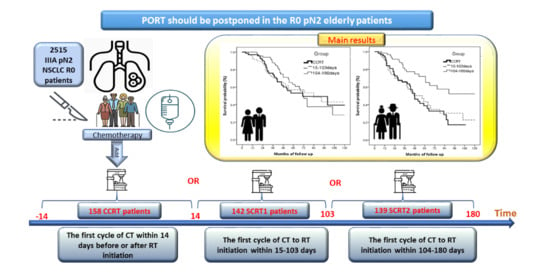
Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient Selection and Characteristics
3.2. Factors Associated with Patient Survival
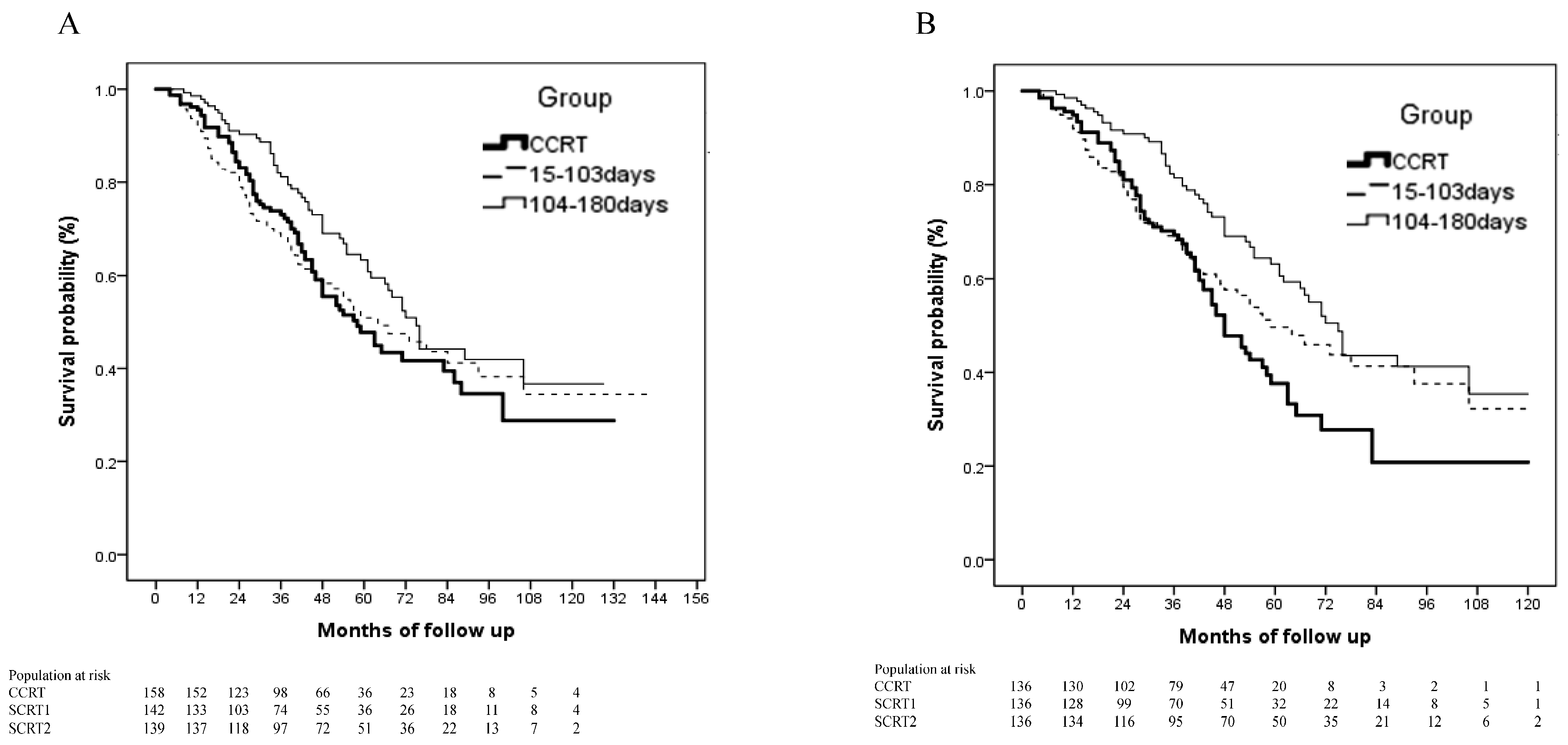
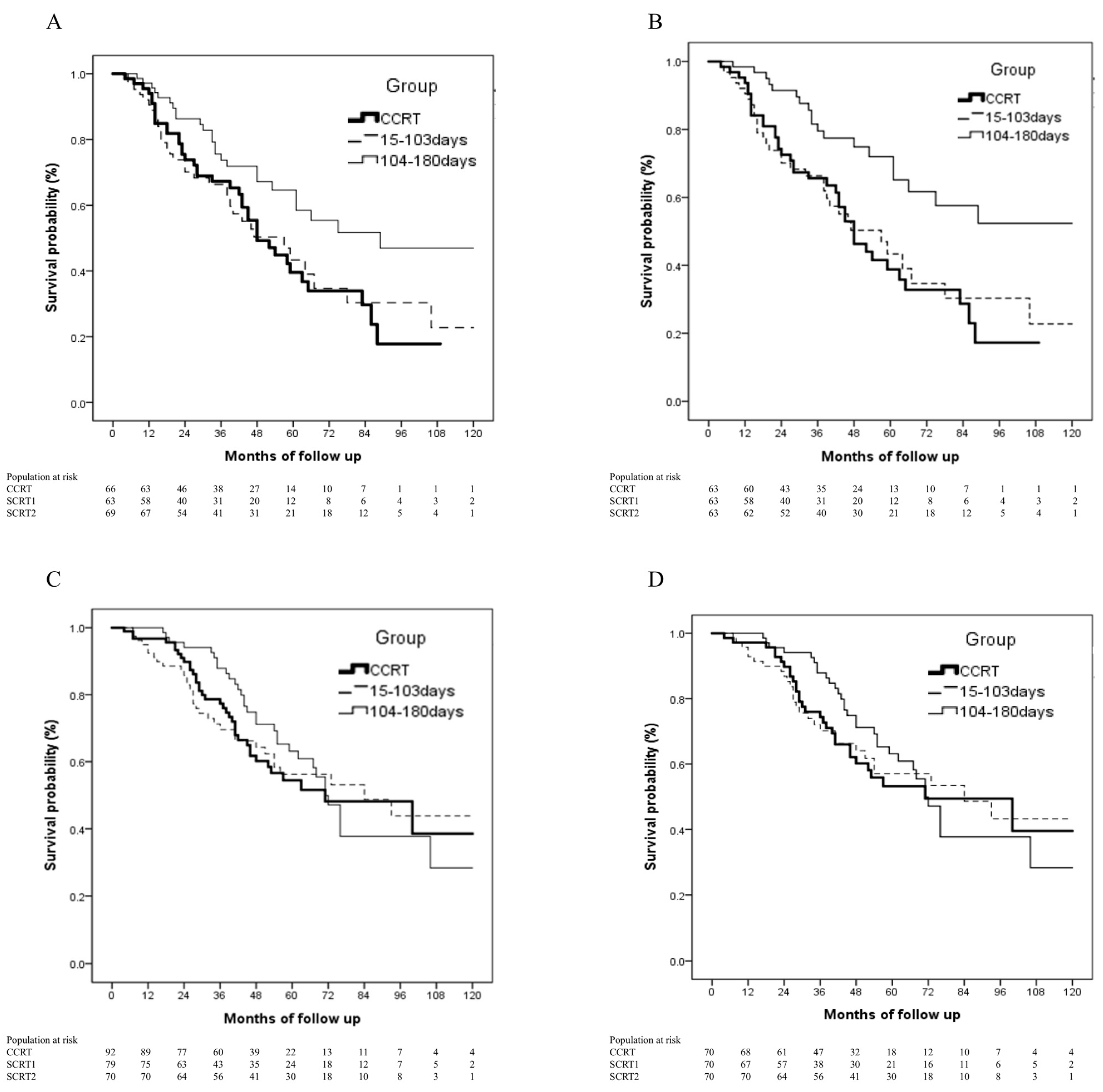
3.3. Impact of Interval between Post-Operative Chemotherapy and Radiotherapy on Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CIs | confidence intervals |
| aHR | adjusted hazard ratio |
| HR | hazard ratio |
| ASC | adenosquamous cell carcinoma |
| CCRT | concurrent chemoradiation |
| ECOG | The Eastern Cooperative Oncology Group |
| EGFR | Epidermal growth factor receptor |
| IMRT | intensity-modulated radiation therapy |
| IQR | interquartile range |
| RT | radiotherapy |
| SD | standard deviation |
| SCRT1 | sequential chemoradiation group 1 |
| SCRT2 | sequential chemoradiation group 2 |
| SqCC | Squamous cell carcinoma |
| TCRD | Taiwan Cancer Registry Database |
| NSCLC | non-small-cell lung cancer |
| OS | overall survival |
| PORT | postoperative radiotherapy |
| PSM | propensity score matching |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pignon, J.E.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.; Shepherd, F.A.; Dunant, R.J.S.; Torri, V.; Rosell, R.; Seymour, L.; Spiro, S.G.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Arriagada, R.; Bergman, B.; Dunant, A.; le Chevalier, T.; Pignon, J.; Vansteenkiste, J.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar] [CrossRef] [PubMed]
- PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998, 352, 257–263. [Google Scholar] [CrossRef]
- Bekelman, J.E.; Rosenzweig, K.E.; Bach, P.B.; Schrag, D. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 492–499. [Google Scholar] [CrossRef]
- Urban, D.; Bar, J.; Solomon, B.; Ball, D. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Le Péchoux, C. Role of postoperative radiotherapy in resected non-small cell lung cancer: A reassessment based on new data. Oncologist 2011, 16, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Corso, C.D.; Rutter, C.E.; Wilson, L.D.; Kim, A.W.; Decker, R.H.; Husain, Z.A. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. J. Thorac. Oncol. 2015, 10, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mikell, J.L.; Gillespie, T.W.; Hall, W.A.; Nickleach, D.C.; Liu, Y.; Lipscomb, J.; Ramalingam, S.S.; Rajpara, R.S.; Force, S.D.; Fernandez, G.F.; et al. Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes: Results of an analysis of the National Cancer Data Base. J. Thorac. Oncol. 2015, 10, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.G.; Patel, A.P.; Bradley, J.D.; DeWees, T.; Waqar, S.N.; Morgensztern, D.; Baggstrom, M.Q.; Go-vindan, R.; Bell, J.M.; Guthrie, T.J.; et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: A review of the National Cancer Data Base. J. Clin. Oncol. 2015, 33, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Herskovic, A.; Mauer, E.; Christos, P.; Nagar, H. Role of postoperative radiotherapy in pathologic stage IIIA (N2) Non-Small Cell Lung Cancer in a Prospective Nationwide Oncology Outcomes Database. J. Thorac. Oncol. 2017, 12, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Noh, O.K.; Oh, Y.-T.; Choi, J.-H.; Chun, M.; Kim, H.-I.; Heo, J.; Ahn, M.S.; Park, S.Y.; Park, R.W.; et al. Radiation therapy-first strategy after surgery with or without adjuvant chemotherapy in stage IIIA-N2 non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 621–627. [Google Scholar] [CrossRef]
- Wang, H.-H.; Deng, L.; Wen, Q.-L.; Zhang, C.-Z.; Zaorsky, N.G.; Zhang, B.-L.; Chen, J.; Zeng, X.-L.; Cui, Y.-L.; Shi, Y.-Y.; et al. Early postoperative radiotherapy is associated with improved outcomes over late postoperative radiotherapy in the management of completely resected (R0) Stage IIIA-N2 non-small cell lung cancer. Oncotarget 2017, 8, 62998–63013. [Google Scholar] [CrossRef][Green Version]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Riggi, M.; Hurteloup, P.; Mahe, M.-A. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The Adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 695–701. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Faivre-Finn, C.; Lerouge, D.; Zalcman, G.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann. Oncol. 2020, 31, S1178. [Google Scholar]
- Hirsch, F.R.; Bunn, P.A., Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009, 10, 432–433. [Google Scholar] [CrossRef]
- Chiang, C.J.; You, S.L.; Chen, C.J.; Yang, Y.W.; Lo, W.C.; Lai, M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am. Stat. 1985, 39, 33–38. [Google Scholar] [CrossRef]
