Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
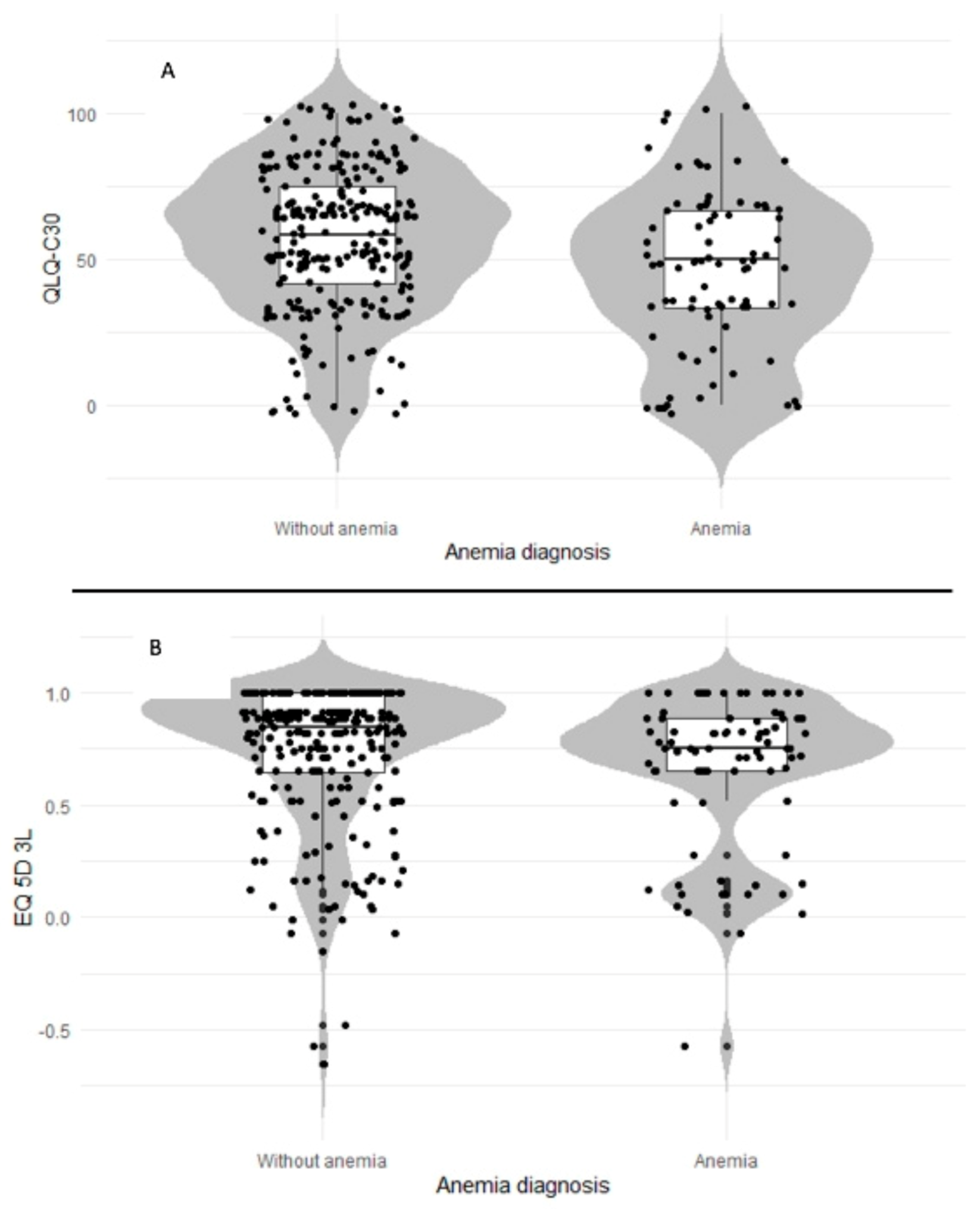
2.1. Impact of Anemia on Quality of Life in Patients with Cancer
2.2. Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Alcázar, L. The economic impact of anaemia in Peru. Lima: Group for the Analysis of Development and Action Against Hunger. Animal Genetics 2013, 39, 561–563. [Google Scholar]
- De Benoist, B.; Mclean, E. Worldwide Prevalence of Anaemia 1993–2005 Who Global Database on Anaemia; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Knight, K.; Wade, S.; Balducci, L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am. J. Med. 2004, 116, 11–26. [Google Scholar] [CrossRef]
- Mercadante, S.; Gebbia, V.; Marrazzo, A.; Filosto, S. Anaemia in cancer: Pathophysiology and treatment. Cancer Treat. Rev. 2000, 26, 303–311. [Google Scholar] [CrossRef]
- Birgegård, G.; Aapro, M.S.; Bokemeyer, C.; Dicato, M.; Drings, P.; Hornedo, J.; Krzakowski, M.; Ludwig, H.; Pecorelli, S.; Schmoll, H.-J.; et al. Cancer-related anemia: Pathogenesis, prevalence and treatment. Oncology 2005, 68, 3–11. [Google Scholar] [CrossRef]
- Gilreath, J.A.; Stenehjem, D.D.; Rodgers, G.M. Diagnosis and treatment of cancer-related anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef]
- Blanc, B.; Finch, C.A.; Hallberg, L.; Herbert, V.; Lawkowicz, W.; Layrisse, M. Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech. Rep. Ser. 1968, 405, 5–37. [Google Scholar]
- Shasha, D.; Cremieux, P.; Harrison, L. Relationship between hemoglobin levels and quality of life during radiation therapy plus concomitant or sequential chemotherapy in patients with cancer and anemia treated with epoetin alfa. J. Natl. Compr. Cancer Netw. 2004, 2, 509–517. [Google Scholar] [CrossRef]
- Crawford, J.; Cella, D.; Cleeland, C.S.; Cremieux, P.-Y.; Demetri, G.D.; Sarokhan, B.J.; Slavin, M.B.; Glaspy, J.A. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002, 95, 888–895. [Google Scholar] [CrossRef]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwing, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, 96–110. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Feeny, D.H.; Patrick, D.L. Measuring health-related quality of life. Ann. Intern. Med. 1993, 118, 622–629. [Google Scholar] [CrossRef]
- Fitzpatrick, R. The International Assessment of Health-Related Quality of Life: Theory, Translation, Measurement and Analysis. J. Med Ethics 1996, 22, 248. [Google Scholar] [CrossRef]
- Palasamudram, K.; Kaiser, J. Study on the impact of anemia on the Quality of Life (QoL) of cancer patients. Eur. J. Oncol. 2014, 19, 43–51. [Google Scholar]
- Holzner, B.; Kemmler, G.; Greil, R.; Kopp, M.; Zeimet, A.; Raderer, M.; Hejna, M.; Zochbauer, S.; Krajnik, G.; Huber, H.; et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann. Oncol. 2002, 13, 965–973. [Google Scholar] [CrossRef]
