.; Kordek, R. Raman “optical Biopsy” of Human Breast Cancer. Prog. Biophys. Mol. Biol. 2012, 108, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Brozek-Pluska, B.; Musial, J.; Kordek, R.; Bailo, E.; Dieing, T.; Abramczyk, H. Raman Spectroscopy and Imaging: Applications in Human Breast Cancer Diagnosis. Analyst 2012, 137, 3773–3780. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J. Antitumor Activity of Dietary Carotenoids, and Prospects for Applications in Therapy. In Carotenoids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 31–42. ISBN 978-1-118-62222-3. [Google Scholar]
- Consani, C.; Bräm, O.; van Mourik, F.; Cannizzo, A.; Chergui, M. Energy Transfer and Relaxation Mechanisms in Cytochrome c. Chem. Phys. 2012, 396, 108–115. [Google Scholar] [CrossRef]
- Jadasz, J.J.; Kremer, D.; Göttle, P.; Tzekova, N.; Domke, J.; Rivera, F.J.; Adjaye, J.; Hartung, H.-P.; Aigner, L.; Küry, P. Mesenchymal Stem Cell Conditioning Promotes Rat Oligodendroglial Cell Maturation. PLoS ONE 2013, 8, e71814. [Google Scholar] [CrossRef]
- Brazhe, N.A.; Treiman, M.; Brazhe, A.R.; Find, N.L.; Maksimov, G.V.; Sosnovtseva, O.V. Mapping of Redox State of Mitochondrial Cytochromes in Live Cardiomyocytes Using Raman Microspectroscopy. PLoS ONE 2012, 7, e41990. [Google Scholar] [CrossRef]
- Tumor Grade Fact Sheet—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/prognosis/tumor-grade-fact-sheet (accessed on 3 December 2020).
- Strickland, M.; Stoll, E.A. Metabolic Reprogramming in Glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Moraes, C.T.; Murphy, M.P.; Budinger, G.R.S.; Chandel, N.S. The Qo Site of the Mitochondrial Complex III Is Required for the Transduction of Hypoxic Signaling via Reactive Oxygen Species Production. J. Cell Biol. 2007, 177, 1029–1036. [Google Scholar] [CrossRef]
- Danielson, P.B. The Cytochrome P450 Superfamily: Biochemistry, Evolution and Drug Metabolism in Humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef]
- Singh, B.; Berry, J.A.; Shoher, A.; Ramakrishnan, V.; Lucci, A. COX-2 Overexpression Increases Motility and Invasion of Breast Cancer Cells. Int. J. Oncol. 2005, 26, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in Glioblastoma Multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.R.; Wolfe, M.M. COX-2–Selective NSAIDs: New and Improved? JAMA 2000, 284, 1297–1299. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Hernandez-Diaz, S.; Honein, M.A. Use of Over-the-Counter Medications during Pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J.; Kopeć, M.; Olejnik, A.K.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The Role of Lipid Droplets and Adipocytes in Cancer. Raman Imaging of Cell Cultures: MCF10A, MCF7, and MDA-MB-231 Compared to Adipocytes in Cancerous Human Breast Tissue. Analyst 2015, 140, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L.; et al. Fatty Acid Uptake and Lipid Storage Induced by HIF-1α Contribute to Cell Growth and Survival after Hypoxia-Reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on Fat: The Role of Lipid Synthesis in Cancer Metabolism and Tumour Development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Adar, F.; Erecinska, M. Spectral Evidence for Interactions between Membrane-Bound Hemes: Resonance Raman Spectra of Mitochondrial Cytochrome b--C1 Complex as a Function of Redox Potential. FEBS Lett. 1977, 80, 195–200. [Google Scholar] [CrossRef]
- Kakita, M.; Kaliaperumal, V.; Hamaguchi, H. Resonance Raman Quantification of the Redox State of Cytochromes b and c In-Vivo and in-Vitro. J. Biophotonics 2012, 5, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Guppy, M.; Leedman, P.; Zu, X.; Russell, V. Contribution by Different Fuels and Metabolic Pathways to the Total ATP Turnover of Proliferating MCF-7 Breast Cancer Cells. Biochem. J. 2002, 364, 309–315. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramczyk, H.; Surmacki, J.M.; Brozek-Pluska, B.; Kopec, M. Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging. Cancers 2021, 13, 2599. https://doi.org/10.3390/cancers13112599
Abramczyk H, Surmacki JM, Brozek-Pluska B, Kopec M. Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging. Cancers. 2021; 13(11):2599. https://doi.org/10.3390/cancers13112599
Chicago/Turabian StyleAbramczyk, Halina, Jakub Maciej Surmacki, Beata Brozek-Pluska, and Monika Kopec. 2021. "Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging" Cancers 13, no. 11: 2599. https://doi.org/10.3390/cancers13112599
APA StyleAbramczyk, H., Surmacki, J. M., Brozek-Pluska, B., & Kopec, M. (2021). Revision of Commonly Accepted Warburg Mechanism of Cancer Development: Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging. Cancers, 13(11), 2599. https://doi.org/10.3390/cancers13112599