- Moreno, A.C.; Haque, W.; Verma, V.; Fang, P.; Lin, S.H. Concurrent versus sequential chemoradiation therapy in completely resected pathologic N2 non-small cell lung cancer: Propensity-matched analysis of the National cancer data Base. Ann. Surg. Oncol. 2018, 25, 1245–1253. [Google Scholar] [CrossRef]
- Francis, S.; Orton, A.; Stoddard, G.; Tao, R.; Hitchcock, Y.J.; Akerley, W.; Kokeny, K.E. Sequencing of postoperative radiotherapy and chemotherapy for locally advanced or incompletely resected non-small-cell lung cancer. J. Clin. Oncol. 2018, 36, 333–341. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef]
- Ou, W.; Sun, H.-B.; Ye, X.; Zhang, B.-B.; Yang, H.; Fang, Q.; Li, P.; Wang, S.-Y. Adjuvant carboplatin-based chemotherapy in resected stage IIIA-N2 non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Burdett, S.; Rydzewska, L.; Tierney, J.F.; Fisher, D.J. PORT Meta-analysis Trialist Group. A closer look at the effects of postoperative radiotherapy by stage and nodal status: Updated results of an individual participant data meta-analysis in non-small-cell lung cancer. Lung Cancer 2013, 80, 350–352. [Google Scholar] [CrossRef]
- Grills, I.S.; Yan, D.; Martinez, A.A.; A Vicini, F.; Wong, J.W.; Kestin, L.L. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: A comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 875–890. [Google Scholar] [CrossRef]
- Liao, B.-C.; Shao, Y.-Y.; Chen, H.-M.; Shau, W.-Y.; Lin, Z.-Z.; Kuo, R.N.; Lai, C.-L.; Chen, K.-H.; Cheng, A.-L.; Yang, J.C.-H.; et al. Comparative effectiveness of first-line platinum-based chemotherapy regimens for advanced lung squamous cell carcinoma. Clin. Lung Cancer 2015, 16, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-H.; Shao, Y.-Y.; Liao, B.-C.; Lee, H.-S.; Yang, J.C.-H.; Chen, H.-M.; Chiang, C.-J.; Cheng, A.-L.; Lai, M.-S. Cytotoxic chemotherapy as first-line therapy for advanced non-small-cell lung cancer in Taiwan: Daily practice. J. Cancer 2016, 7, 1515–1523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.-L.; Lu, S.; Cheng, Y.; Zhou, C.; Wang, M.; Qin, S.; Lu, Y.; Zhang, Y.; Zhu, Y.; Song, X.; et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 2014, 85, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Kang, J.H.; Mok, T.; Ahn, M.-J.; Srimuninnimit, V.; Lin, C.-C.; Kim, N.-W.; Tsai, C.-M.; Barraclough, H.; Altug, S.; et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: A randomised, phase 3 trial. Eur. J. Cancer 2014, 50, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.; Parikh, P.; Von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Friedlaender, A.; Addeo, A.; Russo, A.; Gregorc, V.; Cortinovis, D.; Rolfo, C.D. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int. J. Mol. Sci. 2020, 21, 6329. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.F.; Kelsey, C.R.; Kirkpatrick, J.P.; Marks, L.B.; Kelsey, C. Estimating the magnitude and field-size dependence of radiotherapy-induced mortality and tumor control after postoperative radiotherapy for non-small-cell lung cancer: Calculations from clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Billiet, C.; Decaluwé, H.; Peeters, S.; Vansteenkiste, J.; Dooms, C.; Haustermans, K.; De Leyn, P.; De Ruysscher, D. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: A meta-analysis. Radiother. Oncol. 2014, 110, 3–8. [Google Scholar] [CrossRef] [PubMed]


| Variables | CCRT | SCRT1 | SCRT2 | p Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | 0.882 | ||||||
| Male | 77 | 48.7 | 72 | 50.7 | 71 | 51.1 | |
| Female | 81 | 51.3 | 70 | 49.3 | 68 | 48.9 | |
| Age at diagnosis, years | |||||||
| Mean ± SD | 57.42 ± 10.75 | 58.54 ± 10.13 | 60.55 ± 9.37 | 0.029 | |||
| Year of diagnosis | 0.816 | ||||||
| 2007–2010 | 35 | 22.2 | 33 | 23.2 | 28 | 20.1 | |
| 2011–2017 | 123 | 77.8 | 109 | 76.8 | 111 | 79.9 | |
| Facility type | 0.033 | ||||||
| Regional hospital | 71 | 44.9 | 69 | 48.6 | 47 | 33.8 | |
| Medical center | 87 | 55.1 | 73 | 51.4 | 92 | 66.2 | |
| Surgery | 0.718 | ||||||
| Lobectomy | 152 | 96.2 | 137 | 96.5 | 133 | 95.7 | |
| Pneumonectomy | 2 | 1.3 | 0 | 0.0 | 1 | 0.7 | |
| Segmental resection | 4 | 2.5 | 5 | 3.5 | 5. | 3.6 | |
| Histology | 0.633 | ||||||
| Adenocarcinoma | 120 | 75.9 | 117 | 82.4 | 107 | 77.0 | |
| SqCC | 19 | 12.0 | 12 | 8.5 | 16 | 11.5 | |
| ASC | 4 | 2.5 | 4 | 2.8 | 7 | 5.0 | |
| Others | 15 | 9.5 | 9 | 6.3 | 9 | 6.5 | |
| Grade (differentiation) | <0.001 | ||||||
| Well, moderately | 87 | 55.1 | 72 | 50.7 | 70 | 50.4 | |
| Poorly | 49 | 31.0 | 65 | 45.8 | 68 | 48.9 | |
| Undifferentiated and unknown | 22 | 13.9 | 5 | 3.5 | 1 | 0.7 | |
| Tumor size (cm) | 0.595 | ||||||
| ≤3 | 69 | 43.7 | 62 | 44.0 | 62 | 44.6 | |
| >3–5 | 70 | 44.3 | 56 | 39.7 | 52 | 37.4 | |
| >5 | 19 | 12.0 | 23 | 16.3 | 25 | 18.0 | |
| Pathologic T stage | 0.072 | ||||||
| I | 35 | 22.2 | 47 | 33.1 | 29 | 20.9 | |