- Wedding, U.; Röhrig, B.; Pientka, L.; Höffken, K. Anaemia-related impairment in quality of life in elderly cancer patients prior to chemotherapy. J. Cancer Res. Clin. Oncol. 2007, 133, 279–286. [Google Scholar] [CrossRef]
- Farag, Y.; Keithi-Reddy, S.; Mittal, B.; Surana, S.; Addabbo, F.; Goligorsky, M.; Singh, A. Anemia, inflammation and health-related quality of life in chronic kidney disease patients. Clin. Nephrol. 2011, 75, 524–533. [Google Scholar] [CrossRef]
- Ferrari, M.; Manea, L.; Anton, K.; Bruzzone, P.; Meneghello, M.; Zamboni, F.; Purgato, L.; Cazzoletti, L.; Ferrari, P.; Testi, R. Anemia and hemoglobin serum levels are associated with exercise capacity and quality of life in chronic obstructive pulmonary disease. BMC Pulm. Med. 2015, 15. [Google Scholar] [CrossRef]
- Kraai, I.; Luttik, M.; Johansson, P.; De Jong, R.; Van Veldhuisen, D.; Hillege, H.; Jaarsma, T. Health-related quality of life and anemia in hospitalized patients with heart failure. Int. J. Cardiol. 2012, 161, 151–155. [Google Scholar] [CrossRef]
- Cella, D. The functional assessment of cancer therapy-anemia (FACT-An) scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin. Hematol. 1997, 34, 13–19. [Google Scholar]
- Demetri, G.D. Anaemia and its functional consequences in cancer patients: Current challenges in management and prospects for improving therapy. Br. J. Cancer 2001, 84, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Wasada, I.; Eguchi, H.; Kurita, M.; Kudo, S.; Shishida, T.; Mishima, Y.; Saito, Y.; Ushiorozawa, N.; Seto, T.; Shimozuma, K.; et al. Anemia affects the quality of life of japanese cancer patients. Tokai J. Exp. Clin. Med. 2013, 38, 7–11. [Google Scholar] [PubMed]
- Blohmer, J.-U.; Dunst, J.; Harrison, L.; Johnston, P.; Khayat, D.; Ludwig, H.; O’Brien, M.; Van Belle, S.; Vaupel, P. Cancer-related anemia: Biological findings, clinical implications and impact on quality of life. Oncology 2005, 68, 12–21. [Google Scholar] [CrossRef]
- Bremberg, E.R.; Brandberg, Y.; Hising, C.; Friesland, S.; Eksborg, S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med. Oncol. 2007, 24, 95–102. [Google Scholar] [CrossRef]
- Paitan, V.; Alcarraz, C.; Leornado, A.; Valencia, G.; Mantilla, R.; Morante, Z.; Oscanoa, T.J.; Mas, L. Anemia como factor pronóstico en pacientes con cáncer. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 250. [Google Scholar] [CrossRef]
- Doni, L.; Perin, A.; Manzione, L.; Gebbia, V.; Mattioli, R.; Speranza, G.B.; Latini, L.; Iop, A.; Bertetto, O.; Ferraù, F.; et al. The impact of anemia on quality of life and hospitalisation in elderly cancer patients undergoing chemotherapy. Crit. Rev. Oncol. Hematol. 2011, 77, 70–77. [Google Scholar] [CrossRef]
- Marin-Barrera, L.; Muñoz-Martin, A.J.; Rios-Herranz, E.; Garcia-Escobar, I.; Beato, C.; Font, C.; Oncala-Sibajas, E.; Revuelta-Rodriguez, A.; Areses, M.C.; Rivas-Jimenez, V.; et al. A case-control analysis of the impact of venous thromboembolic disease on quality of life of patients with cancer: Quality of life in cancer (QCA) study. Cancers 2020, 12, 75. [Google Scholar] [CrossRef]
