Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment—Oncological and Surgical Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Preoperative Verification of LN Status
2.1. Computed Tomography
2.2. Endoscopic Ultrasonography (EUS)
2.3. Magnetic Resonance Imaging
2.4. Fluorodeoxyglucose Positron Emission Tomography
2.5. Staging Laparoscopy
3. Nodal Status from an Oncological Perspective
Neoadjuvant Chemotherapy
4. Immunotherapy
5. Status of Radio- and Radiochemo-Therapy in Perioperative Setting
6. Outcome of Neoadjuvant Therapy on LN Status
6.1. cN0/ypN0—Natural N0
6.2. cN+/ypN0—Downstaged N0
6.3. ypN+—Node-Positive
7. Nodal Status from the Surgical Perspective
8. East vs. West Perspective
9. Nodal Regression Grade
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Wagner, A.D.; Lordick, F.; Grabsch, H.I.; Terashima, M.; Terada, M.; Yoshikawa, T.; Boku, N.; Kataoka, K.; Smyth, E.C.; Mauer, M.; et al. Multidisciplinary management of stage II–III gastric and gastro-oesophageal junction cancer. Eur. J. Cancer 2020, 124, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; De Manzoni, G.; Di Leo, A.; D’Ugo, D.; Galasso, E.; Marrelli, D.; Petrioli, R.; Polom, K.; Roviello, F.; Santullo, F.; et al. Gastric cancer: Current status of lymph node dissection. World J. Gastroenterol. 2016, 22, 2875–2893. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Katayama, H.; Tsuburaya, A.; Mizusawa, J.; Nakamura, K.; Katai, H.; Imamura, H.; Nashimoto, A.; Fukushima, N.; Sano, T.; Sasako, M. An integrated analysis of two phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer 2019, 22, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ito, Y.; Misawa, K.; Shimizu, Y.; Kinoshita, T. Neoadjuvant chemotherapy followed by surgery in gastric cancer patients with extensive lymph node metastasis. World J. Clin. Oncol. 2015, 6, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Hashemzadeh, S.; Pourzand, A.; Somi, M.H.; Zarrintan, S.; Javad-Rashid, R.; Esfahani, A. The effects of neoadjuvant chemotherapy on resectability of locally-advanced gastric adenocarcinoma: A clinical trial. Int. J. Surg. 2014, 12, 1061–1069. [Google Scholar] [CrossRef]
- Eto, K.; Hiki, N.; Kumagai, K.; Shoji, Y.; Tsuda, Y.; Kano, Y.; Yasufuku, I.; Okumura, Y.; Tsujiura, M.; Ida, S.; et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 2018, 21, 703–709. [Google Scholar] [CrossRef]
- NCCN Gastric Cancer Guidelines, Version 2.2021; National Comprehensive Cancer Network: Plymouth, PA, USA, 2021.
- Lowy, A.M.; Mansfield, P.F.; Leach, S.D.; Pazdur, R.; Dumas, P.; Ajani, J.A. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann. Surg. 1999, 229, 303–308. [Google Scholar] [CrossRef]
- Mansour, J.C.; Tang, L.; Shah, M.; Bentrem, D.; Klimstra, D.S.; Gonen, M.; Kelsen, D.P.; Brennan, M.F.; Coit, D.G. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann. Surg. Oncol. 2007, 14, 3412–3418. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, N.; Blum, M.; Estrella, J.S.; Das, P.; Hofstetter, W.L.; Fournier, K.F.; Mansfield, P.; Ajani, J.A.; Badgwell, B.D. Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric Cancer 2018, 21, 74–83. [Google Scholar] [CrossRef]
