Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of Human Polyomavirus in Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue
2.3. Immunohistochemistry of MCC Tumor Samples
2.4. Microscopic Evaluation
2.5. Multiplex-PCR and HRCE
2.6. Statistics
3. Results
3.1. Patients’ Characteristics
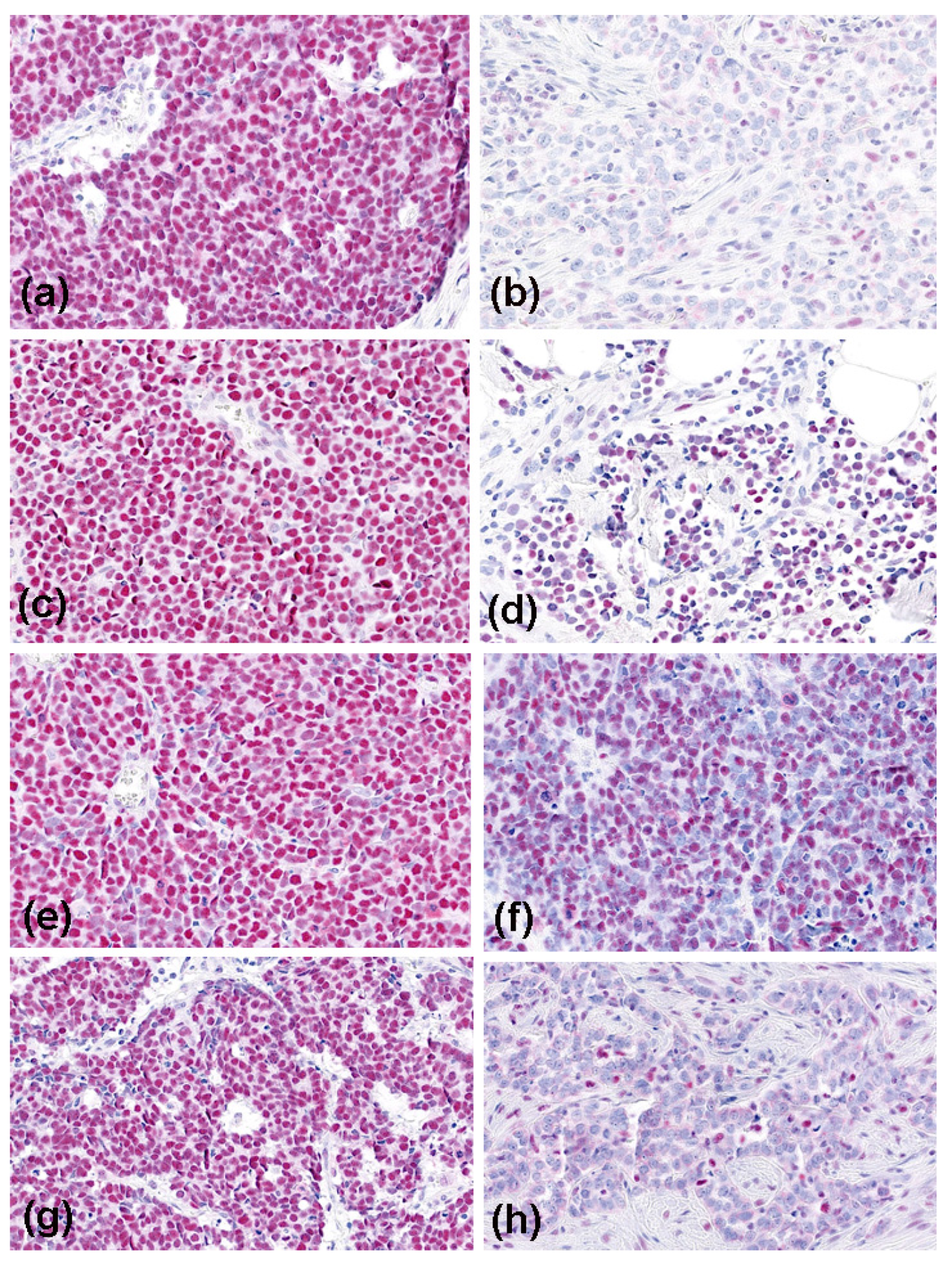
3.2. Expression of MMR Proteins in MCC
3.3. Results of MSI Testing
3.4. Patients’ Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M. Merkel cell carcinoma. Nat. Rev. Dis Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical Factors Associated With Merkel Cell Polyomavirus Infection in Merkel Cell Carcinoma. J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Schrama, D.; Ugurel, S.; Becker, J.C. Merkel cell carcinoma: Recent insights and new treatment options. Curr Opin Oncol. 2012, 24, 141–149. [Google Scholar] [CrossRef]
- Becker, J.C.; Eigentler, T.; Frerich, B.; Gambichler, T.; Grabbe, S.; Höller, U.; Klumpp, B.; Loquai, C.; Krause-Bergmann, A.; Müller-Richter, U.; et al. S2k guidelines for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—Update 2018. J. der Dtsch. Dermatol. Ges. 2019, 17, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Dreißigacker, M.; Kasakovski, D.; Skrygan, M.; Wieland, U.; Silling, S.; Gravemeyer, J.; Melior, A.; Cherouny, A.; Stücker, M.; et al. Patched 1 expression in Merkel cell carcinoma. J. Dermatol. 2021, 48, 64–74. [Google Scholar] [CrossRef]
- Nghiem, P.; Kaufman, H.L.; Bharmal, M.; Mahnke, L.; Phatak, H.; Becker, J.C. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Futur. Oncol. 2017, 13, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Lorenz, E.; Ugurel, S.; Eigentler, T.K.; Kiecker, F.; Pföhler, C.; Kellner, I.; Meier, F.; Kähler, K.; Mohr, P.; et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 2017, 8, 79731–79741. [Google Scholar] [CrossRef] [PubMed]
- Angeles, C.V.; Sabel, M.S. Immunotherapy for Merkel cell carcinoma. J. Surg. Oncol. 2021, 123, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.J.; Dharaneeswaran, H.; Starrett, G.J.; Thakuria, M.; LeBoeuf, N.R.; Silk, A.W.; DeCaprio, J.A.; Hanna, G.J. Predictors of immu-notherapy benefit in Merkel cell carcinoma. Oncotarget 2020, 24, 4401–4410. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Morgan, S.; Slodkowska, E.; Parra-Herran, C.; Mirkovic, J. PD-L1, RB1 and mismatch repair protein immunohistochemical ex-pression in neuroendocrine carcinoma, small cell type, of the uterine cervix. Histopathology 2019, 74, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Handbook; Merkel cell carcinoma; Springer: New York, NY, USA, 2017. [Google Scholar]