- Littlewood, T.J.; Bajetta, E.; Nortier, J.W.R.; Vercammen, E.; Rapoport, B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2001, 19, 2865–2874. [Google Scholar] [CrossRef]
- Hudis, C.A.; Vogel, C.L.; Gralow, J.R.; Williams, D. Weekly epoetin alfa during adjuvant chemotherapy for breast cancer: Effect on hemoglobin levels and quality of life. Clin. Breast Cancer 2005, 6, 132–142. [Google Scholar] [CrossRef]
- Grote, T.; Yeilding, A.L.; Castillo, R.; Butler, D.; Fishkin, E.; Henry, D.H.; DeLeo, M.; Fink, K.; Sullivan, D.J. Efficacy and safety analysis of epoetin alfa in patients with small-cell lung cancer: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2005, 23, 9377–9386. [Google Scholar] [CrossRef]
- Nowrousian, M.R.; Kasper, C.; Oberhoff, C.; Essers, U.; Voigtmann, R.; Gallash, W.; Quarder, O. Pathophysiology of cancer-related anaemia. Erythrop. Cancer Supportive Treat. 1996, 13–34. [Google Scholar]
- Marín Barrera, L.; Muñoz Martín, A.; Ríos Herranz, E.; García-Escobar, I.; Beato, C.; Font, C.; Oncala Sibajas, A.E.; Revuelta Rodríguez, M.C.; Areses, V.; Jiménez, R.; et al. Health-related quality of life in oncological patients with acute symptomatic venous thromboembolic disease. QCa study protocol. Cases and controls study. Rev. Esp. Patol. Torac. 2019, 31, 249–258. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.; et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Brooks, R.; EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual; European Organisation for Research and Treatment of Cancer (EORTC): Brussels, Belgium, 2001; ISBN 2-9300 64-22-6. [Google Scholar]
- Bjordal, K.; de Graeff, A.; Fayers, P.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.; Meyza, J.; Brédart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H and N35) in head and neck patients. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of- life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Maringwa, J.T.; Quinten, C.; King, M.; Ringash, J.; Osoba, D.; Coens, C.; Martinelli, F.; Vercauteren, J.; Cleeland, C.S.; Flechtner, H.; et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer 2011, 19, 1753–1760. [Google Scholar] [CrossRef]
- Jayadevappa, R.; Malkowicz, S.B.; Wittink, M.; Wein, A.J.; Chhatre, S. Comparison of distribution- and anchor-based approaches to infer changes in health-related quality of life of prostate cancer survivors. Health Serv. Res. 2012, 47, 1902–1925. [Google Scholar] [CrossRef]
- Keeler, B.D.; Dickson, E.A.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Banerjea, A.; Walter, C.; Maxwell-Armstrong, C.; et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: Outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia 2019, 74, 714–725. [Google Scholar] [CrossRef]