- Park, J.M.; Ryu, W.S.; Kim, J.H.; Park, S.S.; Kim, S.J.; Kim, C.S.; Mok, Y.J. Prognostic factors for advanced gastric cancer: Stage-stratified analysis of patients who underwent curative resection. Cancer Res. Treat. 2006, 38, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wang, S.; Wang, Z.; Li, L.; Huang, Z.; Yu, W.; Chen, Z.; Wu, Q.F. Clinicopathological risk factors for gastric cancer: A retrospective cohort study in China. BMJ Open 2019, 9, e030639. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Choi, Y.Y.; An, J.Y.; Chung, H.; Seo, S.H.; Shin, H.B.; Bang, H.J.; Li, S.; Kim, H.I.; Cheong, J.H.; et al. Difficulty of predicting the presence of lymph node metastases in patients with clinical early stage gastric cancer: A case control study. BMC Cancer 2015, 15, 943. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, A.S.; Goense, L.; Brenkman, H.J.F.; Mook, S.; Meijer, G.J.; Wessels, F.J.; Verheij, M.; Jansen, E.P.M.; van Hillegersberg, R.; van Rossum, P.S.N.; et al. Imaging strategies in the management of gastric cancer: Current role and future potential of MRI. Br. J. Radiol. 2019, 92, 20181044. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, T.; Katai, H.; Mizusawa, J.; Nakamura, K.; Sano, T.; Terashima, M.; Ito, S.; Yoshikawa, T.; Fukushima, N.; Kawachi, Y.; et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer 2018, 21, 68–73. [Google Scholar] [CrossRef]
- Mueller, C.L.; Lisbona, R.; Sorial, R.; Siblini, A.; Ferri, L.E. Sentinel Lymph Node Sampling for Early Gastric Cancer-Preliminary Results of A North American Prospective Study. J. Gastrointest. Surg. 2019, 23, 1113–1121. [Google Scholar] [CrossRef]
- Berlth, F.; Chon, S.H.; Chevallay, M.; Jung, M.K.; Monig, S.P. Preoperative staging of nodal status in gastric cancer. Transl. Gastroenterol. Hepatol. 2017, 2, 8. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kawaguchi, Y.; Shiraishi, K.; Akaike, H.; Shimizu, H.; Furuya, S.; Hosomura, N.; Amemiya, H.; Kawaida, H.; Sudo, M.; et al. The impact of histological type on the accuracy of preoperative N staging in patients with gastric cancer. World J. Surg. Oncol. 2019, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lv, Y.; Guo, X.; Song, H.; Su, G.; Chen, B. Value and impact factors of multidetector computed tomography in diagnosis of preoperative lymph node metastasis in gastric cancer: A PRISMA-compliant systematic review and meta-analysis. Medicine 2017, 96, e7769. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Joo, I.; Lee, J.M. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J. Gastroenterol. 2014, 20, 4546–4557. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009, 12, 6–22. [Google Scholar] [CrossRef]
- Monig, S.P.; Zirbes, T.K.; Schroder, W.; Baldus, S.E.; Lindemann, D.G.; Dienes, H.P.; Holscher, A.H. Staging of gastric cancer: Correlation of lymph node size and metastatic infiltration. AJR Am. J. Roentgenol. 1999, 173, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Sanjeevaiah, A.; Park, H.; Fangman, B.; Porembka, M. Gastric Cancer with Radiographically Occult Metastatic Disease: Biology, Challenges, and Diagnostic Approaches. Cancers 2020, 12, 592. [Google Scholar] [CrossRef]
- Power, D.G.; Schattner, M.A.; Gerdes, H.; Brenner, B.; Markowitz, A.J.; Capanu, M.; Coit, D.G.; Brennan, M.; Kelsen, D.P.; Shah, M.A. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. J. Am. Coll. Surg. 2009, 208, 173–178. [Google Scholar] [CrossRef]
- Gertsen, E.C.; de Jongh, C.; Brenkman, H.J.F.; Mertens, A.C.; Broeders, I.; Los, M.; Boerma, D.; Ten Bokkel Huinink, D.; van Leeuwen, L.; Wessels, F.J.; et al. The additive value of restaging-CT during neoadjuvant chemotherapy for gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 1247–1253. [Google Scholar] [CrossRef]