- Wieland, U.; Mauch, C.; Kreuter, A.; Krieg, T.; Pfister, H. Merkel Cell Polyomavirus DNA in Persons without Merkel Cell Carcinoma. Emerg. Infect. Dis. 2009, 15, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Wieland, U.; Scola, N.; Stolte, B.; Stücker, M.; Silling, S.; Kreuter, A. No evidence for a causal role of Merkel cell polyomavirus in keratoacanthoma. J. Am. Acad. Dermatol. 2012, 67, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; De La Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Ali, R.; Hussain, Z.F.; Faridi, N.; Khan, E.Y.; Bakar, S.M.A.; Edhi, M.M.; Khan, M. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with his-topathological parameters. World J. Surg. Oncol. 2017, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Vatrano, S.; Pettinato, A.; Randazzo, V.; Zagami, M.; Agueli, C.; Cannella, S.; Banna, G.L.; Fraggetta, F.; Santoro, A. Diagnostic test assessment. Validation study of an alternative system to detect microsatellite instability in colorectal carcinoma. Pathologica 2020, 112, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Jin, Z.; Hubbard, J. Management of Non-Colorectal Digestive Cancers with Microsatellite Instability. Cancers 2021, 13, 651. [Google Scholar] [CrossRef]
- Perrett, C.; Harwood, C.; McGregor, J.; Warwick, J.; Cerio, R.; Karran, P. Expression of DNA mismatch repair proteins and MSH2 polymorphisms in nonmelanoma skin cancers of organ transplant recipients. Br. J. Dermatol. 2009, 162, 732–742. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Sommerer, C.; Hartschuh, W.; Zeier, M.; Doeberitz, M.V.K.; Kloor, M. Absence of Mismatch Repair Deficiency–Related Microsatellite Instability in Non-Melanoma Skin Cancer. J. Investig. Dermatol. 2012, 132, 491–493. [Google Scholar] [CrossRef]
- Saetta, A.A.; Stamatelli, A.; Karlou, M.; Michalopoulos, N.V.; Patsouris, E.; Aroni, K. Mutations of microsatellite instability target genes in sporadic basal cell carcinomas. Pathol. Res. Pr. 2007, 203, 849–855. [Google Scholar] [CrossRef]
- Kubeček, O.; Kopecký, J. Microsatellite instability in melanoma: A comprehensive review. Melanoma Res. 2016, 26, 545–550. [Google Scholar] [CrossRef]
- Korabiowska, M.; Cordon-Cardo, C.; Jaenckel, F.; Stachura, J.; Fischer, G.; Brinck, U. Application of in situ hybridization probes for MLH-1 and MSH-2 in tissue microarrays of paraffin-embedded malignant melanomas: Correlation with immunohisto-chemistry and tumor stage. Hum. Pathol. 2004, 12, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Alvino, E.; Passarelli, F.; Cannavò, E.; Fortes, C.; Mastroeni, S.; Caporali, S.; Jiricny, J.; Cappellini, G.C.A.; Scoppola, A.; Marchetti, P.; et al. High Expression of the Mismatch Repair Protein MSH6 Is Associated With Poor Patient Survival in Melanoma. Am. J. Clin. Pathol. 2014, 142, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L. Microsatellite Instability Predicts Response to Anti-PD1 Immunotherapy in Metastatic Melanoma. Acta Dermatovenerol. Croat. ADC 2018, 26, 341–343. [Google Scholar] [PubMed]
- Ponti, G.; Pellacani, G.; Tomasi, A.; Depenni, R.; Maccaferri, M.; Maiorana, A.; Orsi, G.; Giusti, F.; Cascinu, S.; Manfredini, M. Immuno-histochemical mismatch repair proteins expression as a tool to predict the melanoma immunotherapy response. Mol. Clin. Oncol. 2020, 12, 3–8. [Google Scholar]