- Pickard, A.S.; Wilke, C.T.; Lin, H.W.; Lloyd, A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 2007, 25, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.F.; Birnie, E.; Bonsel, G.J. Quantification of the level descriptors for the standard EQ-5D three-level system and a five-level version according to two methods. Qual. Life Res. 2008, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5. [Google Scholar] [CrossRef]
| Variable | Anemia Present (Cases) | Anemia Absent (Controls) | Entire Cohort |
|---|---|---|---|
| Male sex, n (%) | 43 (47.8%) | 161 (58.5%) | 204 (55.9%) |
| Age, years (n = 365); mean ± SD | 63.2 (11.8) | 60.8 (15.9) | 61.4 (15) |
| Body mass index, kg/m2 (n = 312), mean ± SD | 26.2 (4.9) | 26.4 (5.3) | 26.4 (5.1) |
| Arterial hypertension (n = 364), n (%) | 42 (46.7%) | 95 (34.7%) | 137 (37.6%) |
| Dyslipidemia (n = 363), n (%) | 23 (25.8%) | 65 (23.7%) | 88 (24.2%) |
| Diabetes mellitus (n = 364), n (%) | 16 (17.8%) | 42 (15.3%) | 58 (15.9%) |
| Asthma (n = 364), n (%) | 5 (5.6%) | 2 (0.7%) | 7 (1.9%) |
| Acute coronary syndrome (n = 364), n (%) | 4 (4.4%) | 5 (1.8%) | 9 (2.5%) |
| Stroke (n = 364), n (%) | 5 (5.6%) | 8 (2.9%) | 13 (3.6%) |
| Chronic kidney disease (n = 364), n (%) | 3 (3.3%) | 9 (3.3.%) | 12 (3.3%) |
| Smoking (n = 364), n (%) | 20 (22.2%) | 68 (24.8%) | 88 (24.2%) |
| VTE (n = 365), n (%) | 39 (43.3%) | 79 (28.7%) | 118 (32.3%) |
| Non-steroidal anti-inflammatory drugs (n = 364), n (%) | 9 (10%) | 23 (8.4%) | 32 (8.8%) |
| Statins (n = 364), n (%) | 16 (17.8%) | 35 (12.8%) | 51 (14%) |
| Active anticancer treatment, n (%) | 71 (79.8%) | 208 (76.8%) | 279 (77.5%) |
| Central venous catheter, n (%) | 13 (14.8%) | 62 (22.8%) | 75 (20.8%) |
| ECOG performance status (n = 339) | |||
| 0, n (%) | 16 (19%) | 109 (42.7%) | 125 (36.9%) |
| 1, n (%) | 54 (64.3%) | 128 (50.2%) | 182 (53.7%) |
| 2, n (%) | 13 (15.5%) | 13 (5.1%) | 26 (7.7%) |
| 3, n (%) | 1 (1.2%) | 4 (1.6%) | 5 (1.5%) |
| 4, n (%) | 0 (0%) | 1 (0.4%) | 1 (0.3%) |
| Metastasis (n =345), n (%) | 58 (68.2%) | 176 (67.7%) | 234 (67.8%) |
| Tumor site (n = 365) | |||
| Gynecologic, n (%) | 20 (22.2%) | 22 (8.0%) | 42 (12) |
| Lung, n (%) | 17 (18.9%) | 59 (21.5%) | 76 (20.8%) |
| Digestive, n (%) | 20 (22.2%) | 72 (26.2%) | 92 (25.2%) |
| Genitourinary, n (%) | 8 (8.9%) | 19 (6.9%) | 27 (7.4%) |
| Lymphoma, n (%) | 9 (10%) | 43 (15.6%) | 52 (14.2%) |
| Other sites, n (%) | 16 (17.8%) | 60 (21.8%) | 76 (20.8%) |
| EORTC QLQ-C30 Functional Scale 1 | Cases (Cancer with Anemia) | Controls (Cancer without Anemia) | Mean Difference | Effect Size (95% CI) | Relative Efficiency (95% CI) |
|---|---|---|---|---|---|
| Global health status | 45.6 | 58 | −12.4 ** | 0.78 (0.54; 1.1) | −0.48 (−0.23; −0.73) |
| Physical functioning | 67.2 | 78.9 | −11.7 ** | 0.71 (0.49; 1) | −0.50 (−0.25; −0.75) |