- Ahn, H.S.; Lee, H.J.; Yoo, M.W.; Kim, S.G.; Im, J.P.; Kim, S.H.; Kim, W.H.; Lee, K.U.; Yang, H.K. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J. Surg. Oncol. 2009, 99, 20–27. [Google Scholar] [CrossRef]
- Kagedan, D.J.; Frankul, F.; El-Sedfy, A.; McGregor, C.; Elmi, M.; Zagorski, B.; Dixon, M.E.; Mahar, A.L.; Vasilevska-Ristovska, J.; Helyer, L.; et al. Negative predictive value of preoperative computed tomography in determining pathologic local invasion, nodal disease, and abdominal metastases in gastric cancer. Curr. Oncol. 2016, 23, 273–279. [Google Scholar] [CrossRef]
- Fornaro, L.; Spallanzani, A.; de Vita, F.; D’Ugo, D.; Falcone, A.; Lorenzon, L.; Tirino, G.; Cascinu, S.; on behalf of GAIN (GAstric Cancer Italian Network). Beyond the Guidelines: The Grey Zones of the Management of Gastric Cancer. Consensus Statements from the Gastric Cancer Italian Network (GAIN). Cancers 2021, 13, 1304. [Google Scholar] [CrossRef] [PubMed]
- Hoibian, S.; Giovannini, M.; Autret, A.; Pesenti, C.; Bories, E.; Ratone, J.P.; Dahel, Y.; Dermeche, S.; Meillat, H.; Guiramand, J.; et al. Preoperative EUS evaluation of the response to neoadjuvant therapy for gastric and esophagogastric junction cancer is correlated with survival: A single retrospective study of 97 patients. Endosc. Ultrasound 2021. [Google Scholar] [CrossRef] [PubMed]
- MingHua, Z.; KeCheng, Z.; ZhenYu, C.; Lin, C.; ChunXi, W.; ZeLong, Y. Impact of Lymph Nodes Examined on Survival in ypN0 Gastric Cancer Patients: A Population-Based Study. J. Gastrointest. Surg. 2021, 25, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Seevaratnam, R.; Cardoso, R.; McGregor, C.; Lourenco, L.; Mahar, A.; Sutradhar, R.; Law, C.; Paszat, L.; Coburn, N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012, 15 (Suppl. 1), 3–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, D.H.; Guo, L.; Hu, C.H.; Fang, X.; Meng, Q.; Ping, X.X.; Lu, Z.W. The utility of MRI for pre-operative T and N staging of gastric carcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20140552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J. The role of MRI in the diagnosis and treatment of gastric cancer. Diagn. Interv. Radiol. 2020, 26, 176–182. [Google Scholar] [CrossRef]
- Chen, J.; Cheong, J.H.; Yun, M.J.; Kim, J.; Lim, J.S.; Hyung, W.J.; Noh, S.H. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005, 103, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, W.J.; Choi, D.; Lee, S.J.; Choi, J.Y.; Kim, B.T.; Kim, H.S. The value of PET/CT for preoperative staging of advanced gastric cancer: Comparison with contrast-enhanced CT. Eur. J. Radiol. 2011, 79, 183–188. [Google Scholar] [CrossRef]
- Wieder, H.A.; Krause, B.J.; Herrmann, K. PET and PET-CT in esophageal and gastric cancer. Methods Mol. Biol. 2011, 727, 59–76. [Google Scholar] [CrossRef]
- Smyth, E.; Schoder, H.; Strong, V.E.; Capanu, M.; Kelsen, D.P.; Coit, D.G.; Shah, M.A. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012, 118, 5481–5488. [Google Scholar] [CrossRef]
- Nakajo, M.; Kajiya, Y.; Jinguji, M.; Nakabeppu, Y.; Nakajo, M.; Nihara, T.; Yoshiura, T. Current clinical status of (18)F-FLT PET or PET/CT in digestive and abdominal organ oncology. Abdom. Radiol. 2017, 42, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.M.; Eshmuminov, D.; Rordorf, T.; Vetter, D.; Veit-Haibach, P.; Weber, A.; Bauerfeind, P.; Samaras, P.; Lehmann, K. (18)FDG-PET-CT identifies histopathological non-responders after neoadjuvant chemotherapy in locally advanced gastric and cardia cancer: Cohort study. BMC Cancer 2018, 18, 548. [Google Scholar] [CrossRef] [PubMed]