- Shen, J.; Ju, Z.; Zhao, W. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity un-leashed by immune checkpoint blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Samstein, R.M.; Lee, K.-W.; Havel, J.J.; Wang, H.; Krishna, C.; Sabio, E.Y.; Makarov, V.; Kuo, F.; Blecua, P.; et al. Genetic diversity of tumors with mismatch repair deficiency influences anti–PD-1 immunotherapy response. Science 2019, 364, 485–491. [Google Scholar] [CrossRef]
- Richman, S. Deficient mismatch repair: Read all about it (Review). Int. J. Oncol. 2015, 47, 1189–1202. [Google Scholar] [CrossRef]
- Arora, S.; Velichinskii, R.; Lesh, R.W.; Ali, U.; Kubiak, M.; Bansal, P.; Borghaei, H.; Edelman, M.J.; Boumber, Y. Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv. Ther. 2019, 36, 2638–2678. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Data |
|---|---|
| Age at diagnosis * (years) | 77.5 (51–95) |
| Gender m/f | 27/29 (48.2%/51.8%) |
| Primary MCC localization Head/neck (no/yes) MCPyV (negative/positive) | 32/24 (57.1%/42.9%) 11/45 (19.6%/80.4%) |
| Tumor stage at diagnosis (AJCC 2017) | I 21 (37.5%) IIA 19 (33.9%) IIB 1 (1.8%) |
| IIIA 4 (7.2%) IIIB 6 (10.7%) IV 5 (8.9%) | |
| Mismatch repair protein expression * | |
| (% positive tumor cells) | 96.3% (8.3–99.8) |
| MLH1 | 58% |
| 10th percentile | 6 (56/10.7%), all MCPyV-negative |
| Patients with low-level ** | 94.7% (7.2–99.6) |
| MSH2 | 74% |
| 10th percentile | 6 (55/10.9%), all MCPyV-negative |
| Patients with low-level ** | 91.6% (16.2–99.1) |
| MSH6 | 52% |
| 10th percentile | 5 (56/8.9%), 4 MCPyV-negative |
| Patients with low-level ** | |
| PMS2 | 93.1% (6.4–99.3) |
| 10th percentile | 32% |
| Patients with low-level ** | 6 (54/11.1%), all MCPyV-negative |
| Outcome | |
| 5-year MCC relapse (no/yes) | 34/22 (60.7%/39.3%) |
| Median time to relapse (months) * | 12 (2–60) |
| 5-year MCC (survived/deceased) | 38/18 (67.9%/32.1%) |
| Median time to death (months) * | 26 (3–60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Abu Rached, N.; Tannapfel, A.; Becker, J.C.; Vogt, M.; Skrygan, M.; Wieland, U.; Silling, S.; Susok, L.; Stücker, M.; et al. Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma. Cancers 2021, 13, 2524. https://doi.org/10.3390/cancers13112524
Gambichler T, Abu Rached N, Tannapfel A, Becker JC, Vogt M, Skrygan M, Wieland U, Silling S, Susok L, Stücker M, et al. Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma. Cancers. 2021; 13(11):2524. https://doi.org/10.3390/cancers13112524
Chicago/Turabian StyleGambichler, Thilo, Nessr Abu Rached, Andrea Tannapfel, Jürgen C. Becker, Markus Vogt, Marina Skrygan, Ulrike Wieland, Steffi Silling, Laura Susok, Markus Stücker, and et al. 2021. "Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma" Cancers 13, no. 11: 2524. https://doi.org/10.3390/cancers13112524
APA StyleGambichler, T., Abu Rached, N., Tannapfel, A., Becker, J. C., Vogt, M., Skrygan, M., Wieland, U., Silling, S., Susok, L., Stücker, M., Meyer, T., Stockfleth, E., Junker, K., Käfferlein, H. U., Brüning, T., & Lang, K. (2021). Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma. Cancers, 13(11), 2524. https://doi.org/10.3390/cancers13112524