| II | 101 | 63.9 | 84 | 59.2 | 91 | 65.5 | |
| III | 22 | 13.9 | 11 | 7.7 | 19 | 13.7 | |
| Tumor site | 0.325 | ||||||
| Upper lobe | 73 | 46.2 | 78 | 54.9 | 80 | 57.6 | |
| Middle lobe | 17 | 10.8 | 15 | 10.6 | 8 | 5.8 | |
| Lower lobe | 65 | 41.1 | 48 | 33.8 | 50 | 36.0 | |
| Central region | 3 | 1.9 | 1 | 0.7 | 1 | 0.7 | |
| RT technique | 0.025 | ||||||
| 2D and 3D | 18 | 11.4 | 33 | 23.2 | 24 | 17.3 | |
| IMRT | 140 | 88.6 | 109 | 76.8 | 115 | 82.7 | |
| Radiation dose (cGy) | |||||||
| 4500–5500 | 115 | 72.8 | 99 | 69.7 | 107 | 77.0 | 0.415 |
| 5501–6000 | 28 | 17.7 | 27 | 19.0 | 25 | 18.0 | |
| >6000 | 15 | 9.5 | 16 | 11.3 | 7 | 5.0 | |
| RT treatment time | |||||||
| Mean ± SD | 41.1 ± 6.4 | 39.8 ± 6.6 | 38.0 ± 4.5 | <0.001 | |||
| EGFR mutation status | 0.297 | ||||||
| Wild type | 38 | 24.1 | 35 | 24.7 | 24 | 17.3 | |
| Mutation | 31 | 19.6 | 34 | 23.9 | 34 | 24.5 | |
| Unknown | 89 | 56.3 | 73 | 51.4 | 81 | 58.2 | |
| ECOG scale of performance status | 0.567 | ||||||
| 0–1 | 112 | 70.9 | 93 | 65.5 | 100 | 72.0 | |
| ≥2 | 3 | 1.9 | 3 | 2.1 | 1 | 0.7 | |
| Unknown | 43 | 27.2 | 46 | 32.4 | 38 | 27.3 | |
| Smoking habit | 0.168 | ||||||
| Non-smoker | 79 | 50.0 | 68 | 47.9 | 61 | 43.9 | |
| Smoker | 27 | 17.1 | 16 | 11.3 | 27 | 19.4 | |
| Quit smoking | 17 | 10.8 | 25 | 17.6 | 23 | 16.6 | |
| Unknown | 35 | 22.1 | 33 | 23.2 | 28 | 20.1 | |
| Median follow time (months) | |||||||
| Median [IQR] | 42.0 (25.5–58) | 38.0 (20–60) | 48.0 (33–72) | 0.029 | |||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Group | ||||
| CCRT | 1 | 1 | ||
| SCRT1 | 0.98 (0.70–1.37) | 0.906 | 0.97 (0.69–1.36) | 0.850 |
| SCRT2 | 0.71 (0.50–1.00) | 0.050 | 0.64 (0.44–0.92) | 0.017 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.75 (0.57–0.99) | 0.046 | 0.91 (0.68–1.22) | 0.520 |
| Age at diagnosis (year) | ||||
| ≤60 | 1 | 1 | ||
| >60 | 1.37 (1.04–1.81) | 0.027 | 1.02 (1.00–1.03) | 0.016 |
| Year of diagnosis | ||||
| 2007–2010 | 1 | |||
| 2011–2017 | 0.79 (0.58–1.07) | 0.129 | ||
| Facility Type | ||||
| Medical center | 1 | 1 | ||
| Regional hospital | 1.31 (0.99–1.73) | 0.059 | 1.41 (1.06–1.89) | 0.019 |
| Surgery | ||||
| Segmental resection | 1 | 1 | ||
| Lobectomy | 1.71 (0.55–5.34) | 0.359 | 1.53 (0.48–4.92) | 0.473 |
| Pneumonectomy | 4.81 (0.80–28.84) | 0.086 | 3.05 (0.47–19.85) | 0.244 |
| Histology | ||||
| Adenocarcinoma | 1 | |||