- Morgagni, P.; Bencivenga, M.; Colciago, E.; Tringali, D.; Giacopuzzi, S.; Framarini, M.; Saragoni, L.; Mura, G.; Graziosi, L.; Marino, E.; et al. Limited Usefulness of 18F-FDG PET/CT in Predicting Tumor Regression After Preoperative Chemotherapy for Noncardia Gastric Cancer: The Italian Research Group for Gastric Cancer (GIRCG) Experience. Clin. Nucl. Med. 2020, 45, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Ott, K.; Krause, B.J.; Weber, W.A.; Becker, K.; Stein, H.J.; Lorenzen, S.; Schuster, T.; Wieder, H.; Herrmann, K.; et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007, 8, 797–805. [Google Scholar] [CrossRef]
- Gertsen, E.C.; Borggreve, A.S.; Brenkman, H.J.F.; Verhoeven, R.H.A.; Vegt, E.; van Hillegersberg, R.; Siersema, P.D.; Ruurda, J.P.; Dutch Upper Gastrointestinal Cancer Audit, G. Evaluation of the Implementation of FDG-PET/CT and Staging Laparoscopy for Gastric Cancer in The Netherlands. Ann. Surg. Oncol. 2021, 28, 2384–2393. [Google Scholar] [CrossRef]
- Brenkman, H.J.F.; Gertsen, E.C.; Vegt, E.; van Hillegersberg, R.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Lanschot, J.J.B.; Lagarde, S.M.; et al. Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: A multicenter prospective study (PLASTIC-study). BMC Cancer 2018, 18, 450. [Google Scholar] [CrossRef]
- Leake, P.A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Law, C.; Coburn, N.G. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012, 15 (Suppl. 1), S38–S47. [Google Scholar] [CrossRef]
- Mizrak Kaya, D.; Nogueras-Gonzalez, G.M.; Harada, K.; Amlashi, F.G.; Roy-Chowdhuri, S.; Estrella, J.S.; Das, P.; Lee, J.H.; Weston, B.; Bhutani, M.S.; et al. Risk of peritoneal metastases in patients who had negative peritoneal staging and received therapy for localized gastric adenocarcinoma. J. Surg. Oncol. 2018, 117, 678–684. [Google Scholar] [CrossRef]
- Ramos, R.F.; Scalon, F.M.; Scalon, M.M.; Dias, D.I. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 1315–1321. [Google Scholar] [CrossRef]
- Fukagawa, T. Role of staging laparoscopy for gastric cancer patients. Ann. Gastroenterol. Surg. 2019, 3, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Bintintan, V.V.; Cordos, A.; Chira, R.; Cocu, S.; Rus, P.; Bintintan, A.; Nagy, G.; Ciule, L.; Cata, E.; Pop, A.; et al. The Value of Staging Laparoscopy for Optimal Multidisciplinary Treatment in Patients with Gastric Cancer. Chirurgia 2018, 113, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Hosogi, H.; Shinohara, H.; Tsunoda, S.; Hisamori, S.; Sumida, H.; Hida, K.; Obama, K.; Okabe, H.; Sakai, Y. Staging laparoscopy for advanced gastric cancer: Significance of preoperative clinicopathological factors. Langenbecks Arch. Surg. 2017, 402, 33–39. [Google Scholar] [CrossRef]
- Machairas, N.; Charalampoudis, P.; Molmenti, E.P.; Kykalos, S.; Tsaparas, P.; Stamopoulos, P.; Sotiropoulos, G.C. The value of staging laparoscopy in gastric cancer. Ann. Gastroenterol. 2017, 30, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Vergadis, C.; Schizas, D. Is Accurate N—Staging for Gastric Cancer Possible? Front. Surg. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhao, W.; Ren, F.; Qi, S.; Wang, X.; Lv, T.; Su, Z.; Yin, H.; Ren, J.; Huan, Y. Lymph node metastasis in patients with gastric cancer: A multi-modality, morphologic and functional imaging study. Am. J. Transl. Res. 2016, 8, 5601–5609. [Google Scholar]