| Role functioning | 59.4 | 71.5 | −12 * | 0.86 (0.59; 1.2) | −0.37 (−0.12; −0.62) |
| Emotional functioning | 62.7 | 72.2 | −9.8 * | 0.67 * (0.46; 0.94) | −0.38 (−0.13; −0.63) |
| Cognitive functioning | 80.7 | 84.1 | −3.4 | 0.86 (0.6; 1.2) | −0.14 (−0.39; 0.1) |
| Social functioning | 64.2 | 72.8 | −8.3 | 0.75 (0.52; 1.05) | −0.27 (−0.02; −0.52) |
| EORTC QLQ-C30 symptoms scale 2 | Cases (Cancer with anemia) | Controls (Cancer without anemia) | Mean difference | Effect size (95% CI) | Relative efficiency (95% CI) |
| Fatigue | 48 | 35 | 13 ** | 0.91 (0.3; 1.3) | 0.48 (0.2; 0.7) |
| Nausea and vomiting | 20.6 | 9.6 | 11 ** | 0.34 ** (0.2; 0.5) | 0.52 (0.3; 0.7) |
| Pain | 35.5 | 23.8 | 11.7 ** | 0.8 (0.5; 1.1.) | 0.41 (0.1; 0.6) |
| Dyspnea | 21.6 | 14.6 | 7 * | 0.75 (0.5; 1) | 0.25 (0.1; 0.5) |
| Insomnia | 34.1 | 30.1 | 4 | 0.9 (0.6; 1.3) | 0.12 (0.1; 0.4) |
| Appetite loss | 35.7 | 21.5 | 14.2 ** | 0.6 * (0.4; 0.9) | 0.45 (0.2; 0.7) |
| Constipation | 26.6 | 22.3 | 4.3 | 0.87 (0.6; 1.2) | 0.14 (0.1; 0.4) |
| Diarrhea | 21.5 | 14.4 | 7.1 * | 0.57 * (0.4; 0.8) | 0.26 (0.01; 0.5) |
| Financial difficulties | 17.4 | 19.9 | −2.5 | 1.12 (0.7; 1.5) | −0.08 (−0.3; 0.1) |
| EQ–5D–3L 3 | Cases (Cancer with anemia) | Controls (Cancer without anemia) | Mean difference | Effect size (95% CI) | Relative efficiency (95% CI) |
| Index score | 0.65 | 0.73 | −0.08 | 0.88 (0.6; 1.2) | −0.24 (−0.4; 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca-Hernando, M.; Muñoz-Martin, A.J.; Rios-Herranz, E.; Garcia-Escobar, I.; Beato, C.; Font, C.; Oncala-Sibajas, E.; Revuelta-Rodriguez, A.; Areses, M.C.; Rivas-Jimenez, V.; et al. Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers 2021, 13, 2517. https://doi.org/10.3390/cancers13112517
Barca-Hernando M, Muñoz-Martin AJ, Rios-Herranz E, Garcia-Escobar I, Beato C, Font C, Oncala-Sibajas E, Revuelta-Rodriguez A, Areses MC, Rivas-Jimenez V, et al. Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers. 2021; 13(11):2517. https://doi.org/10.3390/cancers13112517
Chicago/Turabian StyleBarca-Hernando, Maria, Andres J. Muñoz-Martin, Eduardo Rios-Herranz, Ignacio Garcia-Escobar, Carmen Beato, Carme Font, Estefania Oncala-Sibajas, Alfonso Revuelta-Rodriguez, Maria Carmen Areses, Victor Rivas-Jimenez, and et al. 2021. "Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis" Cancers 13, no. 11: 2517. https://doi.org/10.3390/cancers13112517
APA StyleBarca-Hernando, M., Muñoz-Martin, A. J., Rios-Herranz, E., Garcia-Escobar, I., Beato, C., Font, C., Oncala-Sibajas, E., Revuelta-Rodriguez, A., Areses, M. C., Rivas-Jimenez, V., Ballaz-Quincoces, A., Moreno-Santos, M. A., Lopez-Saez, J.-B., Gallego-Gallego, I., Elias-Hernandez, T., Asensio-Cruz, M. I., Chasco-Eguilaz, L., Garcia-Gonzalez, G., Estevez-Garcia, P., ... Jara-Palomares, L. (2021). Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers, 13(11), 2517. https://doi.org/10.3390/cancers13112517