| SqCC | 1.06 (0.67–1.70) | 0.795 | ||
| ASC | 0.93 (0.41–2.10) | 0.856 | ||
| Others | 0.63 (0.33–1.19) | 0.152 | ||
| Grade (differentiation) | ||||
| Well and moderately | 1 | |||
| Poorly | 0.84 (0.62–1.12) | 0.233 | ||
| Undifferentiated and unknown | 0.71 (0.97–1.35) | 0.297 | ||
| Tumor size (cm) | ||||
| ≤3 | 1 | 1 | ||
| >3–5 | 1.14 (0.83–1.55) | 0.423 | 1.18 (0.86–1.63) | 0.308 |
| >5 | 2.26 (1.55–3.29) | <0.001 | 2.50 (1.68–3.73) | <0.001 |
| Pathologic T stage | ||||
| I | 1 | |||
| II | 1.23 (0.88–1.72) | 0.226 | ||
| III | 1.44 (0.87–2.38) | 0.153 | ||
| Tumor site | ||||
| Upper lobe | 1 | 1 | ||
| Middle lobe | 1.22 (0.77–1.95) | 0.399 | 1.36 (0.85–2.19) | 0.202 |
| Lower lobe | 0.75 (0.55–1.02) | 0.063 | 0.71 (0.51–0.98) | 0.037 |
| Central region | 1.87 (0.59–5.90) | 0.286 | 1.61(0.47–5.48) | 0.447 |
| Radiotherapy technique | ||||
| 2D+3D | 1 | |||
| IMRT | 1.06 (0.75–1.50) | 0.735 | ||
| Radiation dose (cGy) | ||||
| 4500–5500 | 1 | |||
| 5501–6000 | 0.78 (0.53–1.14) | 0.200 | ||
| >6000 | 0.95 (0.57–1.59) | 0.842 | ||
| RT treatment time | 1.01(0.98–1.03) | 0.631 | 0.99 (0.969–1.018) | 0.573 |
| EGFR mutation status | ||||
| Wild type | 1 | |||
| Mutation | 0.76 (0.48–1.21) | 0.245 | ||
| ECOG scale of performance status | ||||
| 0–1 | 1 | |||
| ≥2 | 4.89 (1.98–12.10) | 0.001 | ||
| Smoking habit | ||||
| Non-smoker | 1 | |||
| Smoker | 0.87 (0.55–1.38) | 0.550 | ||
| Quit smoking | 1.43 (0.93–2.19) | 0.100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-M.; Ku, H.-Y.; Hsu, C.-Y.; Wang, C.-L.; Chang, G.-C.; Chang, C.-S.; Liu, T.-W. Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer. Cancers 2021, 13, 2494. https://doi.org/10.3390/cancers13102494
Lin S-M, Ku H-Y, Hsu C-Y, Wang C-L, Chang G-C, Chang C-S, Liu T-W. Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer. Cancers. 2021; 13(10):2494. https://doi.org/10.3390/cancers13102494
Chicago/Turabian StyleLin, Shih-Min, Hsiu-Ying Ku, Che-Yu Hsu, Chih-Liang Wang, Gee-Chen Chang, Cheng-Shyong Chang, and Tsang-Wu Liu. 2021. "Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer" Cancers 13, no. 10: 2494. https://doi.org/10.3390/cancers13102494
APA StyleLin, S.-M., Ku, H.-Y., Hsu, C.-Y., Wang, C.-L., Chang, G.-C., Chang, C.-S., & Liu, T.-W. (2021). Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer. Cancers, 13(10), 2494. https://doi.org/10.3390/cancers13102494