- Wang, X.; Wei, Y.; Xue, Y.; Lu, P.; Yu, L.; Shen, B. Predictive Role of the Number of 18F-FDG-Positive Lymph Nodes Detected by PET/CT for Pre-Treatment Evaluation of Locally Advanced Gastric Cancer. PLoS ONE 2016, 11, e0166836. [Google Scholar] [CrossRef] [PubMed]
- Kawanaka, Y.; Kitajima, K.; Fukushima, K.; Mouri, M.; Doi, H.; Oshima, T.; Niwa, H.; Kaibe, N.; Sasako, M.; Tomita, T.; et al. Added value of pretreatment (18)F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur. J. Radiol. 2016, 85, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.; Venkatesh, S.K. Gastric carcinoma: Imaging diagnosis, staging and assessment of treatment response. Cancer Imaging 2013, 13, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Kakroo, S.M.; Rashid, A.; Wani, A.A.; Akhtar, Z.; Chalkoo, M.A.; Laharwal, A.R. Staging Laparoscopy in Carcinoma of Stomach: A Comparison with CECT Staging. Int. J. Surg. Oncol. 2013, 2013, 674965. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Morgagni, P.; Nanni, O.; Framarini, M.; Saragoni, L.; Marrelli, D.; Roviello, F.; Petrioli, R.; Fumagalli Romario, U.; Rimassa, L.; et al. Preoperative or Perioperative Docetaxel, Oxaliplatin, and Capecitabine (GASTRODOC Regimen) in Patients with Locally-Advanced Resectable Gastric Cancer: A Randomized Phase-II Trial. Cancers 2020, 12, 2790. [Google Scholar] [CrossRef] [PubMed]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; FREGAT Working Group. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: A multicenter comparative study. Ann. Surg. 2011, 254, 684–693; discussion 693. [Google Scholar] [CrossRef] [PubMed]
- Piessen, G.; Messager, M.; Le Malicot, K.; Robb, W.B.; Di Fiore, F.; Guilbert, M.; Moreau, M.; Christophe, V.; Adenis, A.; Mariette, C. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas—PRODIGE 19—FFCD1103—ADCI002. BMC Cancer 2013, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Pompella, L.; Tirino, G.; Pappalardo, A.; Laterza, M.M.; Caterino, M.; Orditura, M.; Ciardiello, F.; Lieto, E.; Galizia, G.; et al. Perioperative Treatment in Resectable Gastric Cancer: Current Perspectives and Future Directions. Cancers 2019, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, J.; Zhou, K.; Jin, C.; Wang, A.; Ji, K.; Ji, X.; Zhang, J.; Wu, X.; Li, X.; et al. Effect of Additional Trastuzumab in Neoadjuvant and Adjuvant Treatment for Patients with Resectable HER2-Positive Gastric Cancer. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Wagner, A.D.; Grabsch, H.I.; Mauer, M.; Marreaud, S.; Caballero, C.; Thuss-Patience, P.; Mueller, L.; Elme, A.; Moehler, M.H.; Martens, U.; et al. EORTC-1203-GITCG—The “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: A randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019, 19, 494. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Haag, G.M.; Ettrich, T.J.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.M.; Ebert, M.P.; Goekkurt, E.; Welslau, M.; et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trial of the AIO. J. Clin. Oncol. 2020, 38, 4502. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Fuchs, C.S.; Ohtsu, A.; Tabernero, J.; Ilson, D.H.; Hyung, W.J.; Strong, V.E.; Goetze, T.O.; Yoshikawa, T.; et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019, 15, 943–952. [Google Scholar] [CrossRef]
- Mansukhani, S.; Davidson, M.; Gillbanks, A.; Peckitt, C.; Musallam, A.; Begum, R.; Morganstein, D.; Wotherspoon, A.; Riddell, A.M.; Kinross, J.M.; et al. Iconic: Peri-operative immuno-chemotherapy in operable oesophageal and gastric cancer. J. Clin. Oncol. 2018, 36, TPS4139. [Google Scholar] [CrossRef]
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0182692. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Yasuta, S.; Yamauchi, J.; Miyazaki, K.; Sato, M.; Ikeda, T.; Fujita, S.; Shirasaki, K.; Kobayashi, S.; Ajiki, T.; Tsuchihara, K.; et al. A Case of Advanced Gastric Cancer with Extensive Lymph Node Metastases Treated by Capecitabine plus Cisplatin plus Trastuzumab Chemotherapy, Followed by Conversion Surgery. Gan Kagaku Ryoho 2016, 43, 1923–1925. [Google Scholar]
- Smyth, E.; Knodler, M.; Giraut, A.; Mauer, M.; Nilsson, M.; Van Grieken, N.; Wagner, A.D.; Moehler, M.; Lordick, F. VESTIGE: Adjuvant Immunotherapy in Patients with Resected Esophageal, Gastroesophageal Junction and Gastric Cancer Following Preoperative Chemotherapy with High Risk for Recurrence (N+ and/or R1): An Open Label Randomized Controlled Phase-2-Study. Front. Oncol. 2019, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, Y.; Ninomiya, M.; Hirahara, S.; Doi, H.; Kenji, S.; Toyota, K.; Yano, R.; Kobayashi, H.; Hashimoto, Y.; Yokoyama, Y.; et al. A long-term survival case of unresectable gastric cancer with multidisciplinary therapy including immunotherapy and abscopal effect. Int. Cancer Conf. J. 2020, 9, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Leong, T.; Smithers, B.M.; Michael, M.; Gebski, V.; Boussioutas, A.; Miller, D.; Simes, J.; Zalcberg, J.; Haustermans, K.; Lordick, F.; et al. TOPGEAR: A randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015, 15, 532. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.; Smithers, B.M.; Haustermans, K.; Michael, M.; Gebski, V.; Miller, D.; Zalcberg, J.; Boussioutas, A.; Findlay, M.; O’Connell, R.L.; et al. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann. Surg. Oncol. 2017, 24, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Sada, Y.H.; Smaglo, B.G.; Tan, J.C.; Tran Cao, H.S.; Musher, B.L.; Massarweh, N.N. Prognostic Value of Nodal Response After Preoperative Treatment of Gastric Adenocarcinoma. J. Natl. Compr. Cancer Netw. 2019, 17, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.P.; Blum, M.M.; Chiang, Y.J.; Das, P.; Minsky, B.D.; Estrella, J.S.; Ajani, J.A.; Badgwell, B.D.; Mansfield, P.; Ikoma, N. Preoperative Therapy Regimen Influences the Incidence and Implication of Nodal Downstaging in Patients with Gastric Cancer. J. Gastric Cancer 2020, 20, 313–327. [Google Scholar] [CrossRef]
- Lee, J.; Lim, D.H.; Kim, S.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Choi, M.G.; Sohn, T.S.; Noh, J.H.; et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J. Clin. Oncol. 2012, 30, 268–273. [Google Scholar] [CrossRef]
- Park, S.H.; Zang, D.Y.; Han, B.; Ji, J.H.; Kim, T.G.; Oh, S.Y.; Hwang, I.G.; Kim, J.H.; Shin, D.; Lim, D.H.; et al. ARTIST 2: Interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2-gastrectomy in stage II/III gastric cancer (GC). J. Clin. Oncol. 2019, 37, 4001. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Meershoek-Klein Kranenbarg, E.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.M.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- Ikoma, N.; Estrella, J.S.; Hofstetter, W.; Das, P.; Minsky, B.D.; Ajani, J.A.; Fournier, K.F.; Mansfield, P.; Badgwell, B.D. Nodal Downstaging in Gastric Cancer Patients: Promising Survival if ypN0 is Achieved. Ann. Surg. Oncol. 2018, 25, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Wen, X.; Maciel, J.; Carneiro, F.; Dinis-Ribeiro, M. Occult Tumour Cells in Lymph Nodes from Gastric Cancer Patients: Should Isolated Tumour Cells Also Be Considered? Ann. Surg. Oncol. 2020, 27, 4204–4215. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Tani, T. Clinical significance of molecular diagnosis for gastric cancer lymph node micrometastasis. World J. Gastroenterol. 2014, 20, 13728–13733. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Park, S.S.; Kim, J.H. Current status and scope of lymph node micrometastasis in gastric cancer. J. Gastric Cancer 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Yamamoto, M.; Rashid, O.M.; Wong, J. Surgical management of gastric cancer: The East vs. West perspective. J. Gastrointest. Oncol. 2015, 6, 79–88. [Google Scholar] [CrossRef]
- Russo, A.; Li, P.; Strong, V.E. Differences in the multimodal treatment of gastric cancer: East versus west. J. Surg. Oncol. 2017, 115, 603–614. [Google Scholar] [CrossRef]
- Marrelli, D.; De Franco, L.; Iudici, L.; Polom, K.; Roviello, F. Lymphadenectomy: State of the art. Transl. Gastroenterol. Hepatol. 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020. [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Cuschieri, A.; Weeden, S.; Fielding, J.; Bancewicz, J.; Craven, J.; Joypaul, V.; Sydes, M.; Fayers, P. Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br. J. Cancer 1999, 79, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; of the Italian Gastric Cancer Study Group. D2 dissection improves disease-specific survival in advanced gastric cancer patients: 15-year follow-up results of the Italian Gastric Cancer Study Group D1 versus D2 randomised controlled trial. Eur. J. Cancer 2021, 150, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H. Lymph Node Ratio System for N Staging of Gastric Cancer: Challenging for Universal Application But Useful for the Prognostic Prediction of Individual Patients. J. Gastric Cancer 2021, 21, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xue, Z.; Zhang, S.; Guo, X.; Zhai, L.; Shang, S.; Zhang, Y.; Lu, H. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: A meta-analysis. Int. J. Surg. 2018, 57, 76–83. [Google Scholar] [CrossRef]
- Rawicz-Pruszynski, K.; Cisel, B.; Mlak, R.; Mielko, J.; Skorzewska, M.; Kwietniewska, M.; Pikula, A.; Geca, K.; Sedlak, K.; Kurylcio, A.; et al. The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Cancers 2019, 11, 1914. [Google Scholar] [CrossRef]
- Agnes, A.; Biondi, A.; Laurino, A.; Persiani, R.; D’Ugo, D. Global updates in the treatment of gastric cancer: A systematic review. Part 1: Staging, classification and surgical treatment. Updates Surg. 2020, 72, 341–353. [Google Scholar] [CrossRef]
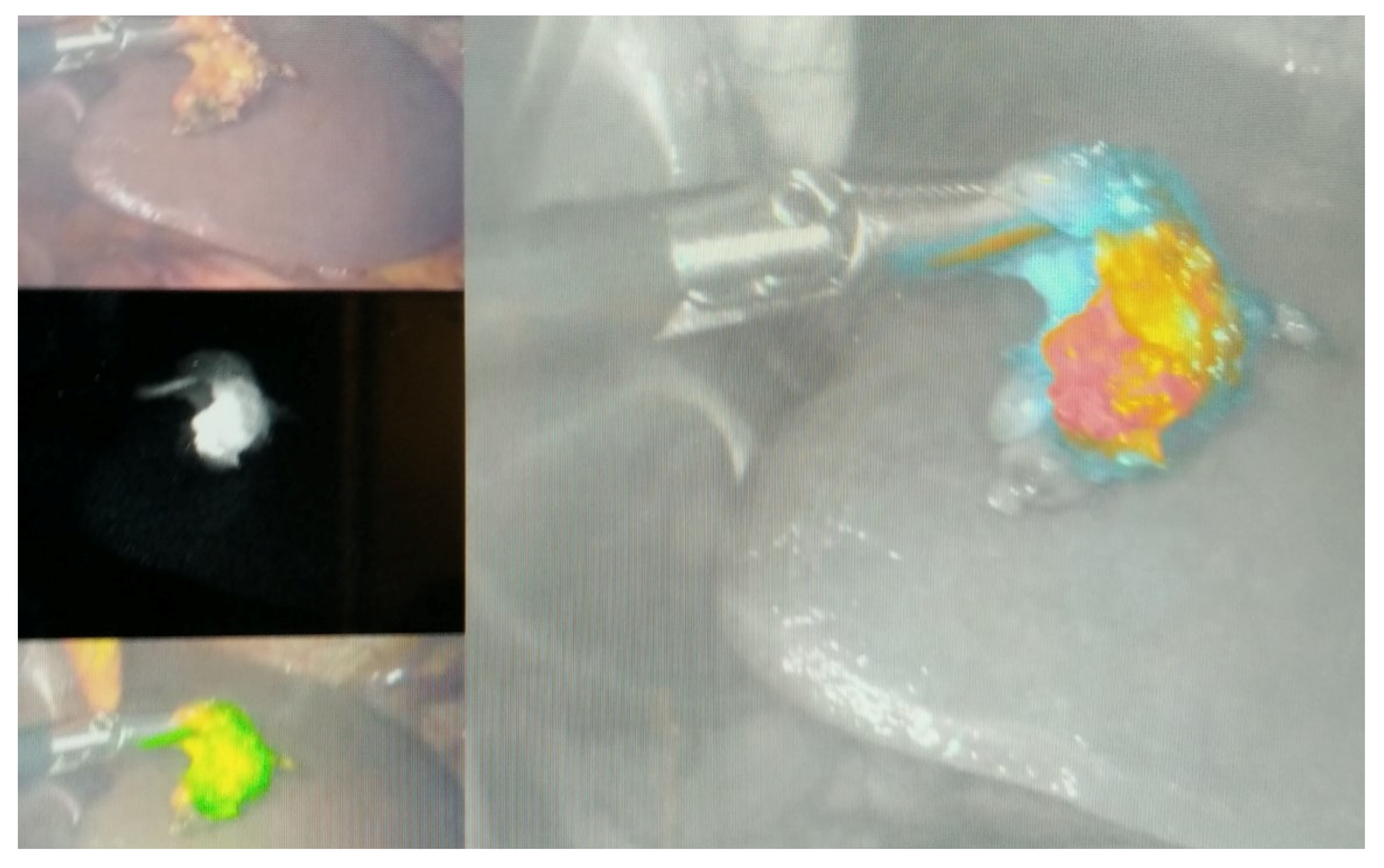
- Baiocchi, G.L.; Molfino, S.; Molteni, B.; Quarti, L.; Arcangeli, G.; Manenti, S.; Arru, L.; Botticini, M.; Gheza, F. Fluorescence-guided lymphadenectomy in gastric cancer: A prospective western series. Updates Surg. 2020, 72, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Cho, M.; Roh, C.K.; Seo, W.J.; Choi, S.; Son, T.; Kim, H.I.; Hyung, W.J. Assessment of diagnostic value of fluorescent lymphography-guided lymphadenectomy for gastric cancer. Gastric Cancer 2021, 24, 515–525. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xie, J.W.; Zhong, Q.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; Lin, M.; Tu, R.H.; Huang, Z.N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients with Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef]
- Chan, W.L.; Lam, K.O.; Lee, V.H.F.; Davidson, M.; So, T.H.; Li, J.S.; Chau, I.; Kwong, D.L.W. Gastric Cancer—From Aetiology to Management: Differences Between the East and the West. Clin. Oncol. 2019, 31, 570–577. [Google Scholar] [CrossRef]
- Zhang, C.D.; Yamashita, H.; Seto, Y. Gastric cancer surgery: Historical background and perspective in Western countries versus Japan. Ann. Transl. Med. 2019, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Brind’Amour, A.; Gagne, J.P.; Hogue, J.C.; Poirier, E. Impact of the introduction of formal D2 lymphadenectomy for gastric cancer in a Western setting. Can. J. Surg. 2021, 64, E119–E126. [Google Scholar] [CrossRef] [PubMed]
- Bausys, A.; Senina, V.; Luksta, M.; Anglickiene, G.; Molnikaite, G.; Bausys, B.; Rybakovas, A.; Baltruskeviciene, E.; Laurinavicius, A.; Poskus, T.; et al. Histologic Lymph Nodes Regression after Preoperative Chemotherapy as Prognostic Factor in Non-metastatic Advanced Gastric Adenocarcinoma. J. Cancer 2021, 12, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Sun, Y.K.; Xue, X.M.; Yue, J.Y.; Yang, L.; Xue, L.Y. Unnecessity of lymph node regression evaluation for predicting gastric adenocarcinoma outcome after neoadjuvant chemotherapy. World J. Gastrointest. Oncol. 2019, 11, 48–58. [Google Scholar] [CrossRef]
- Pereira, M.A.; Ramos, M.; Dias, A.R.; Cardili, L.; Ribeiro, R.R.E.; Charruf, A.Z.; de Castria, T.B.; Zilberstein, B.; Ceconello, I.; Avancini Ferreira Alves, V.; et al. Lymph node regression after neoadjuvant chemotherapy: A predictor of survival in gastric cancer. J. Surg. Oncol. 2020, 121, 795–803. [Google Scholar] [CrossRef]
- Reim, D.; Novotny, A.; Friess, H.; Slotta-Huspenina, J.; Weichert, W.; Ott, K.; Dislich, B.; Lorenzen, S.; Becker, K.; Langer, R. Significance of tumour regression in lymph node metastases of gastric and gastro-oesophageal junction adenocarcinomas. J. Pathol. Clin. Res. 2020, 6, 263–272. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Sola, J.J.; Diaz-Gonzalez, J.A.; Chopitea, A.; Iragorri, Y.; Martinez-Regueira, F.; Ponz-Sarvise, M.; Arbea, L.; Subtil, J.C.; Cano, D.; et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br. J. Cancer 2016, 115, 655–663. [Google Scholar] [CrossRef]
| Imaging Technique | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| CT | 44.4 [22]–92% [21] | 78.8 [35]–93.4% [22] | 62.8–83.1% [21] | 80.00% [22] | 43.3 [31]–90.1% [21] |
| MRI | 86% [36] | 67% [36] | 78% [55] | 64% [56] | 77.8% [56] |
| PET | 40.3–61% [21] | 97.7% [35] | 69.9 [57]–85.8% [58] | 95.2% [57] | 46.1% [57] |
| EUS | 63–83% [21] | 80–95% [21] | 75% [21] | 98% [59] | 94.1% [59] |
| SL | 53% [60] | 91% [60] | 88% [60] | 53% [60] | 90% [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelc, Z.; Skórzewska, M.; Rawicz-Pruszyński, K.; Polkowski, W.P. Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment—Oncological and Surgical Perspective. Cancers 2021, 13, 2509. https://doi.org/10.3390/cancers13102509
Pelc Z, Skórzewska M, Rawicz-Pruszyński K, Polkowski WP. Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment—Oncological and Surgical Perspective. Cancers. 2021; 13(10):2509. https://doi.org/10.3390/cancers13102509
Chicago/Turabian StylePelc, Zuzanna, Magdalena Skórzewska, Karol Rawicz-Pruszyński, and Wojciech P. Polkowski. 2021. "Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment—Oncological and Surgical Perspective" Cancers 13, no. 10: 2509. https://doi.org/10.3390/cancers13102509
APA StylePelc, Z., Skórzewska, M., Rawicz-Pruszyński, K., & Polkowski, W. P. (2021). Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment—Oncological and Surgical Perspective. Cancers, 13(10), 2509. https://doi.org/10.3390/cancers13102509







